Abstract
INTRODUCTION
The most common mesenchymal tumour of the gastrointestinal tract is stromal tumours (GISTs). Symptomatic GISTs can present with complications such as haemorrhage, obstruction and perforation. Complete surgical resection with negative margins is the mainstay of treatment but may be imprudent on emergent occasion. Tyrosine-kinase inhibitors (TKIs) have been revolutionary in the treatment of GISTs and have resulted in improved outcomes.
PRESENTATION OF CASE
A 41 year old HIV positive male presented with an acute history of abdominal pain and obstructive symptoms. Clinical examination revealed sepsis and peritonitis. One of the several small bowel tumours discovered at exploratory laparotomy was necrotic and perforated. The perforated tumour alone was resected and a small bowel internal hernia reduced. The patient made an uneventful recovery and will be considered for TKI therapy with a view to later re-operation.
DISCUSSION
GISTs very rarely perforate. The pathophysiology of stromal tumour necrosis is poorly understood. Multifocality and small bowel location are poor prognosticators and may occur in the setting of familial GISTs, specific syndromes and sporadic cases. There is no established association between HIV and GISTs.
CONCLUSION
Perforation occurs infrequently in ≤8% of symptomatic cases and poses increased risk of local recurrence. The surgical management of perforation takes precedence in an emergency. The surgeon must however take cognisance of the adherence to ideal oncologic principles where feasible. TKI therapy is invaluable if a re-exploration is to be later considered.
Keywords: GIST, Peritonitis, Multifocal, Small bowel, Tyrosine-kinase, Imatinib
1. Introduction1–3
Gastrointestinal stromal tumours (GISTs) are the most common mesenchymal tumours of the gastrointestinal tract. Developments in tissue evaluation with specific reference to immunohistochemistry and molecular analysis have led to a deeper understanding of the lesions previously considered to be neurogenic or smooth muscle in origin. Small bowel is involved in 30% of cases, second only to the stomach (60%). 25% of GISTs are asymptomatic and incidentally discovered. Symptomatic GISTs have the potential to present with catastrophic complications. 10–20% of GISTs have metastatic disease upon initial diagnosis. Complete resection with a 1–2 cm negative margin is the mainstay of treatment. Routine regional lymphadenectomy is unnecessary. The role of chemoradiotherapy has been disappointing. Tyrosine-kinase inhibitors (TKIs) have improved survival and reduced local recurrence rates.
The aim of this paper is to describe a rare case of multifocal small bowel GIST presenting with peritonitis secondary to tumour necrosis and to address the surgical issues relating thereto.
2. Case presentation
A 41 year old male presented with a one day history of severe generalised abdominal pain associated with bile-stained vomiting. He denied antecedent trauma, surgery or a family history of gastrointestinal malignancy. The patient was HIV positive. Clinical examination revealed a septic patient with a distended and generally peritonitic abdomen. Neurofibromas and other syndromic features were absent. Blood profile confirmed a leucocytosis and the abdominal radiograph showed features consistent with small bowel obstruction (Fig. 1).
Fig. 1.
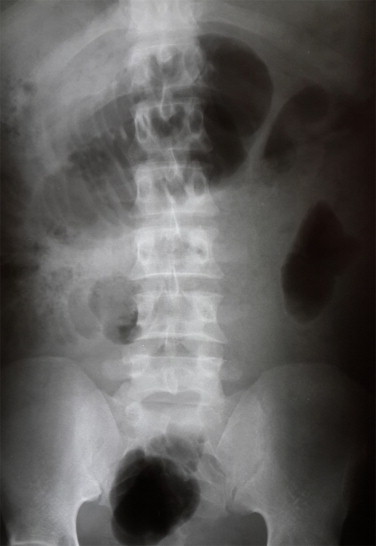
Erect abdominal radiograph showing distended small bowel loops.
An exploratory laparotomy via a midline incision was made (Figs. 2 and 3). Multiple small bowel exophytic masses were discovered, one of which was necrotic and perforated. Large firm mesenteric lymph nodes were present. No masses were found in the remainder of the intra-abdominal gastrointestinal tract and the liver was free of metastatic spread.
Fig. 2.
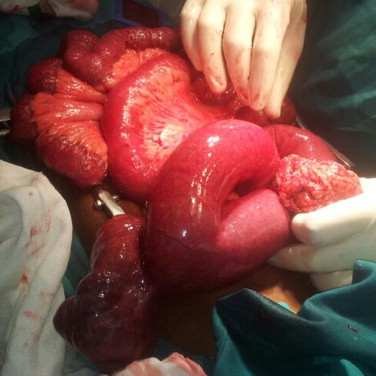
Small bowel volvulus with internal herniation through omental defect. Large associated hanging small bowel tumour is noted.
Fig. 3.
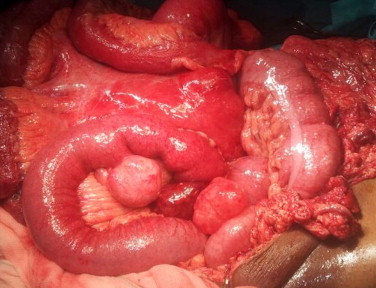
Trio of exophytic small bowel tumours. Central tumour necrosis is evident.
The necrotic mass was amputated at its base and the small bowel defect closed primarily. Thorough peritoneal irrigation preceded routine abdominal closure.
3. Discussion1–4
GISTs are unique mesenchymal tumours arising from the interstitial cells of Cajal and occur anywhere from oesophagus to anorectum. CD117 mutations are characteristic (Table 1). Lymph node and metastatic disease generally appear late. Multifocality, perforation and small bowel location are poor prognosticators present in our patient.5 Accepted poor prognosticators are listed in Table 2. No association between HIV and GISTs has been established and it is believed to be purely incidental in this instance.
Table 1.
GIST immunohistochemical markers.5,8
| Marker | Positivity |
|---|---|
| KIT (CD117) | 100% |
| CD34 | 70% |
| SMA | 20–30% |
| S-100 | 10% |
| Desmin | <5% |
Table 2.
GIST poor prognostic factors.1,3,5,8,10
| Size (>10 cm) | Mitotic index |
| Anatomical location | Advanced/metastatic disease |
| Rupture/perforation | Multifocality |
| Exon 9 mutation | Age extremes |
GISTs display a multitude of presentations depending on site and up to 40% are discovered upon a gastrointestinal bleed.6 Perforation and subsequent peritonitis are exceedingly rare. Tumour necrosis and perforation is poorly understood but is nevertheless theorised to occur from replacement of bowel wall with tumour cells followed by ischaemia from tumour embolisation.7 Perforation is associated with higher rates of local recurrence and reduces 5-year survival to 24%.5,7,8
Multifocal small bowel GISTs has been described in the setting of familial GISTs, specific syndromes (viz. neurofibromatosis type 1 and the Carney-Stratakis Syndrome) and sporadic cases.9 Adult multifocal sporadic GISTs occur in 11% of all multifocal disease, have a predilection for small bowel and stomach and generally display CD117 and CD34 positivity.10
When pre-operative evaluation is possible, small bowel GISTs should be assessed with a CT scan, enteroscopy or capsule endoscopy. Endoscopic biopsy may be precluded by technical factors relating to submucosal tumour position.11 Percutaneous biopsy risks tumour seeding and must be avoided unless the tumour is irresectable or there is radiologic diagnostic doubt.4,8 Complicated GISTs (haemorrhage, obstruction, perforation) warrant urgent open surgical exploration of the abdomen.
Intraoperatively, a decision is made to completely resect all tumours with negative margins where possible. An abbreviated procedure is selectively advocated if the patient is critically ill; liver and peritoneal metastases, synchronous gastrointestinal masses and complications are present.8 In this scenario, the surgeon aims vis-a-vis to acquire tissue for diagnosis with a view to tyrosine-kinase inhibitor therapy initiation on an adjuvant basis. Careful tumour handling cannot be over-emphasised as rupture increases the risk of local recurrence. Distilled water has cytolytic properties and is recommended for peritoneal irrigation in cases of rupture and perforation.7 Re-operation is considered if there is a positive pathological response to TKIs.
Imatinib mesylate has drastically improved survival in patients with GISTs, especially in c-KIT positive patients. Adjuvant therapy is currently administered for three years if there is high histological potential for malignancy adjudged by the Modified National Institute of Health (NIH) criteria (Table 3). Resistance to TKIs is an evolving problem mainly relating to new mutation development.11 Strategies to combat resistance include dose escalation and use of second or third-line agents.
Table 3.
Modified NIH criteria.1
| Tumour characteristics |
|||
|---|---|---|---|
| Diameter (cm) | Mitotic count (per 50HPFs) | Location | |
| Very low risk | <2 | ≤5 | Any |
| Low risk | 2.1–5 | ≤5 | Any |
| Intermediate risk | ≤5 | 6–10 | Gastric |
| Intermediate risk | 5.1–10 | ≤5 | Gastric |
| High risk | >10 | Any | Any |
| High risk | Any size | >10 | Any |
| High risk | >5 | >5 | Any |
| High risk | ≤5 | >5 | Not gastric |
| High risk | 5.1–10 | ≤5 | Not gastric |
4. Conclusion
GISTs present with perforation in ≤8% of symptomatic patients.12 Furthermore, concurrent internal herniation has been reported on only one prior occasion.13 Urgent surgical exploration of the abdomen must be directed to the restoration of physiologic homeostasis and tissue acquisition. The principal goal of an R0 resection of all tumours in an emergent situation is surpassed unless the patient's condition and tumour burden permits a longer operation. TKI therapy and re-operation is a sound surgical strategy.
Conflict of interest statement
No conflict of interest.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Dr. Ebrahim Mansoor – sole contributor and primary author.
References
- 1.Joensuu H., Hohenberger P., Corless C.L. 2013. Gastrointestinal stromal tumour.www.thelancet.com [DOI] [PubMed] [Google Scholar]
- 2.Blackstein, Blay J.Y., Corless C., Driman D.K., Riddell R., Soulières D. Gastrointestinal stromal tumours: consensus statement on diagnosis and treatment. Can J Gastroenterol. 2006;20(3):157–163. doi: 10.1155/2006/434761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan C.B., Zhi W., Shahzad G., Mustacchia P. Gastrointestinal stromal tumors: a review of case reports, diagnosis, treatment, and future directions. ISRN Gastroenterol. 2012 doi: 10.5402/2012/595968. Article ID 595968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rammohan A., Sathyanesan J., Rajendran K., Pitchaimuthu A., Perumal S.K., Srinivasan U.P. A gist of gastrointestinal stromal tumours: a review. World J Gastrointest Oncol. 2013;5(June (6)):102–112. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karagülle E., Türk E., Yildirim E., Göktürk H.S., Kiyici H., Moray G. Multifocal intestinal stromal tumors with jejunal perforation and intra-abdominal abscess: report of a case. Turk J Gastroenterol. 2008;19(4):264–267. [PubMed] [Google Scholar]
- 6.Paramythiotisa D., Panidisa S., Papadopoulosa V., Panagiotoua D., Panteliadoub P., Michalopoulosa A. Perforated gastrointestinal tumour of the small intestine: a case report presenting wit peritonitis. J Curr Surg. 2011;1(1):44–46. [Google Scholar]
- 7.Roy S.D., Khan D., De K.K., De U. Spontaneous perforation of jejunal gastrointestinal stromal tumour (gist). Case report and review of literature. World J Emerg Surg. 2012;7:37. doi: 10.1186/1749-7922-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efremidou E.I., Liratzopoulos N., Papageorgiou M.S., Romanidis K., Manolas K.J., Minopoulos G.J. Perforated GIST of the small intestine as a rare cause of acute abdomen: surgical treatment and adjuvant therapy. Case report. J Gastrointest Liver Dis. 2006;15(3):297–299. [PubMed] [Google Scholar]
- 9.Postow M., Robson M. Inherited gastrointestinal stromal tumor syndromes: mutations, clinical features, and therapeutic implications. Clin Sarcoma Res. 2012;2:16. doi: 10.1186/2045-3329-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Díaz-Delgado M., Hernández-Amate A., Sánchez-León M., Pereira-Gallardo S., Prieto-Sánchez E., Jiménez-Sáenz M. Multiple non-metastatic gastrointestinal stromal tumors. Differential features. Rev Esp Enferm Dig. 2010;102:489–497. doi: 10.4321/s1130-01082010000800006. [DOI] [PubMed] [Google Scholar]
- 11.Lamba G., Gupta R., Lee B., Ambrale S., Liu D. Current management and prognostic features of gastrointestinal stromal tumour (GIST) Exp Hematol Oncol. 2012;1:14. doi: 10.1186/2162-3619-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang S.H., Lam H.B., Liu C.L. A case of gastrointestinal stromal tumor (GIST) of jejunum presenting as hollow organ perforation. J Chin Oncol Soc. 2008;24(1):67–72. [Google Scholar]
- 13.Özben V., Çarkman S., Atasoy D., Doğusoy G., Eyüboğlu E: A case of gastrointestinal stromal tumor presenting with small bowel perforation and internal hernia. Turk J Gastroenterol. 2010;21:470–471. doi: 10.4318/tjg.2010.0142. [DOI] [PubMed] [Google Scholar]


