Abstract
Drosophila melanogaster follicle cells over-replicate the chromosomal domain containing the third chromosome chorion gene cluster. Multiple regions of this cluster are needed in cis for attainment of high levels of amplification. We have confirmed the importance of the proposed amplification control element (ACE3) and demonstrated that it can support low levels of follicular amplification in the absence of other elements, but that it lacks detectable activity as a DNA replication origin. We have also demonstrated the existence of additional amplification-enhancing regions (AERs), by analyzing the amplification levels of a series of in situ induced, nested deletions of the chorion cluster. These deletions were induced by P-transposase perturbation of a chorion transposon in a highly amplifying transformed line, and were not accompanied by re-transposition, making possible a quantitative analysis of amplification levels in the absence of chromosomal position effects. Analysis of endogenous replication intermediates in wild-type follicular DNA suggested that at least one of the AERs may be an origin of replication and that amplification uses at least one additional replication origin.
Full text
PDF






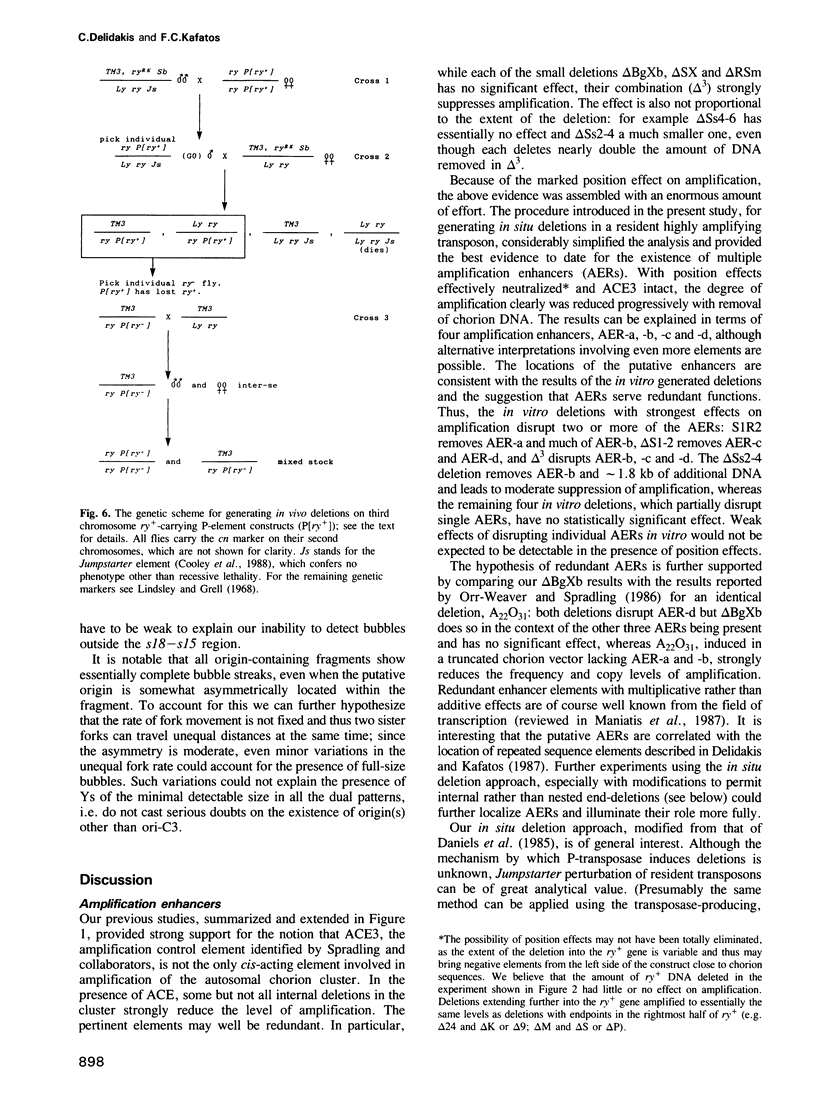



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Isolation of the origin of replication associated with the amplified Chinese hamster dihydrofolate reductase domain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7790–7794. doi: 10.1073/pnas.83.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Gaudray P., De Rose M. L., Emery J. F., Meinkoth J. L., Nakkim E., Subler M., Von Hoff D. D., Wahl G. M. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987 May;7(5):1740–1750. doi: 10.1128/mcb.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Daniels S. B., McCarron M., Love C., Chovnick A. Dysgenesis-induced instability of rosy locus transformation in Drosophila melanogaster: analysis of excision events and the selective recovery of control element deletions. Genetics. 1985 Jan;109(1):95–117. doi: 10.1093/genetics/109.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Transcriptional elements as components of eukaryotic origins of DNA replication. Cell. 1988 Mar 11;52(5):635–638. doi: 10.1016/0092-8674(88)90398-4. [DOI] [PubMed] [Google Scholar]
- Delidakis C., Kafatos F. C. Amplification of a chorion gene cluster in Drosophila is subject to multiple cis-regulatory elements and to long-range position effects. J Mol Biol. 1987 Sep 5;197(1):11–26. doi: 10.1016/0022-2836(87)90605-x. [DOI] [PubMed] [Google Scholar]
- Engels W. R. The P family of transposable elements in Drosophila. Annu Rev Genet. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. DNA replication: direction and rate of chain growth in mammalian cells. J Cell Biol. 1973 Aug;58(2):410–418. doi: 10.1083/jcb.58.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Spotila L. D., Nawotka K. A., el-Assouli S. M., Davis L. R. The in vivo replication origin of the yeast 2 microns plasmid. Cell. 1987 Nov 6;51(3):473–481. doi: 10.1016/0092-8674(87)90643-x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Mitsialis S. A., Spoerel N., Mariani B., Lingappa J. R., Delidakis C. Studies on the developmentally regulated expression and amplification of insect chorion genes. Cold Spring Harb Symp Quant Biol. 1985;50:537–547. doi: 10.1101/sqb.1985.050.01.066. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Kearsey S. E., Mechali M., Dingwall C., Mills A. D., Dilworth S. M., Kleinschmidt J. Chromosome replication in early Xenopus embryos. Cold Spring Harb Symp Quant Biol. 1985;50:657–663. doi: 10.1101/sqb.1985.050.01.080. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Lingappa J. R., Kafatos F. C. Temporal regulation in development: negative and positive cis regulators dictate the precise timing of expression of a Drosophila chorion gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3029–3033. doi: 10.1073/pnas.85.9.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cruzado J. C., Swimmer C., Fenerjian M. G., Kafatos F. C. Evolution of the autosomal chorion locus in Drosophila. I. General organization of the locus and sequence comparisons of genes s15 and s19 in evolutionary distant species. Genetics. 1988 Jul;119(3):663–677. doi: 10.1093/genetics/119.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchali M., Kearsey S. Lack of specific sequence requirement for DNA replication in Xenopus eggs compared with high sequence specificity in yeast. Cell. 1984 Aug;38(1):55–64. doi: 10.1016/0092-8674(84)90526-9. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Spradling A. C. Drosophila chorion gene amplification requires an upstream region regulating s18 transcription. Mol Cell Biol. 1986 Dec;6(12):4624–4633. doi: 10.1128/mcb.6.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W., Komitopoulou K., Kafatos F. C. Mutants suppressing in trans chorion gene amplification in Drosophila. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3773–3777. doi: 10.1073/pnas.81.12.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. Visualization of Drosophila melanogaster chorion genes undergoing amplification. Mol Cell Biol. 1988 Jul;8(7):2811–2821. doi: 10.1128/mcb.8.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr Novel amplification and transcriptional activity of chorion genes in Drosophila melanogaster follicle cells. Cell. 1983 Jun;33(2):543–553. doi: 10.1016/0092-8674(83)90435-x. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Steller H., Bozzetti M. P. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985 Dec 16;4(13A):3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Cis-acting negative control of DNA replication in eukaryotic cells. Cell. 1988 Feb 12;52(3):397–404. doi: 10.1016/s0092-8674(88)80032-1. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer L. D., Miller O. L., Jr Electron microscopic study of Saccharomyces cerevisiae rDNA chromatin replication. Mol Cell Biol. 1986 Apr;6(4):1148–1157. doi: 10.1128/mcb.6.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. A chromosome inversion alters the pattern of specific DNA replication in Drosophila follicle cells. Cell. 1981 Nov;27(1 Pt 2):203–209. doi: 10.1016/0092-8674(81)90374-3. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1096–1100. doi: 10.1073/pnas.77.2.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., de Cicco D. V., Wakimoto B. T., Levine J. F., Kalfayan L. J., Cooley L. Amplification of the X-linked Drosophila chorion gene cluster requires a region upstream from the s38 chorion gene. EMBO J. 1987 Apr;6(4):1045–1053. doi: 10.1002/j.1460-2075.1987.tb04857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Orr-Weaver T. Regulation of DNA replication during Drosophila development. Annu Rev Genet. 1987;21:373–403. doi: 10.1146/annurev.ge.21.120187.002105. [DOI] [PubMed] [Google Scholar]
- Williamson D. H. The yeast ARS element, six years on: a progress report. Yeast. 1985 Sep;1(1):1–14. doi: 10.1002/yea.320010102. [DOI] [PubMed] [Google Scholar]
- Zannis-Hadjopoulos M., Persico M., Martin R. G. The remarkable instability of replication loops provides a general method for the isolation of origins of DNA replication. Cell. 1981 Nov;27(1 Pt 2):155–163. doi: 10.1016/0092-8674(81)90369-x. [DOI] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]






