Abstract
Objective:
To evaluate the treatment outcome of second line drugs used in directly observed treatment, short-course (DOTS)-Plus regimen under Revised National Tuberculosis Control Program (RNTCP).
Materials and Methods:
A prospective, observational study was carried out on multidrug resistant tuberculosis (MDR-TB) patients enrolled for DOTS-Plus regimen at TB and Chest Disease Department from January to December 2009. Demographic details, symptoms, sputum examination and adverse drug reactions were recorded in a case record form. Patients were followed up for 24 months. The data were analysed by Fisher's exact test and paired student's ‘t’ test.
Results:
Out of 130 patients, 51 (39%) were cured, 7 (5%) completed the treatment, 25 (19%) died, 30 (23%) defaulted and 17 (13%) failure. A significant increase in body weight (P < 0.0001) was observed at the end of the 24 months. Out of 89 patients with sputum culture conversion, majority (73) turned negative within first 3 months. Female gender (P < 0.05), conversion of sputum culture from positive to negative (P < 0.0001), and radiological improvement (P < 0.0001) were found to be positive predictors of a successful treatment outcome. While smoking habit (P < 0.05) and alcohol consumption (P < 0.05) were negative predictors of successful treatment outcome. Thirty five (26%) patients developed ADRs that required withdrawal of causal drug. The most common ADR was joint pain due to pyrazinamide (11) followed by neurological and psychiatric disturbances due to cycloserine (9).
Conclusion:
The treatment outcome of standardized regimen in MDR-TB patients was low. The long duration of treatment and defaulters are major challenges for a successful outcome.
Keywords: DOTS-Plus, India, MDR-TB, standardized regimen, treatment outcome
INTRODUCTION
Multidrug resistant tuberculosis (MDR-TB) is defined as tuberculosis (TB) resistant to isoniazid and/or rifampicin, the two most powerful anti-TB drugs. [1] The emergence of mutants that resist anti-TB drugs has been a major challenge to control the disease. [2,3] The estimated number of MDR-TB cases has reached 650,000 globally in 2010 with 99,000 from India. [4]
Treatment and control of MDR-TB requires a sound infrastructure with well equipped laboratory facilities to provide quality and prompt diagnosis. [5] Since the drug treatment of MDR-TB is extended up to 24 months and associated with major adverse drug reactions (ADRs), well trained vigilant healthcare workers are essential to provide the treatment services and ensure compliance. [6]
In India, MDR-TB is treated under directly observed treatment short-course (DOTS)-Plus program launched as pilot project in 2007. DOTS-Plus program follows a standardized regimen. The project has demonstrated feasibility and effectiveness of MDR-TB treatment in resource limited countries with 61% successful treatment outcome. [6] However, these studies were done under controlled and standardized conditions. It is more than 5 years since the pilot project was launched in India. Moreover, there are reports of threats of extensively resistant TB. Thus the present study was undertaken to assess the treatment outcome in real life situations and find out the factors that may determine the treatment outcome.
MATERIALS AND METHODS
This prospective, observational study was approved by the Institutional Ethics Committee of Civil Hospital. Prior permission to conduct the study was obtained from the Head of TB and Chest Disease Department.
All patients diagnosed and treated for MDR-TB (except pregnant patients) under Category IV regimen of The Revised National Tuberculosis Control Program (RNTCP) from municipal corporation area in Western India were recruited from January 2009 to December 2009. A total of 130 patients were recruited in the study.
The baseline data of the patients were recorded in pretested case record form. Patients enrolled in the study were treated with daily supervised regimen. Following initial hospitalization for 2-4 weeks, ambulatory treatment was arranged at the nearest peripheral health centre according to the patient's choice. The medicines were delivered to the patients by a DOT provider at the health centre. The standardized regimen consisted of an intensive phase (IP) of 6-9 months with six drugs, namely kanamycin (Km), ofloxacin (Ofx) or levofloxacin (Lvx), ethionamide (Eto), pyrazinamide (Z), ethambutol (E), and cycloserine (Cs) given daily. This was followed by a continuation phase (CP) of 18 months of four drugs, such as Ofx, Eto, E and Cs. Pyridoxine was administered to all patients as a supplement.
Each patient was followed up every month for clinical assessment (body weight), sputum examination, chest X-ray and ADRs till completion of 24 months. Monthly two early morning sputum specimens were examined for smear microscopy and culture for the first year, followed by every 3 months till the end of the study. Chest X-ray was done at the end of intensive phase (6 months) and at the end of the study. For the first 6 months, hematological and biochemical tests (liver and renal function tests) were done every month and thereafter as and when required.
Treatment outcome was categorized as cured, defaulted, failure and death as per the RNTCP guidelines. A patient was declared cured after complete treatment for at least 24 months with last five cultures negative. However, patient having two or more of last five cultures positive was considered as failure. While completed treatment patients did not meet the criteria for cure or failure due to lack of bacteriological results. A patient who interrupted treatment for two or more consecutive months was labeled as default.
The data was recorded in Microsoft Excel Worksheet and analyzed by Fisher's exact test and paired student's ‘t’ test with the help of GraphPad Prism 5.0 software. P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Out of 130 MDR-TB patients, 81 (62%) were men and 49 (38%) women. The mean age was 31.42 ± 12.29 years and mean weight was 42 ± 9.2 kg. Most of the patients 122 (94%) were Category II treatment failure, while 7 patients were treated with Cat I and one with Cat III regimen. Majority of the patients had habit of tobacco chewing 56 (43%) followed by smoking 47 (36%) and alcohol consumption 27 (21%).
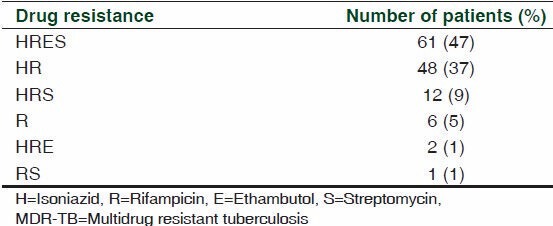
Of 130 MDR-TB patients, 61 (47%) were resistant to all 4 first-line drugs (HRES), 14 (10%) were resistant to 3 first-line drugs (HRS and HRE), 49 (38%) were resistant to 2 first-line drugs (HR and RS) and 6 (5%) were resistant to 1 first-line drug (R) [Table 1].
Table 1.
Pattern of drug resistance of the MDR-TB patients in the study (n=130)
Outcome of second line drugs
Clinical assessment
Following drug treatment, a significant increase in mean body weight was observed at every 3 months till the end of study [Figure 1]. A significant increase in body weight (P < 0.0001) was observed at the end of the 24 months of drug treatment.
Figure 1.
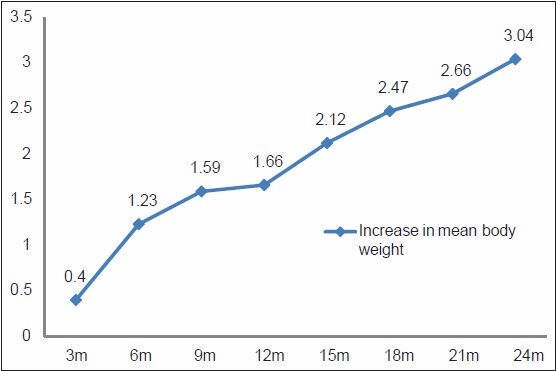
Increase in mean body weight of MDR-TB patients at different time interval (n=130)
Bacteriological improvement
Out of 130 patients, 89 (68%) achieved sputum culture conversion within 9 months. Out of these 89 patients, 73 (82%) became culture-negative within 3 months of treatment, and 84 (94%) within 6 months [Figure 2]. However, sputum remained positive in 41 (32%) patients. Out of 41 patients, 15 died, 18 defaulted, and 8 failed the treatment.
Figure 2.
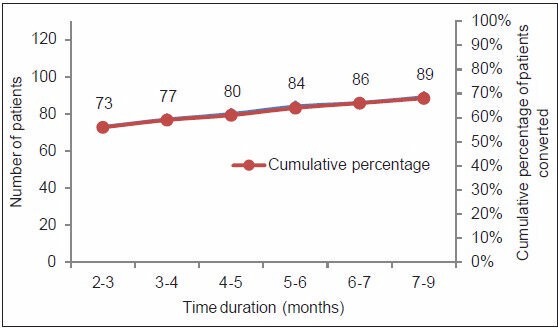
Median time for sputum culture conversion in MDR-TB patients (n=130)
Safety assessment
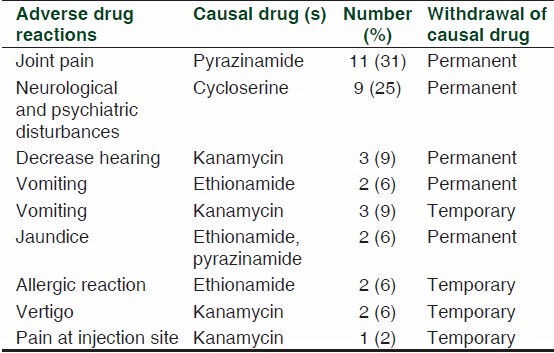
Although the medicines were well tolerated, 35 (26%) patients developed ADRs that required withdrawal of causal drug [Table 2]. Out of 35 ADRs, 27 were serious, while 8 were nonserious as per World Health Organization (WHO) algorithm [Table 2]. The most common ADR was joint pain due to pyrazinamide (11) followed by neurological and psychiatric disturbances due to cycloserine (9). While other ADRs observed were gastrointestinal (vomiting and jaundice) followed by vestibular ototoxicity. It was observed that majority of ADRs were moderately severe (19) followed by mild ADRs (16), while none of the ADR was severe based on modified Hartwig and Siegel scale. Causality assessment showed that majority of ADRs were categorized as probable in nature (27) followed by possible (6) and doubtful (2) by WHO-UMC scale, while 27 ADRs were categorized as probable and 8 as possible in nature by Naranjo scale.
Table 2.
Details of adverse drug reactions observed among MDR-TB patients treated with standardized regime (n=130)
Variables and TB treatment outcome
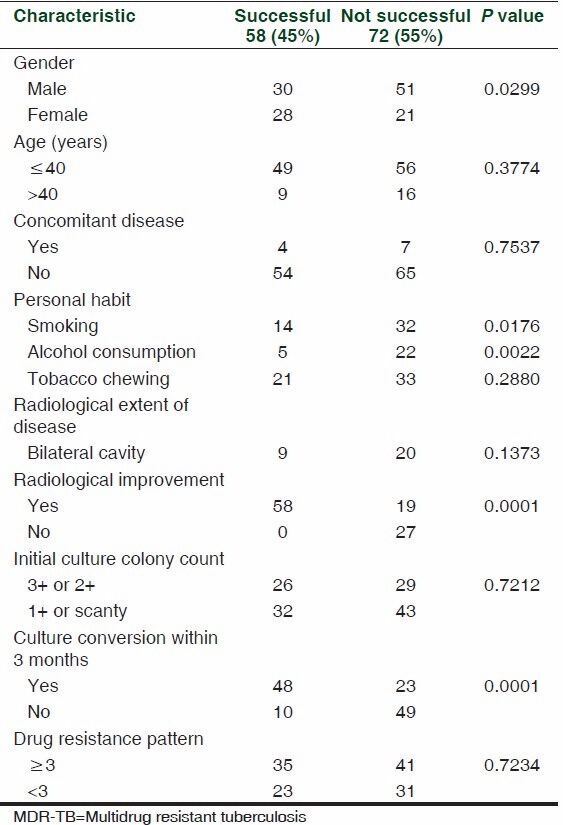
Out of the 130 MDR patients, 58 (45%) had successful outcome, that is, 51 (39%) were cured and 7 (5%) completed the treatment. While 17 (13%) patients had persistent sputum positive, 30 (23%) were defaults, and 25 (19%) died.
An attempt was made to find out the relationship between different variables and treatment outcome in MDR-TB patients. It was observed that variables like gender, personal habit, sputum culture conversion, and radiological improvement may affect the treatment outcome. Female gender (P < 0.05), conversion of sputum culture from positive to negative (P < 0.0001), and radiological improvement during treatment (P < 0.0001) were found to be positively associated with successful treatment outcome in MDR TB. While smoking habit (P < 0.05) and alcohol consumption (P < 0.05) were found to be negatively associated with successful treatment outcome. However, age, concomitant disease, tobacco chewing, extent of the disease radiologically, initial culture colony count and drug resistance pattern at initiation of treatment did not influence the treatment outcome [Table 3].
Table 3.
Factors associated with treatment outcome in MDR-TB patients treated with standardized regimen (n=130)
DISCUSSION
MDR-TB patients received standardized regimen of DOTS-Plus, which includes six medicines for first 6-9 months of intensive phase and four drugs for 18 months of continuation phase. This standardized regimen has shown 61% successful treatment outcome in pilot study. [6] However, our study observed low treatment outcome (45%) and low cure rate as compared with other studies, suggesting discrepancies between pilot study and actual field work. While the cure rate seems to be high from 61% to 77% in other studies [7,8] and 44% to 53% in South Korea, Taiwan and China. [9,10,11] Our study observed that 13% of patients were failure, 23% defaulter, and 19% died. Although DOTS care provider ensures compliance to drug treatment, the defaulter rate was also high. MDR-TB treatment defaulter are potentially harmful to community as these cases can relapse and spread the infection in the community, develop resistance to second line anti-TB drugs, and may result into extensive drug resistant TB (XDR-TB). The long duration of treatment, pill count per day, frequency of ADRs, and lack of education and awareness among patients could be possible reasons for default and are major challenges for a successful treatment outcome. As most of the MDR-TB patients are treated at peripheral DOTS center, this call was to develop strategy to strengthen the treatment care to address these issues.
Sputum smear and culture examination indicates the bacteriological load, indicates infectious status of the patient and bacteriological improvement. Initial sputum culture in first 3 months conversion is essential to label the patients noninfectious. The sputum culture conversion rate varies from 74% to 92% in different studies reported across globally. [6,7,12] Surprisingly, our study observed that out of 73 sputum culture converted patients initially (first 3 months treatment phase), 5 turned back to sputum positive at the end of the study and 9 were defaulter. These 14 patients had a good chance of continuing drug treatment and being considered for successful outcome. However, there were some lapses in continuation phase of therapy, that is, close monitoring, counseling and care by DOT care provider. The long duration of therapy that requires to take medicines daily for more than 6 months and initial improvement in clinical condition may create false impression among patients. Various studies also report a gap between initial sputum culture conversion and successful outcome. Thus, it is essential for a treatment regimen to convert maximum numbers of patients sputum culture negative within first 3 months and subsequently closely following-up the patients in a continuous phase for a successful outcome.
The study also showed that female gender, initial sputum culture conversion within 3 months and radiological improvement were associated with successful outcome. As women are more vigilant and comply to drug treatment as compared with men and they are less liable to default the treatment. Radiological improvement to MDR-TB treatment was a good predictor of a successful outcome with a strong correlation. The successful patients achieved radiological improvement during the first 3 months of treatment. Whereas smoking and alcohol consumption were found to be negative predictors of successful treatment outcome. Some of the factors that did not influence the treatment outcome includes age, concomitant disease, tobacco chewing, extent of the disease radiologically, initial culture colony count, and drug resistance pattern. While other studies report that variables like male gender, alcohol consumption, severe radiological extent of disease, severe clinical condition, previous use of second line anti-TB drugs, and fluoroquinolones resistance were found to be associated with poor treatment outcome. [10,13,14,15] Alcohol consumption has been found to increase the risk of default from treatment and treatment failure. [16] Patients having severe clinical condition with advanced radiological extent of disease might have low immunity status and less likely to respond to the therapy.
Our study had few limitations. For example, it was an observational study conducted at a single center. The patients received treatment at peripheral DOTS center and observed initially by DOTS care provider. The data was retrieved from the treatment cards at the tertiary care centre. Several cards did not mention details of the blood count and liver function tests and thus this information was not analyzed. Also the mild ADRs that did not require any intervention were not reported.
It can be concluded that Category IV regimen of DOTS-Plus under RNTCP program in MDR-TB patients produced significant improvement in body weight, bacteriological, and radiological examination. However, the treatment outcome with standardized regimen has been reduced from 71% to 45% of MDR-TB patients. The long duration of treatment (24 months), pill count per day and lack of education, awareness among patients are major obstacles for successful outcome. To make the program successful, training of DOT care provider and public awareness program of MDR-TB patients needs to be strengthened. There is also a need to modify the design of treatment regimen with respect to drugs, total duration of drug treatment, and number of pills per day.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. Emergency update (WHO/HTM/TB/2008.402) Geneva, Switzerland: 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. [Google Scholar]
- 2.Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, et al. ; Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Epidemiology of antituberculosis drug resistance 2002-07: An updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–73. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 3.Raviglione MC, Smith IM. XDR tuberculosis--implications for global public health. N Engl J Med. 2007;356:656–9. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Control: Report, 2011. [Last accessed on 2012 Apr 21]. Available from: http://www.whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf .
- 5.Nathanson E, Lambregts-van Wezenbeek C, Rich ML, Gupta R, Bayona J, Blöndal K, et al. Multidrug-resistant tuberculosis in resource-limited settings. Emerg Infect Dis. 2006;12:1389–97. doi: 10.3201/eid1209.051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singla R, Sarin R, Khalid UK, Mathuria K, Singla N, Jaiswal A, et al. Seven-year DOTS-Plus pilot experience in India: Results, constraints and issues. Int J Tuberc Lung Dis. 2009;13:976–81. [PubMed] [Google Scholar]
- 7.Van DA, Salim MA, Das AP, Bastian I, Portaels F. Results of a standardised regimen for multidrug-resistant tuberculosis in Bangladesh. Int J Tuberc Lung Dis. 2004;8:560–7. [PubMed] [Google Scholar]
- 8.Shin SS, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Mishustin S, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–8. [PubMed] [Google Scholar]
- 9.Chiang CY, Enarson DA, Yu MC, Bai KJ, Huang RM, Hsu CJ, et al. Outcome of pulmonary multidrug-resistant tuberculosis: A 6-yr follow-up study. Eur Respir J. 2006;28:980–5. doi: 10.1183/09031936.06.00125705. [DOI] [PubMed] [Google Scholar]
- 10.Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004;8:361–8. [PubMed] [Google Scholar]
- 11.Liu CH, Li L, Chen Z, Wang Q, Hu YL, Zhu B, et al. Characteristics and treatment outcomes of patients with MDR and XDR tuberculosis in a TB Referral Hospital in Beijing: A 13-year experience. PLoS One. 2011;6:e19399. doi: 10.1371/journal.pone.0019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad R, Verma SK, Sahai S, Kumar S, Jain A. Efficacy and safety of kanamycin, ethionamide, PAS, and cycloserine in multidrug-resistant pulmonary tuberculosis patients. Indian J Chest Dis Allied Sci. 2006;48:183–6. [PubMed] [Google Scholar]
- 13.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: A systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliiman K, Altraja A. Predictors of poor treatment outcome in multi- and extensively drug-resistant pulmonary TB. Eur Respir J. 2009;33:1085–94. doi: 10.1183/09031936.00155708. [DOI] [PubMed] [Google Scholar]
- 15.Cox HS, Kalon S, Allamuratova S, Sizaire V, Tigay ZN, Rüsch-Gerdes S, et al. Multidrug-resistant tuberculosis treatment outcomes in Karakalpakstan, Uzbekistan: Treatment complexity and XDR-TB among treatment failures. PLoS One. 2007;2:e1126. doi: 10.1371/journal.pone.0001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Kourbatova EV. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]


