Abstract
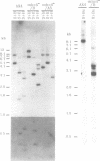
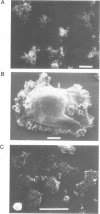
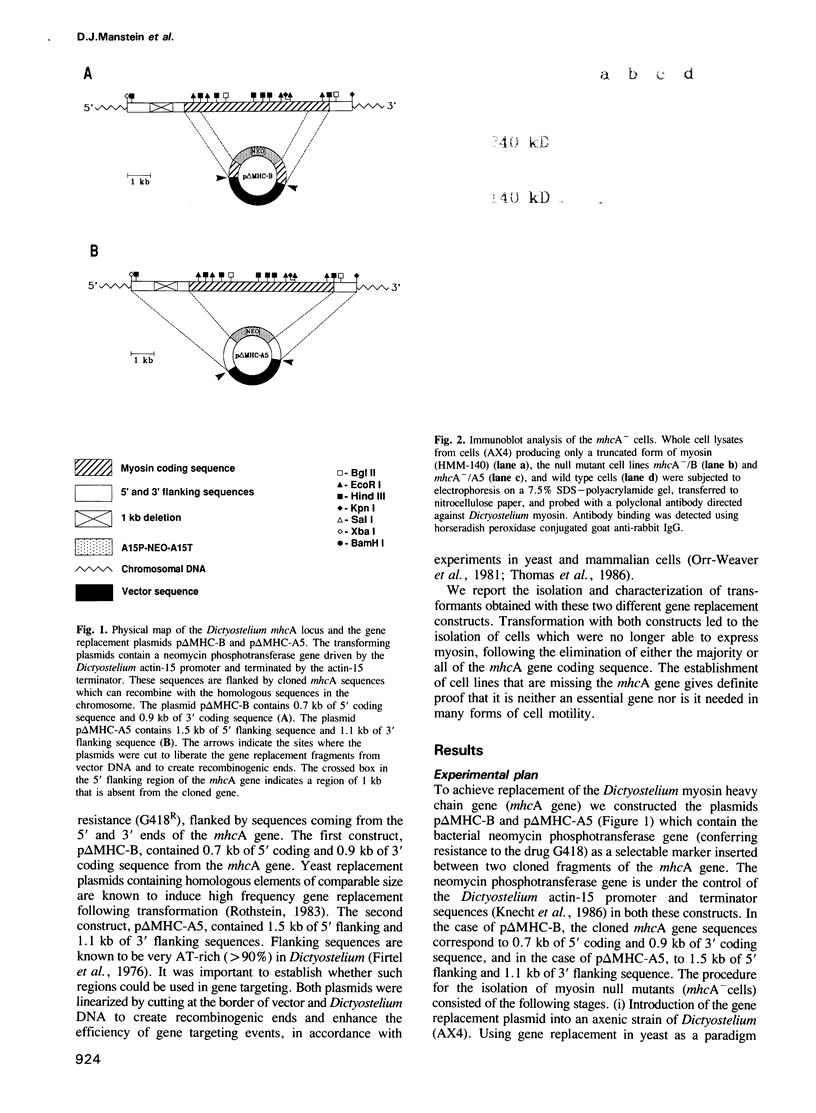
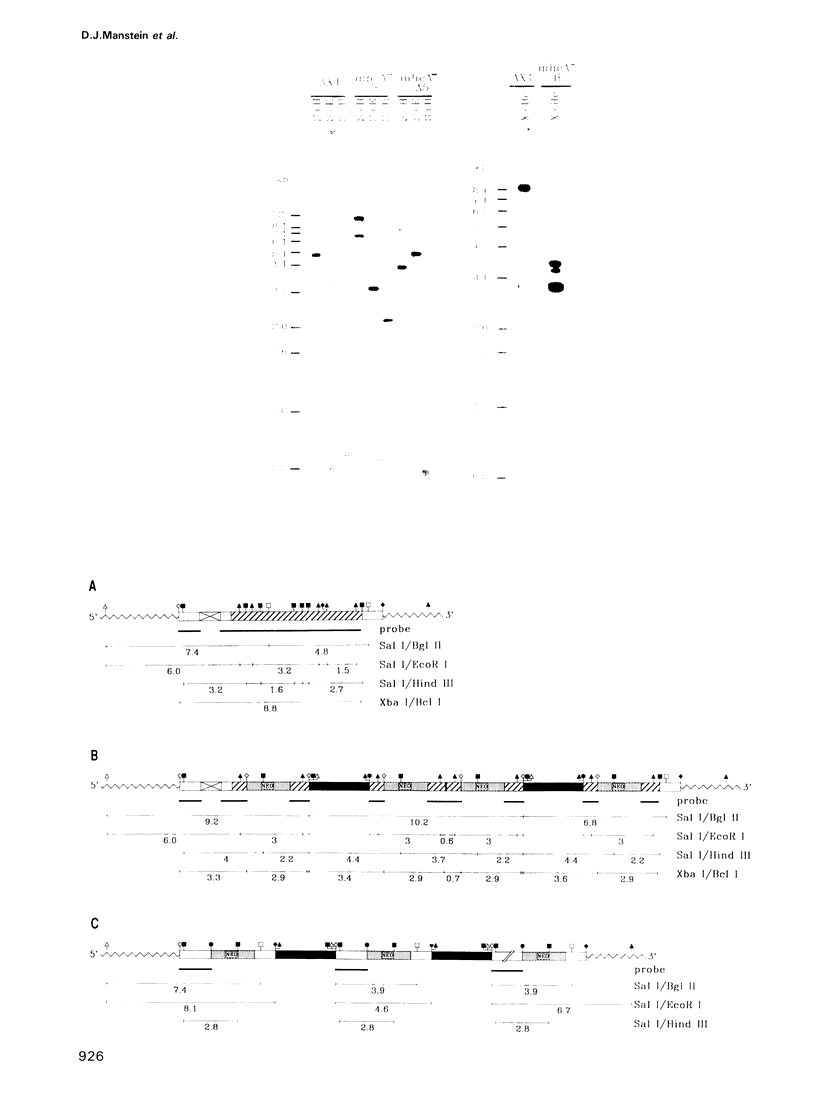
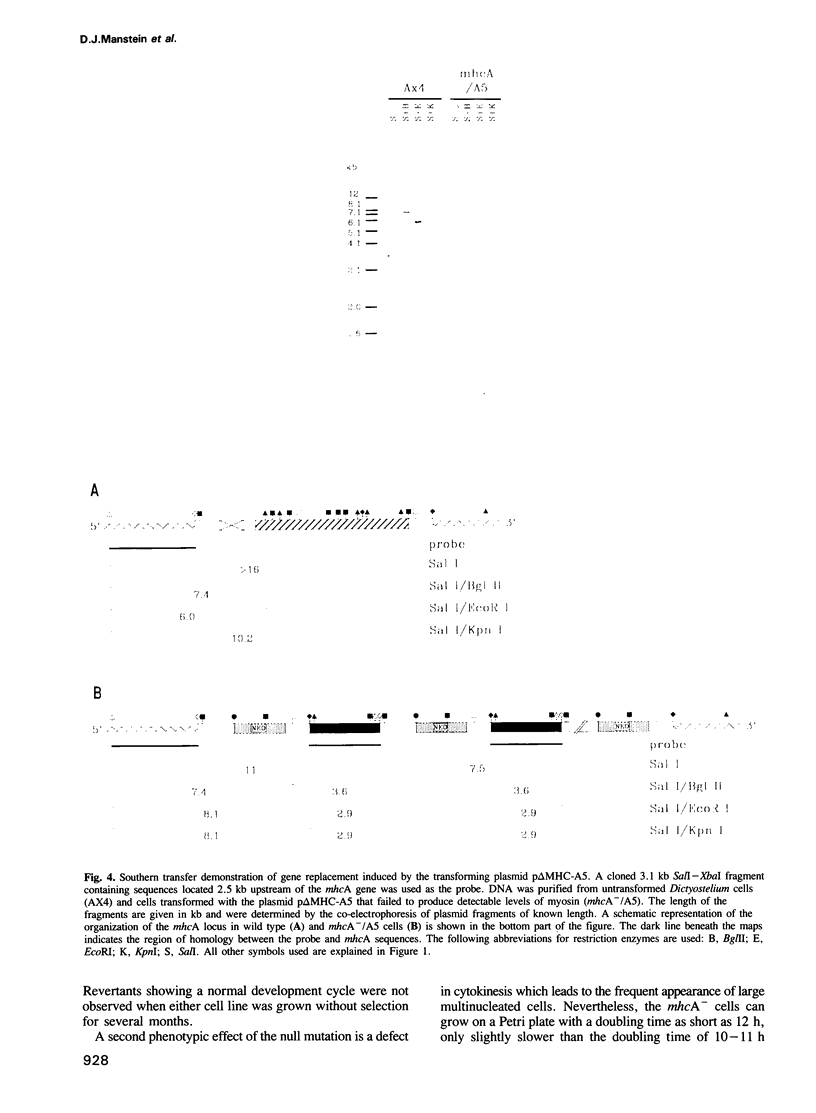
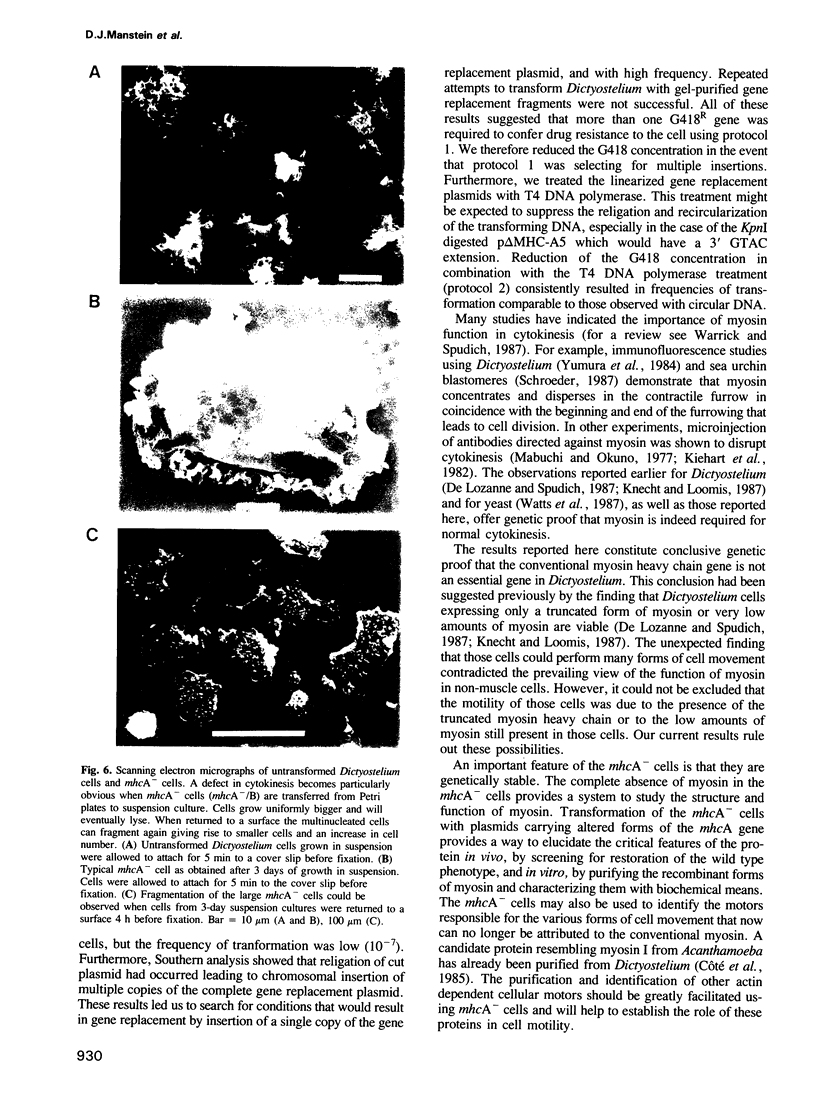
The eukaryotic slime mold Dictyostelium discoideum has a single conventional myosin heavy chain gene (mhcA). The elimination of the mhcA gene was achieved by homologous recombination. Two gene replacement plasmids were constructed, each carrying the G418 resistance gene as a selective marker and flanked by either 0.7 kb of 5' coding sequence and 0.9 kb of 3' coding sequence or 1.5 kb of 5' flanking sequence and 1.1 kb of 3' flanking sequence. Myosin null mutants (mhcA- cells) were obtained after transformation with either of these plasmids. The mhcA- cells are genetically stable and are capable of a variety of motile processes. Our results provide genetic proof that in Dictyostelium the conventional myosin gene is required for growth in suspension, normal cell division and sporogenesis, and illustrate how gene targeting can be used as a tool in Dictyostelium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlot C. H., Spudich J. A., Devreotes P. N. Chemoattractant-elicited increases in myosin phosphorylation in Dictyostelium. Cell. 1985 Nov;43(1):307–314. doi: 10.1016/0092-8674(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Binninger D. M., Skrzynia C., Pukkila P. J., Casselton L. A. DNA-mediated transformation of the basidiomycete Coprinus cinereus. EMBO J. 1987 Apr;6(4):835–840. doi: 10.1002/j.1460-2075.1987.tb04828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Knecht D., Lodish H. F., Loomis W. F. DNA sequences required for expression of a Dictyostelium actin gene. EMBO J. 1986 Dec 1;5(12):3361–3366. doi: 10.1002/j.1460-2075.1986.tb04651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté G. P., Albanesi J. P., Ueno T., Hammer J. A., 3rd, Korn E. D. Purification from Dictyostelium discoideum of a low-molecular-weight myosin that resembles myosin I from Acanthamoeba castellanii. J Biol Chem. 1985 Apr 25;260(8):4543–4546. [PubMed] [Google Scholar]
- Datta S., Firtel R. A. Identification of the sequences controlling cyclic AMP regulation and cell-type-specific expression of a prestalk-specific gene in Dictyostelium discoideum. Mol Cell Biol. 1987 Jan;7(1):149–159. doi: 10.1128/mcb.7.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- DeLozanne A., Lewis M., Spudich J. A., Leinwand L. A. Cloning and characterization of a nonmuscle myosin heavy chain cDNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6807–6810. doi: 10.1073/pnas.82.20.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Cockburn A., Frankel G., Hershfield V. Structural organization of the genome of Dictyostelium discoideum: analysis by EcoR1 restriction endonuclease. J Mol Biol. 1976 Apr 25;102(4):831–852. doi: 10.1016/0022-2836(76)90294-1. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Silan C., Ward T. E., Howard P., Metz B. A., Nellen W., Jacobson A. Extrachromosomal replication of shuttle vectors in Dictyostelium discoideum. Mol Cell Biol. 1985 Nov;5(11):3241–3250. doi: 10.1128/mcb.5.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G. Cyclic AMP and other signals controlling cell development and differentiation in Dictyostelium. Annu Rev Biochem. 1987;56:853–879. doi: 10.1146/annurev.bi.56.070187.004225. [DOI] [PubMed] [Google Scholar]
- Katz K. S., Ratner D. I. Homologous recombination and the repair of double-strand breaks during cotransformation of Dictyostelium discoideum. Mol Cell Biol. 1988 Jul;8(7):2779–2786. doi: 10.1128/mcb.8.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P., Mabuchi I., Inoué S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982 Jul;94(1):165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Cohen S. M., Loomis W. F., Lodish H. F. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol Cell Biol. 1986 Nov;6(11):3973–3983. doi: 10.1128/mcb.6.11.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Lemmon S. K., Jones E. W. Clathrin requirement for normal growth of yeast. Science. 1987 Oct 23;238(4826):504–509. doi: 10.1126/science.3116672. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen W., Datta S., Reymond C., Sivertsen A., Mann S., Crowley T., Firtel R. A. Molecular biology in Dictyostelium: tools and applications. Methods Cell Biol. 1987;28:67–100. doi: 10.1016/s0091-679x(08)61637-4. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Hasson T. B., Hasson M. S., Schekman R. Genetic and biochemical characterization of clathrin-deficient Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):3888–3898. doi: 10.1128/mcb.7.11.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears C. J., Williams J. G. Identification of a DNA sequence element required for efficient expression of a developmentally regulated and cAMP-inducible gene of Dictyostelium discoideum. EMBO J. 1987 Jan;6(1):195–200. doi: 10.1002/j.1460-2075.1987.tb04738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Solomon F., Botstein D. Genetically essential and nonessential alpha-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986 Nov;6(11):3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. Fourth cleavage of sea urchin blastomeres: microtubule patterns and myosin localization in equal and unequal cell divisions. Dev Biol. 1987 Nov;124(1):9–22. doi: 10.1016/0012-1606(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Shortle D., Novick P., Botstein D. Construction and genetic characterization of temperature-sensitive mutant alleles of the yeast actin gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4889–4893. doi: 10.1073/pnas.81.15.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Song K. Y., Schwartz F., Maeda N., Smithies O., Kucherlapati R. Accurate modification of a chromosomal plasmid by homologous recombination in human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6820–6824. doi: 10.1073/pnas.84.19.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. Dictyostelium discoideum: methods and perspectives for study of cell motility. Methods Cell Biol. 1982;25(Pt B):359–364. doi: 10.1016/s0091-679x(08)61433-8. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemez C. L., Heller J. S. Is guanosine diphosphate-D-glucose a precursor of cellulose? Nature. 1970 Jul 4;227(5253):80–81. doi: 10.1038/227080a0. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., De Lozanne A., Leinwand L. A., Spudich J. A. Conserved protein domains in a myosin heavy chain gene from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9433–9437. doi: 10.1073/pnas.83.24.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Watts F. Z., Shiels G., Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987 Nov;6(11):3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witke W., Nellen W., Noegel A. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987 Dec 20;6(13):4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura S., Mori H., Fukui Y. Localization of actin and myosin for the study of ameboid movement in Dictyostelium using improved immunofluorescence. J Cell Biol. 1984 Sep;99(3):894–899. doi: 10.1083/jcb.99.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]