Abstract
Objectives:
To assess the level of homocysteine (tHcy) in children taking AEDs and to study whether daily oral supplementation of folic acid for 1 month will reduce the tHcy level.
Materials and Methods:
This was a double-blinded, randomized control trial conducted in Institute of Maternal and Child Health, Kozhikode, India. Totally 60 children were recruited and of them, 48 were enrolled. Of these children, 32 were assigned to the experimental group and 16 to the control group. Baseline data collection and tHcy estimation were done. One mg folic acid tablets were given to the experimental group and placebo tablets to the control group for 30 days. tHcy levels were re-estimated after 1 month follow-up. Statistical significance was tested by χ2 test, and paired and unpaired t-tests, as appropriate. Correlation was tested by Pearson correlation test and P value less than 0.05 was taken as the cut-off for statistical significance.
Results:
Baseline plasma tHcy concentrations in both groups were comparable [11.90 (6.3) and 13.02 (2.4) μmol/l, respectively]. During the follow-up period, no increase in seizure episodes or no serious adverse reactions were noticed in either group. The reduction of tHcy in the experimental group was 1.92 μmol/l (P = 0.04) and in the control group, there was an increase of 1.05 μmol/l (P = 0.16).
Conclusions:
In children on AED treatment, folic acid supplementation may reduce tHcy level and thus reduce CVD risk.
Keywords: Antiepileptic drugs, cardiovascular disease risk factors, folic acid supplementation, hyperhomocysteinemia
INTRODUCTION
Homocysteine (tHcy) is a thiol-containing amino acid formed as an intermediate product during the metabolism of methionine [Figure 1]. Re-methylation pathway is the most common pathway that recycles tHcy back to methionine and requires vitamin B12 (cyanocobalamin) and folic acid as cofactors [Figure 1]. [1,2] In physiological conditions, homeostasis is usually maintained between the formation and degradation of tHcy, the absence of which would result in hyperhomocysteinemia. [3,4,5] Following McCully et al. in 1969, several epidemiological studies found that elevated plasma concentration of tHcy was an independent risk factor for thrombosis, cardiovascular disorders, and cerebrovascular disorders (CVD). [6,7,8] Several studies during last decade had demonstrated that prolonged treatment with first-generation antiepileptic drugs (AEDs) may potentially cause hyperhomocysteinemia due to depletion in serum folic acid. [9,10,11,12,13,14] AEDs that induce cytochrome P450 isozymes, including carbamazepine (CBZ), phenytoin (PHT), phenobarbital (PB), and oxcarbazepine (OXCZ), may cause a depletion of the cofactors necessary for the metabolism of tHcy. [11,12]
Figure 1.
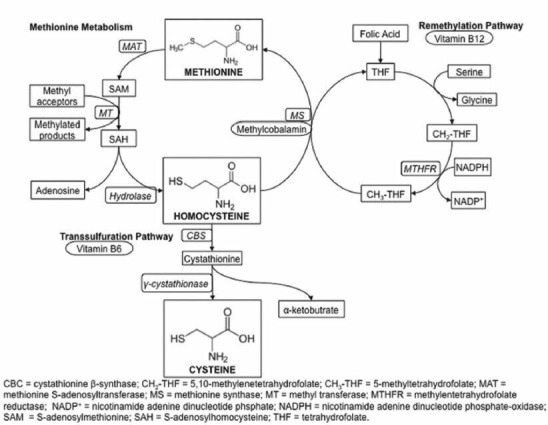
Metabolism of homocysteine
The total tHcy level is the lowest in children and may vary with ethnic background. It increases with age and is higher in male sex. [15] In children, the cut-offs for tHcy level range from 8.3 to 11.3 μmol/l, and hyperhomocysteinemia is defined as tHcy >10.4 μmol/l, which corresponds to 95th centile of a large population group. [8,16] The tendency toward lower folate and higher tHcy concentrations may put children on AEDs at special risk for atherosclerosis. [14] Compared to adults, children taking valproic acid (VPA) suffer from folate depletion and hyperhomocysteinemia, which is an atherosclerotic risk factor. [14]
The factors of early childhood origin of adult cardiovascular risks are now known, which demand early intervention. In Asian Indian children who are genetically more exposed to CVD risks, AED therapy is an additional risk for future development of CVDs. Elevated tHcy levels due to AED use can alter the circulatory markers of vascular risk, such as common carotid artery intima thickness which is positively correlated with the duration of AED therapy. [17] Studies reported that in those on AEDs, the standardized mortality ratio (SMR) due to cerebrovascular disease attributed to atherosclerosis was high. [8] Children born to women taking AEDs during the first trimester of pregnancy have been shown to have higher chances of cardiovascular malformations with relative risk of 4 and neural tube defects with relative risk of 3. [18]
The impacts of folic acid deficiency are more in childhood period when there is increased cell division. [18] The children on AED have to take it for long years, and tHcy elevation itself has got epileptogenic potential and can cause the risk of resistance to treatment leading to development of refractory epilepsy. [19,20] The association of folate depletion and hyperhomocysteinemia with reduced cognitive performance in children was reported. [18]
Few studies reported that in patients on AEDs, increasing tHcy levels were negatively correlated with decreasing serum folic acid levels (P < 0.003), and to normalize the tHcy levels, oral supplementation of folic acid was found to be effective. [8,9,14] Considering the biological plausibility, many doctors are now prescribing folic acid to those undergoing treatment with AEDs, though there is a paucity of data from India. In this context, the present study was conducted with the following objectives: (1) to assess the level of tHcy in children taking AEDs and (2) to study whether daily oral supplementation of folic acid will reduce the tHcy level in them.
MATERIALS AND METHODS
Our study was a double-blinded, randomized controlled trial (RCT) conducted in the Pediatric Neurology Department of Institute of Maternal and Child Health (IMCH) at Government Medical College, Kozhikode, Kerala.
The study subjects were the children belonging to the age-group of 5-12 years with a diagnosis of idiopathic epilepsy, and attending the epilepsy clinic and undergoing AED therapy for more than 6 months. Institutional ethical committee approval was obtained before the study. The enrolment of subjects and randomization were done after taking written informed consents from the legal guardians/parents of the subjects, as well as assent from the children above 7 years of age.
Expecting a mean difference of tHcy value of 2 μmol/l and standard deviation (SD) of 0.2 at an α-error of 5%, power of 80, and a 2:1 ratio (r) between experimental and control groups, the minimum required sample size was 30:15. During the 2 months enrolment period, a total of 60 patients were recruited consecutively, of whom 8 patients who did not meet the inclusion criteria and 4 patients whose parents were not willing to give informed consent were excluded. Thus, 48 patients were enrolled of whom 32 were assigned to the experimental group and 16 to the control group randomly in 2:1 ratio [Figure 2]. The random assignment scheme was created using a table of random numbers. The enrolment and allocation were done by a third person.
Figure 2.
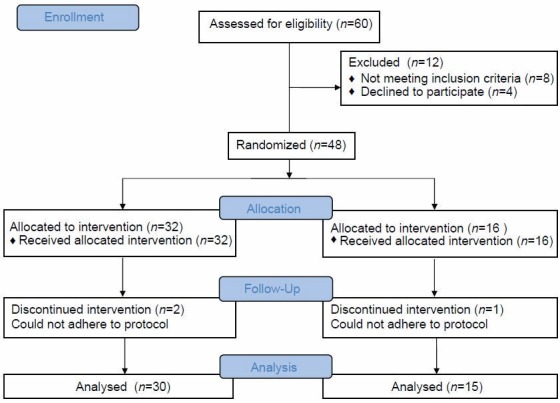
Recruitment, allocation, and follow-up of participants
The diagnosis of epilepsy as well as indication and monitoring of AED treatment were performed by an experienced pediatric neurologist (co-investigator). The patients receiving vitamin supplements or who had clinical evidence for an acute illness, renal dysfunction, thyroid dysfunction, chronic inflammatory diseases, inborn errors of tHcy, cobalamin or folate metabolism, or any other condition known to interfere with tHcy metabolism were excluded (n = 8). The baseline data were collected directly by the investigators using a pre-tested proforma from the informants (mothers in most cases). The morbidity and treatment details were collected from case records at the center. The following variables were recorded by using a standardized data sheet: Age, sex, diagnosis, duration of treatment, past and present treatment regimens, and number of seizures during past 6 months.
One milligram folic acid tablets (Elegant Drugs Pvt Ltd, Karnataka, India; date of manufacture January 2011, expiry date December 2012) were given to the experimental group and placebo tablets (Similia Laboratory, Kerala, India; date of manufacture May 2011) of the same shape, color, and dose were given to the control group (30 tablets for 30 days). Drug diary and appropriate instructions were given to the parents to give the medication orally along with the current AEDs. The ADR monitoring was done through a structured checklist verified during follow-up. Follow-up was done at the end of 2 weeks and 4 weeks at the clinic, and compliance was checked by drug dairy and blister counting. After 1 month, the outcome was evaluated by estimating tHcy levels.
tHcy estimations were done at baseline (day 0) and after 30 days by enzyme immunoassay (EIA) using the kits provided by Axis-Shield Diagnostics Ltd (Dundee DD2 IXA, UK) with a precision of 10%, as per the instruction provided by the manufacturers. [14] Sample of 3 ml blood was taken from antecubital vein using vacutainer with all aseptic precautions. The samples were centrifuged within 30 min after collection and stored at 2-8°C till tHcy assessment was done in the accredited laboratory attached to the Department of Nuclear Medicine on the same day.
Statistical analysis was performed using Statistical Package for Social Sciences (SPSS) 16.0 program for Windows 7 (SPSS Inc., Chicago, IL, USA). Frequencies are reported in percentage measurements in mean and SD. Statistical significance was tested by χ2 test. Fisher's exact test, paired and unpaired t-tests, and Mann–Whitney U test were done as appropriate. Correlation was tested by Pearson correlation test. P value less than 0.05 was taken as the cut-off for the same.
RESULTS
Out of 48 subjects enrolled, 32 were assigned to the experimental group and 16 to the control group. Two from the experimental and one from the control group dropped out/were excluded during the follow-up visit due to their inability to adhere to the protocol due to hospitalization for other morbidities. Thus, the data of 45 patients (30 in the experimental group and 15 in the control group) were included for analysis.
There were no significant differences between the two groups regarding the baseline characteristics like ethnicity, gender, age, body weight, and duration of treatment (P ≥ 0.05) [Table 1]. Baseline plasma tHcy concentrations in the experimental and control groups were comparable [11.90 (6.3) and 13.02 (2.7) μmol/l, respectively] [Table 2].
Table 1.
Characteristics of study subjects
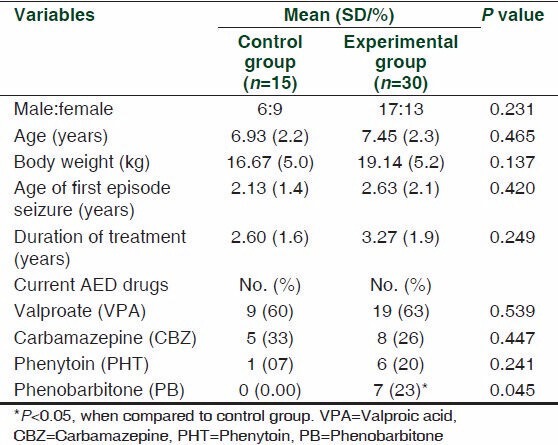
Table 2.
Details of homocysteine values

During the follow-up period, no increase in the frequency of seizure episodes or no serious adverse reactions were noticed in either of the groups. A total of five episodes of seizures were reported in both groups (three in the experimental group and two in the control group, or incidence of 10 and 13/100 persons/month of exposure, respectively). One in each group had fever during this period, which subsided within 3 days and was found to be unrelated to the drugs given.
Mean post-tHcy concentrations in the experimental group and control group were 9.98 (4.7) and 14.07 (4.2) μmol/l, respectively (P = 0.007). The reduction of tHcy in the experimental group was 1.92 μmol/l (P = 0.04) and in the control group, there was an increase of 1.05 μmol/l (P = 0.16) [Table 2]. In both groups, the pre- and post-tHcy values had significant positive correlation (Pearson value r = 0.624 for experimental group and 0.794 for control group, P ≤ 0.0001). Pre-tHcy values in both groups had no significant positive correlation with the age of subjects or the duration of treatment with AEDs (P ≥ 0.05).
DISCUSSION
Out of 45 children, 30 (66%) had shown hyperhomocysteinemia defined as tHcy > 10.4 μmol/l [16] [17 (60%) in the experimental group and 13 (86%) in the control group], which was more than the reported 13-40% (of all patients receiving AEDs) in other studies. [8,20] The tHcy level exceeded the 90th age percentile in 13.8% Japanese and 40.4% of Spanish children taking AEDs. [21,22] A prospective study reported that 5 μmol/l higher tHcy level was associated with a 27% higher risk for venous thrombosis. [23] A meta-analysis of 27 epidemiological studies estimated that a 5 μmol/l increase of tHcy was associated with an odds ratio (OR) of 1.6 for coronary artery disease in men and 1.8 in women, and the same increase was also strongly associated with increased cholesterol and peripheral vascular disease (P ≤ 0.05). [21] An increase in tHcy concentration will promote overproduction of reactive oxygen, enhancement of platelet aggregation, inhibition of protein C, activation of nuclear factor-kB, and increase in the release of inflammatory mediators, all of which can create a prothrombotic environment. [17,24,25] tHcy also affects peripheral arteries by influencing apolipoprotein A-I (Apo A-I) and the high density lipoprotein (HDL)-dependent transport of cholesterol. [9]
The mean ages of the groups were 7.5 years (experimental group) and 6.9 years (control group) and the age of the first episode of seizure was above 2 years. The duration of AED treatment was 3.3 (1.9) and 2.6 (1.6) years, respectively. As reported earlier, there was no positive correlation of age or duration of treatment with tHcy in our study, which may be due to the short duration of treatment in our subjects. [9,17]
In both groups, majority were taking mono-drug therapy (60% and 80%, respectively), which is similar to the results of other studies. [9,16,17] The drugs used in both groups in descending order were VPA, CBZ, and PHT (63%, 26%, and 20% in the experimental group and 60%, 33%, and 7% in the control group, respectively), the proportion of which was similar [Table 1]. It was reported earlier that patients treated with CBZ and PHT had increased biomarkers of CVD risk factors like C-reactive proteins and cholesterolemia, and those on VPA had hyperhomocysteinemia. [17] In a study among adolescents, 12 months of CBZ or VPA treatment resulted in a significant increase of tHcy and decrease of folate. [25] VPA is a broad-spectrum AED that inhibits specific cytochrome P450 isozymes and is the most commonly administered drug in children, along with CBZ. Studies have shown that the use of VPA for treatment led to significant elevation in tHcy concentration and folate depletion compared to baseline data in children, which was not observed in adults. [26] Although VPA has less enzyme-inducing activity than CBZ, it can still impair the intestinal absorption of dietary folic acid. A study done in pregnant Wistar rats has shown that VPA directly impaired the activity of methionine synthase – the enzyme that re-methylates tHcy to methionine – resulting in elevated tHcy. [27] Since the majority of the subjects in our study use VPA and CBZ, this effect may be well reflected.
In our study, after folic acid administration for 30 days, the mean tHcy concentration in the experimental group was reduced from 11.90 (6.3) to 9.98 (4.7) μmol/l, which was significant (P = 0.04), without any observable effect on anticonvulsive efficiency. In the control group, it increased from 13.02 (2.4) to 14.07 (4.2) μmol/l with placebo (nonsignificant). On comparison, contrary to the basal level of tHcy levels, the difference of post-tHcy levels in both groups was significantly different with higher value in the control group (P = 0.007). The reduction of tHcy after folic acid supplementation in our study was 1.92 (2.2) μmol/l, which was lower than the earlier reported 5.2 (0.50) μmol/l possibly due to shorter duration of supplementation. [16,28] In one study, oral supplementation of 0.5-5 mg folic acid daily reduced tHcy by 25% and the reduction was 32% when used in combination with cyanocobalamin. [29,30] In our study, 16% of the subjects had reduction in tHcy levels.
A dosage of 1 mg/day folic acid is considered safe in children, [9,11] and in the National Program of Prophylaxis for Nutritional Anaemia in India, the daily dose for children is 1 mg. Since our study subjects are children from 5 to 12 years of age, we used 1 mg tablets. Since folate stores (5-10 mg) from the body would rapidly become depleted, folic acid supplementation must be given continuously; otherwise, tHcy values would reverse. [14] A meta-analysis reported that in areas where the mean population level of folic acid was lower, the supplementation in AEDs may not be effective. [9] In countries were folic acid was given as nutritional supplements, the population tHcy value was found to be lower, which may be applicable to our subjects. [19] Independent of its tHcy-lowering capacity, folic acid may improve endothelial function; the folate metabolite 5-methyltetrahydrofolate appears to enhance endothelial nitric oxide synthesis in healthy adults. [30] It was reported that after oral supplementation with folic acid, flow-mediated vasodilatation was improved in children with type I diabetes. [31] Therefore, the study suggests that the drug-induced risk for atherosclerosis and associated Non communicable disease NCD risks for children taking AEDs can be prevented by folic acid supplementation.
Formerly, folic acid was considered to be epileptogenic, but recent data disproved this assumption. [32] A published meta-analysis of 12 randomized trials observed that 30% reduction of tHcy concentrations occurred following daily intake of oral multivitamin preparations containing folic acid (0.5-5.0 mg) and cyanocobalamin (0.5 mg), without any adverse effects. [9,33] As the safety and efficacy of folic acid in children has been proved, it can be given cost effectively for long term. Also, since the long-term AED therapy depletes the folate through induction of the hepatic enzymes, folate intake has been proposed to provide benefit in preventing cardiovascular events related to high levels of tHcy in pediatric patients with epilepsy. [9] A daily oral nutritional supplement drink containing physiological amounts of folate significantly reduced plasma tHcy concentrations in older patients recovering from acute illness. [34] Whether dietary supplementation with folate would prove to be equally effective in children undergoing treatment with AEDs is worth exploring.
Limitations of the study
The present study is limited by its short duration of 1 month. It could be improved by a crossover design. Due to resource constraints, we could not estimate the folate levels in subjects before or after treatment. For better evidence, an RCT with larger sample size and longer duration may be needed.
CONCLUSION
Epilepsy is a medical condition that requires long-term therapy with AEDs, which may lead to hyperhomocysteinemia associated with metabolic consequences resulting in an increased risk of cardiovascular (CVD) and Cerebrovascular disorders.
Daily oral folic acid supplementation reduces tHcy level, thus reducing future CVD risks. Further research is necessary to evaluate the long-term effects of folic acid supplementation in patients taking AEDs, with respect to different clinical end points such as anticonvulsive efficiency, cardiovascular disease, mood, and cognitive performance.
ACKNOWLEDGMENTS
We are thankful to Directorate of Medical Education, Government of Kerala, State Board of Medical Research (SBMR) for funding the study. We are thankful to Dr. Riyas, Head of Department of Pediatrics and the staff of Department of Pediatrics,Calicut Medical College, and the laboratory technicians and staff of the Department of Nuclear Medi cine for their help. We also thank all the children and their parents who participated in the study for their cooperation.
Footnotes
Source of Support: State Board of Medical Research, Directorate of Medical Education, Kerala, India
Conflict of Interest: None declared.
REFERENCES
- 1.Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–82. doi: 10.1007/BF03195624. [DOI] [PubMed] [Google Scholar]
- 2.Hoffer LJ. Homocysteine remethylation and trans-sulfuration. Metabolism. 2004;53:1480–3. doi: 10.1016/j.metabol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Ueland PM, Refsum H. Plasma homocysteine, a risk factor for vascular disease: Plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989;114:473–501. [PubMed] [Google Scholar]
- 4.Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000;72:324–32. doi: 10.1093/ajcn/72.2.324. [DOI] [PubMed] [Google Scholar]
- 5.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 6.McCully KS. Vascular pathology of homocysteinemia: Implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–28. [PMC free article] [PubMed] [Google Scholar]
- 7.Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000;72:324–32. doi: 10.1093/ajcn/72.2.324. [DOI] [PubMed] [Google Scholar]
- 8.Coppola G, Ingrosso D, Operto FF, Signoriello G, Lattanzio F, Barone E, et al. Role of folic acid depletion on homocysteine serum level in children and adolescents with epilepsy and different MTHFR C677T genotypes. Seizure. 2012;21:340–3. doi: 10.1016/j.seizure.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Belcastro V, Striano P. Anti-epileptic drugs, hyperhomocysteinemia and B-vitamins supplementation in patients with epilepsy. Epilepsy Res. 2012;102:1–7. doi: 10.1016/j.eplepsyres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Osganian SK, Stampfer MJ, Spiegelman D, Rimm E, Cutler JA, Feldman HA, et al. Distribution of and factors associated with serum homocysteine levels in children: Child and Adolescent Trial for Cardiovascular Health. JAMA. 1999;281:1189–96. doi: 10.1001/jama.281.13.1189. [DOI] [PubMed] [Google Scholar]
- 11.Karabiber H, Sonmezgoz E, Ozerol E, Yakinci C, Otlu B, Yologlu S. Effects of valproate and carbamazepine on serum levels of homocysteine, vitamin B12, and folic acid. Brain Dev. 2003;25:113–5. doi: 10.1016/s0387-7604(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 12.Verrotti A, Pascarella R, Trotta D, Giuva T, Morgese G, Chiarelli F, et al. Hyperhomocysteinemia in children treated with sodium valproate and carbamazepine. Epilepsy Res. 2000;41:253–7. doi: 10.1016/s0920-1211(00)00150-9. [DOI] [PubMed] [Google Scholar]
- 13.Semmler A, Moskau-Hartmann S, Stoffel-Wagner B, Elger C, Linnebank M. Homocysteine plasma levels in patients treated with anti-epileptic drugs depend on folate and vitamin B12 serum levels, but not on genetic variants of homocysteine metabolism. Clin Chem Lab Med. 2013;51:665–9. doi: 10.1515/cclm-2012-0580. [DOI] [PubMed] [Google Scholar]
- 14.Linnebank M, Moskau S, Semmler A, Widman G, Stoffel-Wagner B, Weller M, et al. Anti-epileptic drugs interact with folate and vitamin B12 serum levels. Ann Neurol. 2011;69:352–9. doi: 10.1002/ana.22229. [DOI] [PubMed] [Google Scholar]
- 15.Osganian SK, Stampfer MJ, Spiegelman D, Rimm E, Cutler JA, Feldman HA, et al. Distribution of and factors associated with serum homocysteine levels in children: Child and Adolescent Trial for Cardiovascular Health. JAMA. 1999;281:1189–96. doi: 10.1001/jama.281.13.1189. [DOI] [PubMed] [Google Scholar]
- 16.Huemer M, Vonblon K, Fodinger M, Krumpholz R, Hubmann M, Ulmer H, et al. Total homocysteine, folate, and cobalamin, and their relation to genetic polymorphisms, lifestyle and body mass index in healthy children and adolescents. Pediatr Res. 2006;60:764–9. doi: 10.1203/01.pdr.0000246099.39469.18. [DOI] [PubMed] [Google Scholar]
- 17.Chuang YC, Chuang HY, Lin TK, Chang CC, Lu CH, Chang WN, et al. Effects of long-term anti-epileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia. 2012;53:120–8. doi: 10.1111/j.1528-1167.2011.03316.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng LS, Prasad AN, Rieder MJ. Relationship between anti-epileptic drugs and biological markers affecting long-term cardiovascular function in children and adolescents. Can J Clin Pharmacol. 2010;17:e5–46. [PubMed] [Google Scholar]
- 19.Instructions for Use Homocysteine EIA. Axis–Shield Diagnostics Limited Dundee DD2 IXA, United Kingdom. 2008:3–11. [Google Scholar]
- 20.Huemer M, Ausserer B, Graninger G, Hubmann M, Huemer C, Schlachter K, et al. Hyperhomocysteinemia in children treated with anti-epilepticdrugs is normalized by folic acid supplementation. Epilepsia. 2005;46:1677–83. doi: 10.1111/j.1528-1167.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 21.Verrotti A, Pascarella R, Trotta D, Giuva T, Morgese G, Chiarelli F, et al. Hyperhomocysteinemia in children treated with sodium valproate and carbamazepine. Epilepsy Res. 2000;41:253–7. doi: 10.1016/s0920-1211(00)00150-9. [DOI] [PubMed] [Google Scholar]
- 22.Ono H, Sakamoto A, Mizoguchi N, Sakura N. The C677T mutation in the methylenetetrahydrofolate reductase gene contributes to hyperhomocysteinemia in patients taking anticonvulsants. Brain Dev. 2002;24:223–6. doi: 10.1016/s0387-7604(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 23.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–9. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 24.Doshi S, McDowell I, Goodfellow J, Stabler S, Boger R, Allen R, et al. Relationship between S adenosylmethionine, S adenosylhomocysteine, asymmetric dimethylarginine, and endothelial function in healthy human subjects during experimental hyper- and hypohomocysteinemia. Metabolism. 2005;54:351–60. doi: 10.1016/j.metabol.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Stühlinger MC, Stanger O. Asymmetric dimethyl-L-arginine (ADMA): A possible link between homocysteine and endothelial dysfunction. Curr Drug Metab. 2005;6:3–14. doi: 10.2174/1389200052997393. [DOI] [PubMed] [Google Scholar]
- 26.Verrotti A, Pascarella R, Trotta D, Giuva T, Morgese G, Chiarelli F. Hyperhomocysteinemia in children treated with sodium valproate and carbamazepine. Epilepsy Res. 2000;41:253–7. doi: 10.1016/s0920-1211(00)00150-9. [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Aperte E, Ubeda N, Achon M, Perez-Miguelsanz J, Varela-Moreiras G. Impaired methionine synthesis and hypomethylation in rats exposed to valproate during gestation. Neurology. 1999;52:750–6. doi: 10.1212/wnl.52.4.750. [DOI] [PubMed] [Google Scholar]
- 28.Fowler B. Genetic defects of folate and cobalamin metabolism. Eur J Pediatr. 1998;157:S60–6. doi: 10.1007/pl00014306. [DOI] [PubMed] [Google Scholar]
- 29.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 30.Stühlinger MC, Stanger O. Asymmetric dimethyl-L-arginine (ADMA): A possible link between homocysteine and endothelial dysfunction. Curr Drug Metab. 2005;6:3–14. doi: 10.2174/1389200052997393. [DOI] [PubMed] [Google Scholar]
- 31.McCarty MF. Coping with endothelial superoxide: Potential complementarity of arginine and high-dose folate. Med Hypotheses. 2004;63:709–18. doi: 10.1016/j.mehy.2002.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Pena AS, Wiltshire E, Gent R, Hirte C, Couper J. Folic acid improves endothelial function in children and adolescents with type 1 diabetes. J Pediatr. 2004;144:500–4. doi: 10.1016/j.jpeds.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Arrastia R. Homocysteine and Neurologic Disease. Arch Neurol. 2000;57:1422–7. doi: 10.1001/archneur.57.10.1422. [DOI] [PubMed] [Google Scholar]
- 34.Gariballa SE, Forster SJ, Powers HJ. Effects of mixed dietary supplements on total plasma homocysteine concentrations (tHcy): A randomized, double-blind, placebo-controlled trial. Int J VitamNutr Res. 2012;82:260–6. doi: 10.1024/0300-9831/a000118. [DOI] [PubMed] [Google Scholar]


