Abstract
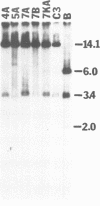
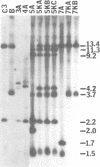
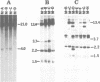
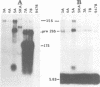
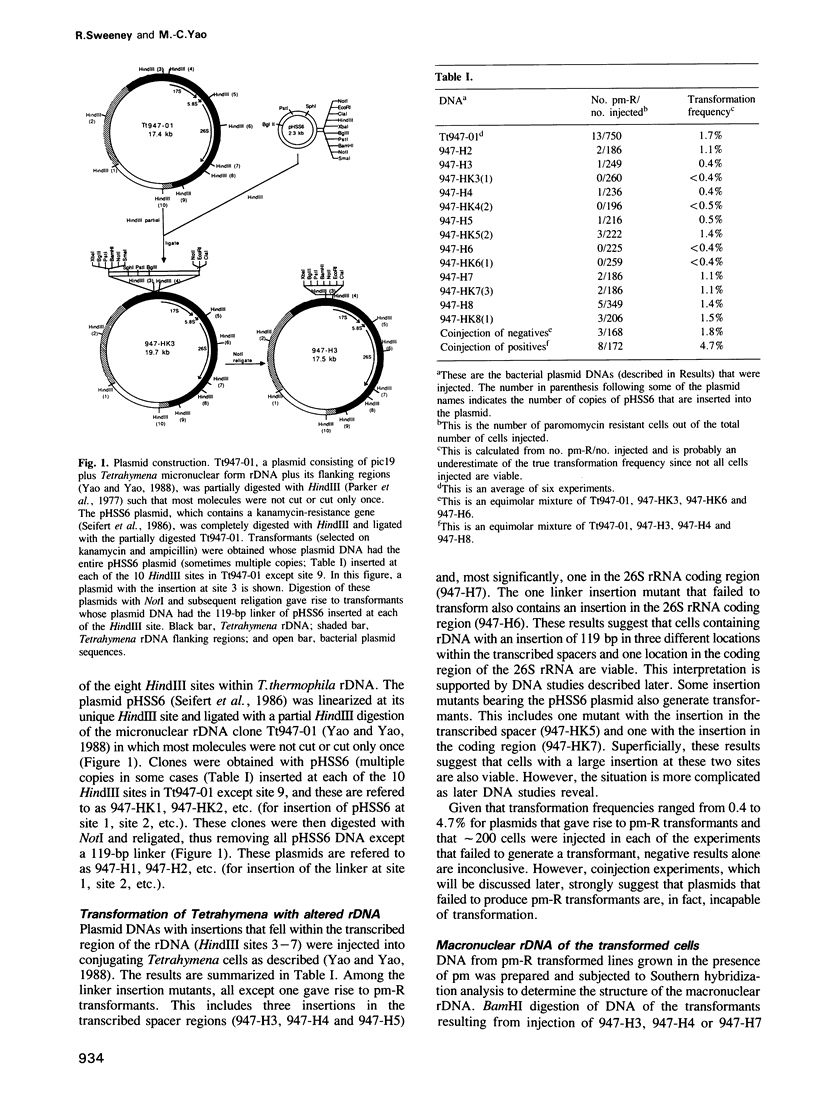
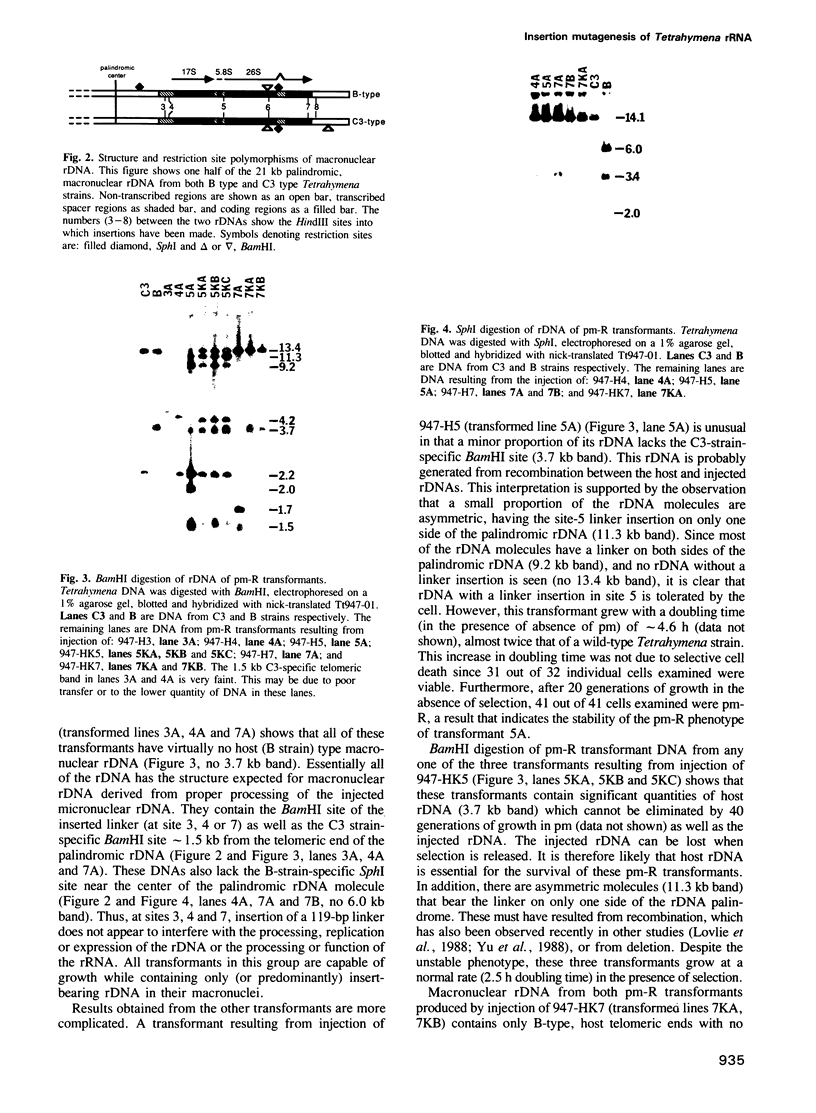
The free, linear macronuclear ribosomal RNA genes (rDNA) of Tetrahymena are derived from a unique copy of micronuclear rDNA during development. We have injected cloned copies of the micronuclear rDNA that have been altered in vitro into developing macronuclei and obtained transformants that express the paromomycin-resistant phenotype specified by the injected rDNA. In most cases, these transformants contain almost exclusively the injected rDNA which has been accurately processed into macronuclear rDNA. Mutants with a 119 bp insertion at three points in the transcribed spacers and at two points in the 26S rRNA coding region were tested. Cells containing these spacer mutant rDNAs are viable, although one of them grows slowly. This slow-growing line contains the insertion between the 5.8S and 26S rRNA coding regions and accumulates more rRNA processing intermediates than control lines. One of the 26S rRNA mutants failed to generate transformants, but the other did. These transformants grew normally, and produced 26S rRNA containing the inserted sequence. A longer insertion (2.3 kb) at the same four points either abolished transformation or generated transformants that retained at least some wild-type rDNA. This study reveals that some rRNA sequences can be altered without significantly affecting cell growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austerberry C. F., Yao M. C. Nucleotide sequence structure and consistency of a developmentally regulated DNA deletion in Tetrahymena thermophila. Mol Cell Biol. 1987 Jan;7(1):435–443. doi: 10.1128/mcb.7.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns P. J., Katzen A. L., Martin L., Blackburn E. H. A drug-resistant mutation in the ribosomal DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1985 May;82(9):2844–2846. doi: 10.1073/pnas.82.9.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din N., Engberg J., Kaffenberger W., Eckert W. A. The intervening sequence in the 26S rRNA coding region of T. thermophila is transcribed within the largest stable precursor for rRNA. Cell. 1979 Oct;18(2):525–532. doi: 10.1016/0092-8674(79)90069-2. [DOI] [PubMed] [Google Scholar]
- Eckert W. A., Kaffenberger W., Krohne G., Franke W. W. Introduction of hidden breaks during rRNA maturation and ageing in Tetrahymena pyriformis. Eur J Biochem. 1978 Jul 3;87(3):607–616. doi: 10.1111/j.1432-1033.1978.tb12413.x. [DOI] [PubMed] [Google Scholar]
- Engberg J., Din N., Saiga H., Higashinakagawa T. Nucleotide sequence of the 5'-terminal coding region for pre-rRNA and mature 17S rRNA in Tetrahymena thermophila rDNA. Nucleic Acids Res. 1984 Jan 25;12(2):959–972. doi: 10.1093/nar/12.2.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Hui A., de Boer H. A. Specialized ribosome system: preferential translation of a single mRNA species by a subpopulation of mutated ribosomes in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4762–4766. doi: 10.1073/pnas.84.14.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob W. F., Santer M., Dahlberg A. E. A single base change in the Shine-Dalgarno region of 16S rRNA of Escherichia coli affects translation of many proteins. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4757–4761. doi: 10.1073/pnas.84.14.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M., Yao M. C. Transformation of Tetrahymena thermophila with hypermethylated rRNA genes. Mol Cell Biol. 1988 Apr;8(4):1664–1669. doi: 10.1128/mcb.8.4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss G. B., Pearlman R. E. Extrachromosomal rDNA of Tetrahymena thermophila is not a perfect palindrome. Gene. 1981 Apr;13(3):281–287. doi: 10.1016/0378-1119(81)90032-9. [DOI] [PubMed] [Google Scholar]
- Kister K. P., Müller B., Eckert W. A. Complex endonucleolytic cleavage pattern during early events in the processing of pre-rRNA in the lower eukaryote, Tetrahymena thermophila. Nucleic Acids Res. 1983 Jun 11;11(11):3487–3502. doi: 10.1093/nar/11.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. Ribosome synthesis in Tetrahymena pyriformis. J Cell Biol. 1970 Jun;45(3):623–634. doi: 10.1083/jcb.45.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Lenaers G., Nielsen H., Engberg J., Herzog M. The secondary structure of large-subunit rRNA divergent domains, a marker for protist evolution. Biosystems. 1988;21(3-4):215–222. doi: 10.1016/0303-2647(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Løvlie A., Haller B. L., Orias E. Molecular evidence for somatic recombination in the ribosomal DNA of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5156–5160. doi: 10.1073/pnas.85.14.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W. C., Orias E., Flacks M., Blackburn E. H. Allele-specific, selective amplification of a ribosomal RNA gene in Tetrahymena thermophila. Cell. 1982 Mar;28(3):595–604. doi: 10.1016/0092-8674(82)90214-8. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Watson R. M., Vinograd J. Mapping of closed circular DNAs by cleavage with restriction endonucleases and calibration by agarose gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):851–855. doi: 10.1073/pnas.74.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Bostock C., Gamow E., Lauth M. Characterization of rapidly labeled RNA in Tetrahymena pyriformis. Exp Cell Res. 1971 Jul;67(1):124–128. doi: 10.1016/0014-4827(71)90627-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Sutton C. A., Sylvan P., Hallberg R. L. Ribosome biosynthesis in Tetrahymena thermophila. IV. Regulation of ribosomal RNA synthesis in growing and growth arrested cells. J Cell Physiol. 1979 Dec;101(3):503–513. doi: 10.1002/jcp.1041010316. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Blackburn E., Gall J. Tandemly repeated C-C-C-C-A-A hexanucleotide of Tetrahymena rDNA is present elsewhere in the genome and may be related to the alteration of the somatic genome. J Cell Biol. 1981 Aug;90(2):515–520. doi: 10.1083/jcb.90.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G. L., Hasson M., Blackburn E. H. Circular ribosomal DNA plasmids transform Tetrahymena thermophila by homologous recombination with endogenous macronuclear ribosomal DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5151–5155. doi: 10.1073/pnas.85.14.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]