Abstract
Cardiac ryanodine receptor type-2 (RyR2) play a key role in excitation-contraction coupling. The RyR2-channel protein is modulated by various posttranslational modifications, including phosphorylation by protein kinase-A and Ca2+/calmodulin protein kinase-II (CaMKII). Despite extensive research in this area, the functional effects of RyR2-phosphorylation remain disputed. In particular, the potential involvement of increased RyR2-phosphorylation in the pathogenesis of heart failure and arrhythmias remains a controversial area, which is discussed in this review article.
Keywords: Atrial fibrillation, calcium handling, heart failure, phosphorylation, ryanodine receptor
INTRODUCTION
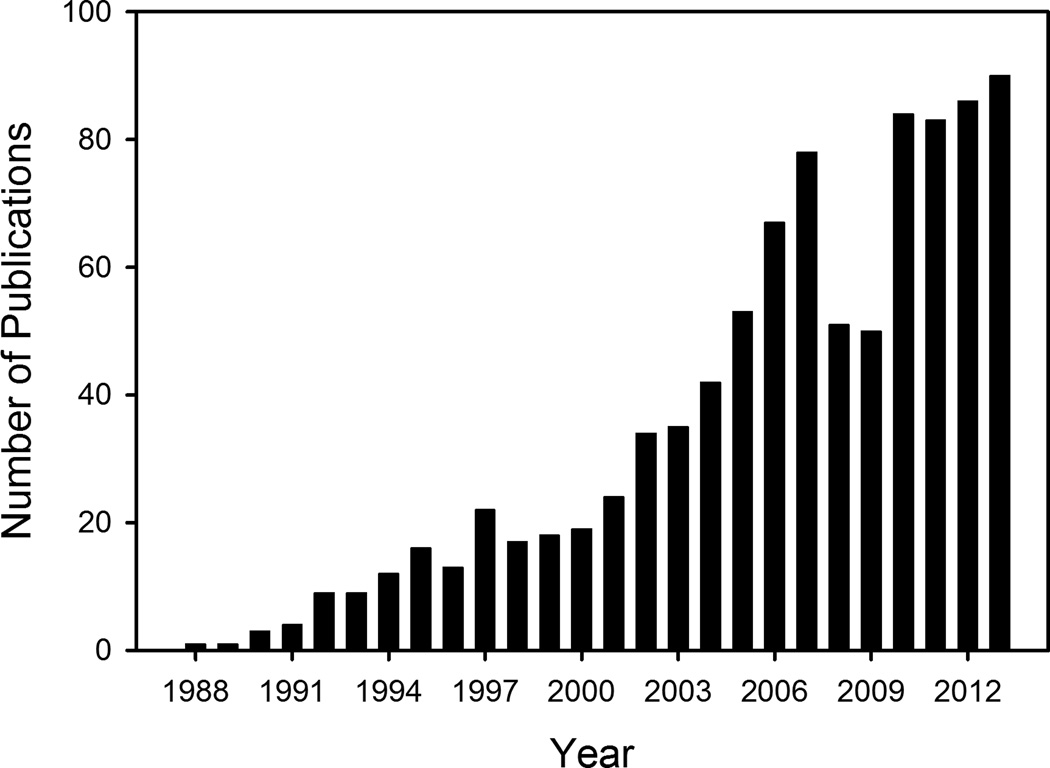
This review focuses on recent controversies surrounding studies addressing the consequences of ryanodine receptor type-2 (RyR2) phosphorylation in heart. Ryanodine receptors serve as Ca2+-release channels on endo/sarcoplasmic reticulum (ER/SR) of excitable tissues, including neurons, skeletal and cardiac muscle.1 During the past 20 years, over 900 publications have focused on the role of RyR-phosphorylation (Fig. 1). Circulation Research has published the largest share of these papers, over 80 in total, including some of the most controversial work on RyR2-phosphorylation in the pathogenesis of cardiac disease. Several recent reviews have summarized major studies in the field, and already described some of the inconsistent and partly opposing conclusions thereof.2–5 Here, we will try to delineate which concepts can be considered as mostly accepted, which require further in-depth investigations, and will discuss major unresolved controversies in the field.
Figure 1.
Number of peer-reviewed papers published between 1988–2013, containing key words ‘ryanodine receptors’ and ‘phosphorylation’ in key bibliographic fields, according to Scopus.
RyR2-phosphorylation and excitation-contraction coupling in normal hearts
RyR2 is the major SR Ca2+-release channel involved in excitation-contraction coupling, the process by which an electrical depolarizing impulse is transduced into a cardiac contraction. The amount of Ca2+ released from the SR via RyR2 largely determines the Ca2+-transient amplitude, which correlates with the strength of systolic contraction.6 The RyR2-channel consists of four pore-forming subunits, which associate with numerous accessory proteins including FK506-binding protein-12.6 (FKBP12.6), calmodulin (CaM), calsequestrin-2 (CSQ2), junctin, triadin, and junctophilin-2 (JPH2), all of which can regulate channel gating.7 In addition, RyR2 is regulated at the posttranslational level by S-nitrosylation, oxidation, and protein phosphorylation.8
RyR2-channels contain several phosphorylation sites. The degree of steady-state phosphorylation of each site depends on a dynamic balance between multiple protein kinases and phosphatases,9 allowing precise control of RyR2-phosphorylation and, consequently, channel activity.10 Alterations in RyR2-phosphorylation play a critical role in various cardiac diseases, including heart failure (HF) as well as atrial and ventricular arrhythmias. Most studies to date have only focused on the effects of protein kinase-A (PKA) and Ca2+/calmodulin-dependent protein kinase-II (CaMKII)-mediated RyR2-phosphorylation, although it is likely that other serine/threonine protein kinases can also phosphorylate RyR2.10–12
Serine-2808 (S2808, or S2809 in some species) was the first RyR2-residue identified as a phosphorylation site,13 and is believed to be the primary target of PKA-phosphorylation.14, 15 Addition of purified PKA to a single RyR2-channel studied in the planar lipid-bilayer system increased RyR2 open probability (Po).14 The increased RyR2-Po was prevented by mutating S2808 to alanine (S2808A), rendering the channel unphosphorylatable.10 Conversely, the phosphomimic mutation S2808D increased RyR2-Po in some10, 15, but not all studies.16 Similarly, studies in ventricular cardiomyocytes from S2808A and S2808D mice showed that the RyR2-phosphorylation level alters channel activity in intact cardiomyocytes, as evidenced by changes in Ca2+-transient amplitudes and Ca2+-spark frequencies, in some,17 but not other studies.18 Additional studies were performed by treating wild-type ventricular cardiomyocytes with PKA-inhibitors.19 However, it is impossible to attribute the observed changes (or lack thereof) to phosphorylation of a specific RyR2-residue, because all PKA-phosphorylation sites and other PKA-target proteins will be simultaneously modified. Therefore, studies using knock-in mouse-models of missense mutations of specific phosphorylation-sites remain invaluable to study specific sites, although they also have intrinsic limitations (see below). Even using similar S2808A knock-in mouse-lines, opposing findings have been reported for the functional effects of S2808-phosphorylation..20 For example, Marks et al.20 reported blunted heart rate and contractile responses to isoproterenol (Iso), and a blunted enhancement of Ca2+-transient amplitude and fractional shortening in S2808A-mice. In contrast, Houser et al.21 showed unaltered chronotropic and inotropic responses to Iso in vivo and in vitro.22 It is possible that there is a threshold below which PKA-phosphorylation of RyR2 (at S2808) does not cause a physiological effect, but that does not appear a sufficient explanation for these clear differences. Nevertheless, it is intriguing why well-established and leading labs in the field using similar mutant recombinant channels and knock-in mouse-models arrive at opposite conclusions regarding the physiological effects of PKA-dependent RyR2-phosphorylation, particularly at the S2808-site.
Several factors might contribute to these discrepancies, including subtle differences in the animal models, reagents, experimental-procedures, and data interpretation (summarized in Table 1). Furthermore, new findings continue to refine our understanding of signaling pathways and RyR2 regulation.5 For example, initially PKA-phosphorylation of S2808 was reported to reduce FKBP12.6-binding.14 However, S2808D knock-in mice with constitutively-phosphorylated RyR2-channels exhibited normal FKBP12.6-binding, at least at a young age.17 We will not discuss the controversies related to FKBP12.6 in relation to RyR2-regulation and phosphorylation due to space limitations, but refer to prior excellent review articles.4, 5, 23 Recent studies revealed that oxidation and/or S-nitrosylation, together with RyR2-phosphorylation at S2808, are required to dissociate FKBP12.6 from RyR2, and to increase RyR2-Po.17, 24 At present, the exact mechanisms underlying the synergy between these different posttranslational RyR2-modifications remain unknown and require follow-up studies.
TABLE 1.
Potential reasons for divergent experimental results and interpretations
| Item | Variability | References |
|---|---|---|
| Animal models | ||
| Species | Results might vary depending on species studied, i.e., RyR2 phosphorylation level at baseline or in heart failure | 10, 14, 15, 38 |
| Genetic background | Mouse strain might contribute to variable responses to β-adrenergic or pathological stress | 15, 18, 20, 21, 40, 94, 95 |
| Environmental conditions | Diet (chow) and lifestyle can affect SR Ca2+ handling | 96–98 |
| Circadian rhythm | Circadian rhythm can modulate pathological phenotypes as well as RyR2 properties itself | 99, 100 |
| Experimental models | ||
| Animal model of disease | Involvement of specific RyR2 phosphorylation sites might depend on the type of experimental heart failure (i.e., LAD ligation vs. transverse aortic banding) and the time points at which phenotypes are evaluated | 15, 18, 20, 21, 27, 28, 40, 54, 94, 95, 101, 102 |
| Anesthesia / surgical techniques | Anesthesia type and surgical techniques might alter study outcomes | 103, 104 |
| Sample analysis methods | Results may dependent on analysis methods, i.e., Western Blotting vs. back-phosphorylation might reveal different results, and SR Ca2+ leak (tetracaine) protocol vs. Ca2+ spark measurements | 10, 25, 105 |
| Scale | Results might vary at the whole animal (in vivo vs. ex vivo), isolated cell (intact vs. permeabilized), and single channel level | 74, 81, 86, 91, 106 |
| Reagents | ||
| Antibodies | Results might vary depending on antibody (i.e., epitope, purification, species). Antibodies are often not well characterized. | 25, 27, 28, 32, 38, 74 |
| Buffers, detergents, fluorophores | Buffer conditions, detergents (for solubilization of RyR2), fluorophores (for Ca2+ imaging) could affects results | 107, 108 |
| Redox levels | S- and Cys-nitrosylation, oxidation, tyrosine nitration levels can alter RyR phosphorylation or Ca2+ handling | 24, 109 |
| Data processing | ||
| Data analysis procedures | Results might vary depending on data analysis procedures (i.e., quantification of western blot signals, processing of confocal Ca2+ imaging data) | 110 |
| Data quality | Proper use of positive and negative controls, etc. | |
| Fitting of data within conceptual models | Different groups might interpret the same data differentially based on the null hypothesis | 15, 18 |
Serine-2814 (S2814, in some species S2815) was the second RyR2-residue identified as a primary CaMKII-target.10 Careful stoichiometric analysis revealed that in vitro CaMKII-phosphorylation could partially phosphorylate a second still-unidentified residue.10 One study claimed that CaMKII can phosphorylate up to 3–4 sites, but these conclusions were not based on stoichiometric studies using quantifications of both total and phosphorylated RyR2.25 Claims in the literature including some review articles, that there are 3–4 or even more phosphorylation residues per RyR2-monomer, are not based on credible data and will not help to resolve this controversy.26 However, there is a broad consensus that CaMKII-mediated RyR2-phosphorylation increases RyR2-Po, both at the single-channel level in bilayers,4, 10 and in ventricular-cardiomyocytes as evidenced by increased Ca2+-spark frequency.27 Mutation S2814A prevents most CaMKII-effects on RyR2, suggesting that S2814 is the major, but probably not the exclusive, RyR2-residue subject to CaMKII-phosphorylation.10, 28 These findings are consistent with studies in CaMKII-knockout and transgenic-animals revealing altered RyR2-phosphorylation at S2814 and altered Ca2+-spark frequency depending on global CaMKII-levels.29, 30
Some data suggest a crosstalk between the S2808 and S2814 phosphorylation-sites. For example, enhanced S2814-phosphorylation was observed in S2808A knock-in mice, suggesting that the phosphorylation-state of one residue might affect the likelihood that neighboring residues are phosphorylated, particularly following adrenergic stimulation.18 Alternatively, this may be an artifact due to changes in the epitope for the phospho-specific antibodies. Therefore, it is important that all known RyR2-phosphorylation sites are monitored when studying (patho)physiological mechanisms using RyR2 knock-in mouse models.
Some years ago Serine-2030 (S2030, or S2031 in some species) was described as a third functional RyR2-phosphorylation site.31 PKA-phosphorylation of this site might enhance RyR2-sensitivity to luminal (intra-SR) Ca2+.31 Other studies, however, have failed to demonstrate a measurable functional effect of this phosphorylation-site, and its physiological significance remains to be determined in vivo.15, 32 Overall, it would be of great interest to identify other RyR2-phosphorylation sites that are regulated under physiological conditions or perhaps altered in cardiac diseases, to determine the exact basal-level of RyR2-phosphorylation at steady-state for each individual residue, to identify the specific kinases and their counterbalancing phosphatases that dynamically phosphorylate/dephosphorylate each respective residue, and to delineate the precise allosteric mechanisms underlying the synergetic effects of concomitant phosphorylation at multiple sites, along with other posttranslational modifications of RyR2-subunits.
Dephosphorylation of RyR2 is mediated by PP1, which is targeted to the complex by the regulatory-subunit spinophilin, and PP2A, targeted through its regulatory-subunit PR130.33, 34 PP2A may be also targeted to RyR2 through B56α binding to ankyrin-B, which also interacts with RyR2,35 or through the muscle-specific A-kinase-anchoring protein (mAKAP), which binds both RyR2 and PP2A-B56α.36 Ssteady-state phosphorylation of individual RyR2 phosphorylation sites is regulated in a highly complex and dynamic manner, which should be seriously considered when quantifying the degree of phosphorylation and studying its consequences for cardiac (dys)function. Several studies have demonstrated that PP-mediated dephosphorylation of RyR2 decreased channel open probability, but other studies have reported the opposite.9, 37–39 Moreover, it was shown that PP increases RyR2 leakiness in cells expressing WT, but not S2808A mutant RyR2 with the disabled PKA phosphorylation site.40 Thus, the PP-mediated regulation of RyR2 remains controversial at this time.
Role of altered RyR2-phosphorylation in heart failure
Enhanced RyR2-mediated SR Ca2+-leak has been observed in HF-patients and various animal models.4, 41 It is likely that diastolic SR Ca2+-leak, together with reduced SERCA2a function and enhanced NCX1 function, contributes to the depletion of SR Ca2+-content in HF.5 The causal role of RyR2-phosphorylation in HF pathogenesis, however, remains highly controversial because different groups have reported contradictory findings using similar approaches and animal models.15, 18, 42 In addition, RyR2 is regulated by various additional posttranslational modifications, and the potential synergistic or antithetic effects with phosphorylation remain largely unexplored.43–45
The first paper demonstrating altered RyR2-phosphorylation in HF-patients was published in 2000 by Dr. Marks’ group.14, 15, 17 These authors14 postulated that the hyperadrenergic state in HF increases PKA-mediated S2808-phosphorylation, reduces FKBP12.6-binding to RyR2, and enhances RyR2-Po. Several (but not all) subsequent papers validated the increased S2808-phosphorylation in HF in various species, as previously summarized.3–5 In some cases, different groups used similar mutant mice (S2808A knock-in) and seemingly similar experimental models, yet obtained opposite results.4 For example, the Marks lab demonstrated in S2808A knock-in mice that S2808-phosphorylation is a critical mediator of progressive cardiac failure following experimental myocardial infarction (MI).15 In contrast, the Houser lab showed no protective effects in S2808A-mice (generated independently by their group), despite a significant increase in S2808-phosphorylation after MI.22 Once again, numerous factors might contribute to the discrepant results (see Table 1). Complementary studies in knock-in mice of the constitutively hyperphosphorylated S2808-site (S2808D-mice) revealed the spontaneous development of an age-dependent cardiomyopathy, which might support a role for this site in some types of HF development.17
It is important to consider several general concepts when comparing studies in this field. For example, since cardiac remodeling is likely heterogeneous throughout the heart, RyR2-phosphorylation levels might vary among different regions.46 Furthermore, some studies suggest that the etiology of HF might affect the relative phosphorylation-level of different RyR2-sites.10, 27, 28 It is also unclear whether RyR2-phosphorylation sites are differentially phosphorylated during different stages of HF or in the context of different etiologies of the disease.47 Recent studies on human hearts revealed that both S2808 and S2814 are hyperphosphorylated during compensated cardiac hypertrophy, whereas only S2814 remained phosphorylated in patients with end-stage HF.47 At present, it is unclear how common risk factors such as metabolic syndrome, diabetes, and ischemia (i.e., oxidative stress) might impact RyR2-phosphorylation. Additional studies in larger patient populations and large-animal models with well-controlled conditions are required to resolve such important questions.
There is now strong evidence that abnormal CaMKII-phosphorylation of RyR2 can contribute to contractile dysfunction in HF.28, 47, 48 First, several studies showed that CaMKII hyperactivity can cause HF, for example in CaMKII-transgenic mice,49 whereas mice overexpressing a CaMKII-inhibitor or deficient in CaMKII-δ are protected from developing HF.50, 51 Second, RyR2 has been identified as a major downstream target of CaMKII involved in abnormal SR Ca2+-leak and contractile dysfunction in HF.48, 52 Our studies of a limited number of human failing hearts revealed increased RyR2-phosphorylation at S2814 in nonischemic dilated cardiomyopathy (DCM) but not in ischemic cardiomyopathy (ICM).28 These findings suggest that CaMKII activation might depend on the type of HF.
Mouse HF-models demonstrated that S2814-phosphorylation was increased in mice subjected to transverse-aortic constriction (TAC) but not after MI.28 S2814 ablation in S2814A-mice prevented progression of cardiomyopathy to severe HF in these mice, whereas no beneficial effect was noted in S2814A mice subjected to MI.28 Moreover, constitutive S2814-phosphorylation in S2814D mice caused spontaneous late-onset HF.27 These data suggest that S2814-phosphorylation might be involved in adverse cardiac remodeling in DCM, although additional studies are required to confirm this. These studies do not exclude the possibility of additional CaMKII-phosphorylation sites on RyR2 playing a role in DCM or other types of HF.
Some studies have demonstrated CaMKII-activation in failing hearts following MI.50, 53 However, consistent with the lack of S2814-hyperphosphorylation following MI in mice,28 it was not too surprising that S2814A knock-in mice were not protected from HF-progression following MI. These findings are also in agreement with a study from another lab showing a lack of protection from MI-induced HF in S2814A-mice.54
Role of RyR2-phosphorylation in ventricular arrhythmogenesis
Many patients with HF die suddenly due to ventricular arrhythmias,55 many of which are thought to be initiated by focal triggered activity, involving spontaneous diastolic SR Ca2+-release events (SCaEs) via RyR2.56 Sudden increases in SR Ca2+-leak can activate a potentially-arrhythmogenic depolarizing inward Na+/Ca2+-exchange (NCX)-current, which can cause delayed afterdepolarizations (DADs) and trigger ventricular arrhythmias. CaMKII, which is upregulated and more active in HF, has been shown to promote SR Ca2+-leak associated with triggered arrhythmias.48, 57, 58
Studies in S2814D-mice with constitutively-phosphorylated RyR2 revealed an increased risk for ventricular arrhythmias, even in the absence of structural heart disease.27 Interestingly, although S2814D-mice show more spontaneous Ca2+-sparks, ectopic activity was not observed under resting conditions, suggesting that a combination of increased SR Ca2+-load (during fast pacing/beta-adrenergic stimulation) is required to initiate arrhythmias.27 It remains to be studied whether concomitant S2808- and S2814-hyperphosphorylation promotes severe arrhythmogenic SR Ca2+-leak, even in the absence of β-adrenergic stimulation. In addition, S2814-phosphorylation might be a key mechanism for triggered arrhythmias in HF, at least following TAC in mice.27 Thus, it is mostly accepted that CaMKII-mediated RyR2-phosphorylation leads to SR Ca2+-leak associated with arrhythmias,7 but the potential role of S2808-phosphorylation in arrhythmogenic SCaEs remains uncertain. Finally, in HF-patients, several mechanisms likely conspire to promote triggered activity and ventricular tachycardia, including enhanced phosphorylation, oxidation and S-nitrosylation of RyR2.5, 7 The mechanisms underlying such synergistic effects are mostly understudied and should be addressed in future studies.
RyR2-phosphorylation is also believed to participate in the pathogenesis of catecholaminergic polymorphic ventricular tachycardia (CPVT), an inherited condition characterized by exercise or stress-induced arrhythmias, syncope and sudden cardiac death.59, 60 Over half the CPVT cases are caused by autosomal-dominant missense RyR2-mutations.59, 61 Although over 150 different RyR2-mutations have been reported, none appear to occur within known phosphorylation-motifs.62 Clinically, potentially-lethal arrhythmias occur predominantly following strenuous exercise or emotional stress, presumably in association with increased adrenergic activity.59 At the cellular level, enhanced adrenergic activity activates PKA and/or CaMKII, leading to enhanced RyR2-phosphorylation.58, 63 Most experimental studies agree that RyR2-phosphorylation can potentiate SR Ca2+-leak, which is associated with ectopic activity and arrhythmias in CPVT. However, some issues remain controversial, as discussed below. We will focus only on the role of RyR2-phosphorylation in CPVT, and refer the reader to prior reviews about other aspects of CPVT including the potential role of reduced FKBP12.6-binding and domain-unzipping as mechanisms of RyR2-destabilization.62, 64
It is somewhat controversial whether CPVT-associated RyR2-mutations cause channel dysfunction in the absence of adrenergic stimulation and channel-phosphorylation.64 Several studies have shown that RyR2-phosphorylation uncovers latent single-channel dysfunction of mutant RyR2.65–67 Other studies have shown that only SR Ca2+ overload, without activation of PKA, can unmask the ‘gain-of-function’ phenotype of CPVT-mutant RyR2 variants.68 However, other studies demonstrate a baseline gain-of-function,69, 70 or even a rare loss-of-function phenotype of CPVT-mutant RyR2-channels.71 These differences might result from different experimental approaches used or may reflect differences in phenotype-severity depending on the specific affected residue. For example, mutation V2475F appears to cause a particularly severe phenotype since homozygous knock-in mice are not viable,72 unlike other mutant gain-of-function RyR2-strains.27, 73–75 Interestingly, the neighboring mutation R2474S also causes a rather severe arrhythmia phenotype, compared to other RyR2-mutants (L433P, N2386I) in side-by-side studies.75 Most studies show that the CPVT-linked mutations do not alter baseline RyR2-phosphorylation at S2808 and S2814.67, 70, 74, 75 However, recombinant RyR2-channels with the V2475F-mutation exhibited significantly increased S2808- and S2030-phosphorylation levels at baseline, that were even more pronounced following exposure to the PKA-catalytic subunit.72 However, the composition of recombinant RyR2 is likely different from native heart channels, so future experiments will need to confirm whether CPVT-mutations alter basal RyR2-phosphorylation levels.
Role of RyR2-phosphorylation in atrial arrhythmogenesis
Atrial fibrillation (AF) is partially characterized by Ca2+-handling abnormalities.76 Initial work showed that the incidence of spontaneous Ca2+-release events (SCaEs, which include Ca2+-sparks and Ca2+-waves) is increased in cardiomyocytes from patients with chronic (persistent) AF (cAF), pointing primarily to RyR2-dysfunction.77 Emerging evidence indicates that abnormal RyR2-phosphorylation indeed plays a prominent role in AF-pathogenesis. RyR2 is hyperphosphorylated at S2808 in both cAF-patients and dogs with sustained AF due to atrial tachycardia remodeling.78 Increased S2808-phosphorylation of RyR2 in cAF-patients was surprising because cytosolic PP1 and PP2A activities are increased, which would be expected to reduce RyR2-phosphorylation levels.79 Despite the larger SR Ca2+-leak, SR Ca2+-load is unaltered in cAF,80 possibly due to increased phospholamban (PLB) phosphorylation.79
In dogs the increase in S2808-phosphorylation of RyR2 was associated with a stronger dissociation of FKBP12.6-subunit from RyR2-channels.78 Mice lacking FKBP12.6 showed larger SR Ca2+-leak and more SCaEs and triggered activity, along with an increased susceptibility to burst pacing-induced AF, validating the causal role of reduced FKBP12.6-levels for RyR2-dysfunction.74, 81, 82 Similar results were obtained in mice with the E169K-mutation in JPH2, which reduced its interaction with RyR2, suggesting an important RyR2-stabilizing role also for JPH2.83 Gain-of-function mutations in RyR2 predispose patients to CPVT and AF, and mice with these CPVT-mutations show RyR2-dysregulation and burst pacing-induced AF.74, 75, 84 The RyR2-FKBP12.6 binding stabilizing compound S107 prevents AF-initiation in CPVT-mice, pointing to a role of FKBP12.6 for RyR2-dysfunction in CPVT-related AF.75
The individual contribution of S2808-phosphorylation to RyR2-dysregulation and SR Ca2+-leak in cAF-patients is uncertain. While RyR2-hyperphosphorylation at S2808 appears a consistent finding in cAF-patients,74, 78, 80, 85 pharmacological PKA-inhibition does not affect the increased RyR2-Po and SR Ca2+-leak in atrial myocytes from cAF patients.80 Although these data intuitively question an important role of S2808-phosphorylation, the apparent lack of S2808 contribution might be due to the atrial tachycardia-induced permanent increase in CaMKII-activity and S2814-hyperphosphorylation, which may mask the impact of S2808-phosphorylation for atrial function. In addition, the S2814-hyperphosphorylation might cause a stronger conformational change of RyR2 resulting in a larger increase in RyR2-Po. Extensive additional work is needed to address these interesting possibilities.
Several papers have demonstrated that increased CaMKII-dependent RyR2-phosphorylation at 2814 is the major cause of SR Ca2+-leak and SCaEs in cAF-patients.74, 80, 85, 86 CaMKII-activity may increase as a result of faster atrial rate, which promotes its auto-phosphorylation10 or from oxidation of methionines 281/282, coupling AF-related oxidative stress to proarrhythmic Ca2+-handling.86 In addition, Thr35-hyperphosphorylation of I-1 in cAF is expected to reduce PP1-activity within the RyR2-complex,79, 87 also increasing S2814-phosphorylation. In goats with sustained AF, CaMKII-dependent RyR2-phosphorylation is increased, likely causing SR Ca2+-leak, potentially contributing to the reduced SR Ca2+-load and decreased atrial contractility associated with AF.88
Several mouse models have validated the causal role of CaMKII-and S2814-mediated RyR2-dysfunction, increased SR Ca2+-leak, and SCaEs in AF-initiation.74, 80, 85, 86 Pharmacological CaMKII-inhibition and genetic inhibition of CaMKII-dependent S2814-phosphorylation prevent AF initiation in FKBP12.6 knock-out mice, further supporting a critical role for S2814-mediated RyR2-dysfunction in AF.74, 82 Mice with cardiac-restricted overexpression of a repressor form of the cAMP-response element modulator (CREM) develop a complex cardiac phenotype including spontaneous-onset AF.89, 90 CREM-Tg mice exhibit changed atrial structure and hypertrophy, along with altered conduction and Ca2+-handling abnormalities including increased incidence of SCaEs and augmented SR Ca2+-leak.90 This mouse model supports a critical role for S2814-phosphorylation dependent RyR2-dysfunction in spontaneous AF.90 CaMKII-dependent RyR2-hyperphosphorylation is likely an early event in atrial CREM-TG-mice dysfunction, since CREM-TG-mice crossed with RyR2-S2814A-mice resistant to CaMKII-dependent RyR2-hyperphosphorylation, are protected from spontaneous AF.90 Overall, these studies support a major role for S2814-phosphorylation of RyR2 in atrial dysfunction and arrhythmogenesis in persistent AF.
Interestingly, patients with paroxysmal AF (pAF) do not exhibit changes in RyR2-phosphorylation at S2808 and S2814, despite increased SCaEs incidence and triggered activity.91 The underlying molecular substrate involves increased SR Ca2+-load and RyR2-dysregulation independent of RyR2-phosphorylation. The increased SR Ca2+-load is due to PKA-dependent PLB-hyperphosphorylation, relieving PLB-inhibition of SERCA2a and increasing SR Ca2+-uptake.91 RyR2-dysregulation involves increased protein expression and higher RyR2-Po, resulting in larger likelihood and amplitude of SCaEs. A relative deficiency of JPH2, resulting from increased RyR2 but unaltered JPH2-expression, might explain RyR2-dysfunction in pAF-patients.83, 91
Conclusions
Many studies have demonstrated that phosphorylation is an important mechanism by which RyR2-mediated SR Ca2+-release is fine-tuned within cardiomyocytes.5 Although our knowledge in this field is still evolving and certain concepts remain controversial due to disagreements among certain studies, it is evident that abnormal PKA- and particularly CaMKII-phosphorylation of RyR2 may contribute to the pathogenesis of HF, atrial and ventricular arrhythmias. The fact that not all studies agree is not uncommon in science, and actually helps to advance the field as a whole. It is important that there will be unrestricted exchange of reagents such as plasmids, antibodies, and knockin mouse models among labs in order to enable external confirmation of published results. As new scientific insights and technologies become available, some of the outstanding questions will undoubtedly be resolved. In the meantime, it is important that we all focus on the generation of new insights rather the perpetuation of (perceived) controversies, especially if our conclusions are based on imperfect experimental approaches. Finally, it needs to be emphasized that advancing our understanding of RyR2 regulation and dysfunction in the context of cardiac diseases is of the utmost importance, as RyR2 represents a unique and very promising therapeutic target for HF and arrhythmias.92, 93
Supplementary Material
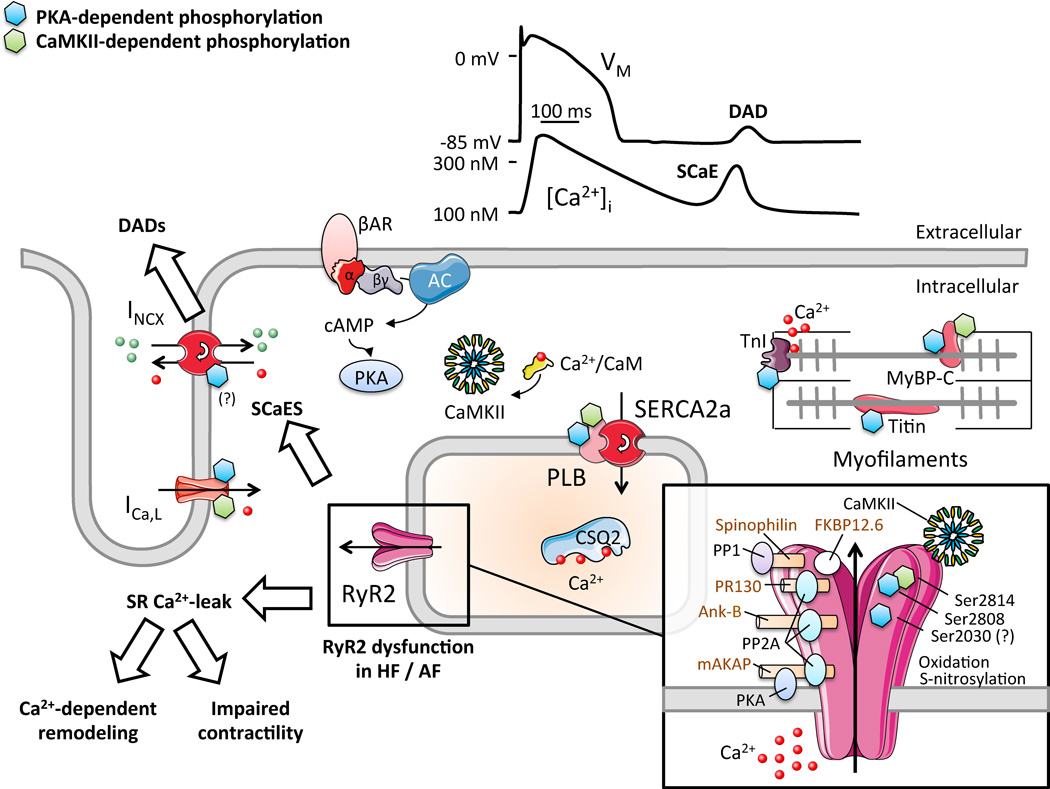
Figure 2.
Schematic representation of the key determinants of excitation-contraction coupling in cardiomyocytes. Physiologicallly, Ca2+-entry through L-type Ca2+-channels (ICa,L) triggers SR Ca2+-release through RyR2. The systolic Ca2+-transient activates myofilaments, initiating contraction. Diastolic relaxation occurs when Ca2+ is transported into SR via SERCA2a and out of the myocyte via NCX1. In heart failure (HF) and atrial fibrillation (AF), altered RyR2-phosphorylation increases SR Ca2+-leak, promotes Ca2+-dependent remodeling and impairs contractility. Spontaneous SR Ca2+-release events (SCaEs) promote delayed afterdepolarizations (DADs) and triggered activity. Inset shows RyR2-macromolecular complex with accessory proteins, protein kinases and phosphatases (and their respective anchoring proteins) that control phosphorylation levels. Protein kinase-A (PKA) and Ca2+-calmodulin-dependent protein kinase-II (CaMKII)-dependent phosphorylation sites are indicated with blue and green “P” symbols, respectively.
ACKNOWLEDGMENTS
The authors thank Dr. Jordi Heijman for assistance with the figures.
SOURCES OF FUNDING
The authors’ work is supported by the Fondation Leducq grants “European–North American Atrial Fibrillation Research Alliance” (07CVD03 to DD) and “Alliance for Calmodulin Kinase Signaling in Heart Disease” (08CVD01, to XHTW), the European Network for Translational Research in Atrial Fibrillation (261057, to DD), the DZHK (German Center for Cardiovascular Research, to DD), the American Heart Association (13EIA14560061 to XHTW), Muscular Dystrophy Association (186530), and National Institutes of Health grants HL089598, HL091947, and HL117641 (to XHTW).
ABBREVIATIONS
- AF
Atrial fibrillation
- CaMKII
Ca2+/calmodulin-dependent protein kinase-II
- CPVT
Catecholaminergic polymorphic ventricular tachycardia
- DCM
Dilated cardiomyopathy
- HF
Heart failure
- JPH2
Junctophilin-2
- MI
Myocardial infarction
- PKA
Protein kinase-A
- PP1
Protein phosphatase type-1
- PP2A
Protein phosphatase type-2A
- RyR2
Ryanodine receptor type-2
- CSQ2
Calsequestrin-2
- SR
Sarcoplasmic-reticulum
- TAC
Transverse-aortic constriction
- VT
Ventricular tachycardia
- WT
Wild-type
Footnotes
DISCLOSURES
Dr. Wehrens is a founding partner of Elex Biotech, a company that develops drugs that modulate ryanodine receptors.
REFERENCES
- 1.Wehrens XH, Lehnart SE, Marks AR. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann N Y Acad Sci. 2005;1047:366–375. doi: 10.1196/annals.1341.032. [DOI] [PubMed] [Google Scholar]
- 2.George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res. 2008;77:302–314. doi: 10.1093/cvr/cvm006. [DOI] [PubMed] [Google Scholar]
- 3.Eschenhagen T. Is ryanodine receptor phosphorylation key to the fight or flight response and heart failure? J Clin Invest. 2010;120:4197–4203. doi: 10.1172/JCI45251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res. 2012;110:796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 5.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. doi: 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 7.McCauley MD, Wehrens XH. Ryanodine receptor phosphorylation, calcium/calmodulin-dependent protein kinase II, and life-threatening ventricular arrhythmias. Trends Cardiovasc Med. 2011;21:48–51. doi: 10.1016/j.tcm.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 2004;35:621–628. doi: 10.1016/j.ceca.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Heijman J, Dewenter M, El-Armouche A, Dobrev D. Function and regulation of serine/threonine phosphatases in the healthy and diseased heart. J Mol Cell Cardiol. 2013;64:90–98. doi: 10.1016/j.yjmcc.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–e70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 11.Marx SO, Marks AR. Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. J Mol Cell Cardiol. 2013;58:225–231. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ather S, Respress JL, Li N, Wehrens XH. Alterations in ryanodine receptors and related proteins in heart failure. Biochim Biophys Acta. 2013;1832:2425–2431. doi: 10.1016/j.bbadis.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 14.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 15.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 17.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 20.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circ Res. 2008;102:e65–e72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, Berretta RM, Koch WJ, Chen X, Gao E, Valdivia HH, Houser SR. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circ Res. 2012;110:831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacMillan D. FK506 binding proteins: cellular regulators of intracellular Ca2+ signalling. Eur J Pharmacol. 2013;700:181–193. doi: 10.1016/j.ejphar.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR, Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:1559–1564. doi: 10.1073/pnas.0908540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on serine 2809 by calmodulin-dependent kinase II and protein kinase A. J Biol Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 26.Valdivia HH. Ryanodine receptor phosphorylation and heart failure: phasing out S2808 and "criminalizing" S2814. Circ Res. 2012;110:1398–1402. doi: 10.1161/CIRCRESAHA.112.270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 30.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. The Journal of clinical investigation. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, Shimoni Y, Cheng H, Ter Keurs H, Chen SR. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huke S, Bers DM. Ryanodine receptor phosphorylation at Serine 2030, 2808 and 2814 in rat cardiomyocytes. Biochem Biophys Res Commun. 2008;376:80–85. doi: 10.1016/j.bbrc.2008.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks AR, Marx SO, Reiken S. Regulation of ryanodine receptors via macromolecular complexes: a novel role for leucine/isoleucine zippers. Trends Cardiovasc Med. 2002;12:166–170. doi: 10.1016/s1050-1738(02)00156-1. [DOI] [PubMed] [Google Scholar]
- 34.Belevych AE, Sansom SE, Terentyeva R, Ho HT, Nishijima Y, Martin MM, Jindal HK, Rochira JA, Kunitomo Y, Abdellatif M, Carnes CA, Elton TS, Gyorke S, Terentyev D. MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex. PLoS One. 2011;6:e28324. doi: 10.1371/journal.pone.0028324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terentyev D, Belevych AE, Terentyeva R, Martin MM, Malana GE, Kuhn DE, Abdellatif M, Feldman DS, Elton TS, Gyorke S. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res. 2009;104:514–521. doi: 10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, McAvoy T, Nairn AC, Kapiloff MS. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem. 2010;285:11078–11086. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terentyev D, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, Gyorke S. Protein phosphatases decrease sarcoplasmic reticulum calcium content by stimulating calcium release in cardiac myocytes. J Physiol. 2003;552:109–118. doi: 10.1113/jphysiol.2003.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter S, Colyer J, Sitsapesan R. Maximum phosphorylation of the cardiac ryanodine receptor at serine-2809 by protein kinase a produces unique modifications to channel gating and conductance not observed at lower levels of phosphorylation. Circ Res. 2006;98:1506–1513. doi: 10.1161/01.RES.0000227506.43292.df. [DOI] [PubMed] [Google Scholar]
- 39.El-Armouche A, Wittkopper K, Degenhardt F, Weinberger F, Didie M, Melnychenko I, Grimm M, Peeck M, Zimmermann WH, Unsold B, Hasenfuss G, Dobrev D, Eschenhagen T. Phosphatase inhibitor-1-deficient mice are protected from catecholamine-induced arrhythmias and myocardial hypertrophy. Cardiovasc Res. 2008;80:396–406. doi: 10.1093/cvr/cvn208. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Ho HT, Velez-Cortes F, Lou Q, Valdivia C, Knollmann B, Valdivia H, Gyorke S. Genetic ablation of ryanodine receptor 2 phosphorylation at Ser-2808 aggravates Ca2+-dependent cardiomyopathy by exacerbating diastolic Ca2+ release. J Physiol. 2014 doi: 10.1113/jphysiol.2013.264689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Makarewich C, Kubo H, Wang W, Duran J, Li Y, Berretta R, Koch WJ, Chen X, Gao E, Valdivia H, Houser SR. Hyperphosphorylation of the Cardiac Ryanodine Receptor at Serine 2808 Is Not Involved in Cardiac Dysfunction After Myocardial Infarction. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.111.255158. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obayashi M, Xiao B, Stuyvers BD, Davidoff AW, Mei J, Chen SR, ter Keurs HE. Spontaneous diastolic contractions and phosphorylation of the cardiac ryanodine receptor at serine-2808 in congestive heart failure in rat. Cardiovasc Res. 2006;69:140–151. doi: 10.1016/j.cardiores.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Fischer TH, Herting J, Tirilomis T, Renner A, Neef S, Toischer K, Ellenberger D, Forster A, Schmitto JD, Gummert J, Schondube FA, Hasenfuss G, Maier LS, Sossalla S. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation. 2013;128:970–981. doi: 10.1161/CIRCULATIONAHA.113.001746. [DOI] [PubMed] [Google Scholar]
- 48.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 49.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 50.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 51.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Brown JH. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curran J, Tang L, Roof SR, Velmurugan S, Millard A, Shonts S, Wang H, Santiago D, Ahmad U, Perryman M, Bers DM, Mohler PJ, Ziolo MT, Shannon TR. Nitric Oxide-Dependent Activation of CaMKII Increases Diastolic Sarcoplasmic Reticulum Calcium Release in Cardiac Myocytes in Response to Adrenergic Stimulation. PLoS One. 2014;9:e87495. doi: 10.1371/journal.pone.0087495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farr MA, Basson CT. Sparking the failing heart. N Engl J Med. 2004;351:185–187. doi: 10.1056/NEJMcibr041466. [DOI] [PubMed] [Google Scholar]
- 56.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 58.Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J, Bers DM. Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation. 2013;127:913–922. doi: 10.1161/CIRCULATIONAHA.12.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 60.Marks AR, Priori S, Memmi M, Kontula K, Laitinen PJ. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J Cell Physiol. 2002;190:1–6. doi: 10.1002/jcp.10031. [DOI] [PubMed] [Google Scholar]
- 61.Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 62.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reiken S, Gaburjakova M, Gaburjakova J, He KlKL, Prieto A, Becker E, Yi GhGH, Wang J, Burkhoff D, Marks AR. beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 64.McCauley MD, Wehrens XH. Targeting ryanodine receptors for anti-arrhythmic therapy. Acta Pharmacol Sin. 2011;32:749–757. doi: 10.1038/aps.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 66.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 67.Meli AC, Refaat MM, Dura M, Reiken S, Wronska A, Wojciak J, Carroll J, Scheinman MM, Marks AR. A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium. Circ Res. 2011;109:281–290. doi: 10.1161/CIRCRESAHA.111.244970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 69.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, Gronau P, Maier LS, Vos MA, Lai FA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–59. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 71.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loaiza R, Benkusky NA, Powers PP, Hacker T, Noujaim S, Ackerman MJ, Jalife J, Valdivia HH. Heterogeneity of ryanodine receptor dysfunction in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2013;112:298–308. doi: 10.1161/CIRCRESAHA.112.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, Kearney DL, Danila CI, De Biasi M, Wehrens XH, Pautler RG, Roden DM, Taffet GE, Dirksen RT, Anderson ME, Hamilton SL. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A, Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 77.Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 78.Vest JA, Wehrens XH, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 79.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 80.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sood S, Chelu MG, van Oort RJ, Skapura D, Santonastasi M, Dobrev D, Wehrens XH. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm. 2008;5:1047–1054. doi: 10.1016/j.hrthm.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, Landstrom AP, Li N, Wang Q, Olivotto I, Dobrev D, Ackerman MJ, Wehrens XH. Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol. 2013;62:2010–2019. doi: 10.1016/j.jacc.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heijman J, Wehrens XH, Dobrev D. Atrial arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia--is there a mechanistic link between sarcoplasmic reticulum Ca(2+) leak and re-entry? Acta Physiol (Oxf) 2013;207:208–211. doi: 10.1111/apha.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–1144. doi: 10.1161/CIRCRESAHA.109.203836. [DOI] [PubMed] [Google Scholar]
- 86.Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wittkopper K, Fabritz L, Neef S, Ort KR, Grefe C, Unsold B, Kirchhof P, Maier LS, Hasenfuss G, Dobrev D, Eschenhagen T, El-Armouche A. Constitutively active phosphatase inhibitor-1 improves cardiac contractility in young mice but is deleterious after catecholaminergic stress and with aging. J Clin Invest. 2010;120:617–626. doi: 10.1172/JCI40545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, Nattel S, Allessie MA, Dobrev D, Schotten U. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–394. doi: 10.1016/j.yjmcc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 89.Kirchhof P, Marijon E, Fabritz L, Li N, Wang W, Wang T, Schulte K, Hanstein J, Schulte JS, Vogel M, Mougenot N, Laakmann S, Fortmueller L, Eckstein J, Verheule S, Kaese S, Staab A, Grote-Wessels S, Schotten U, Moubarak G, Wehrens XH, Schmitz W, Hatem S, Muller FU. Overexpression of cAMP-response element modulator causes abnormal growth and development of the atrial myocardium resulting in a substrate for sustained atrial fibrillation in mice. Int J Cardiol. 2013;166:366–374. doi: 10.1016/j.ijcard.2011.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XH. Ryanodine-Receptor Mediated Calcium Leak Drives Progressive Development of an Atrial Fibrillation Substrate in a Transgenic Mouse Model. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 93.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, Duff HJ, Roden DM, Wilde AA, Knollmann BC. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ullrich ND, Valdivia HH, Niggli E. PKA phosphorylation of cardiac ryanodine receptor modulates SR luminal Ca2+ sensitivity. J Mol Cell Cardiol. 2012;53:33–42. doi: 10.1016/j.yjmcc.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sarma S, Li N, van Oort RJ, Reynolds C, Skapura DG, Wehrens XH. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:13165–13170. doi: 10.1073/pnas.1004509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoyer DP, Gronke S, Frank KF, Addicks K, Wettschureck N, Offermanns S, Erdmann E, Reuter H. Diabetes-related defects in sarcoplasmic Ca2+ release are prevented by inactivation of G(alpha)11 and G(alpha)q in murine cardiomyocytes. Mol Cell Biochem. 2010;341:235–244. doi: 10.1007/s11010-010-0454-1. [DOI] [PubMed] [Google Scholar]
- 97.Paulino EC, Ferreira JC, Bechara LR, Tsutsui JM, Mathias W, Jr, Lima FB, Casarini DE, Cicogna AC, Brum PC, Negrao CE. Exercise training and caloric restriction prevent reduction in cardiac Ca2+-handling protein profile in obese rats. Hypertension. 2010;56:629–635. doi: 10.1161/HYPERTENSIONAHA.110.156141. [DOI] [PubMed] [Google Scholar]
- 98.Zeng B, Chen GL, Xu SZ. Store-independent pathways for cytosolic STIM1 clustering in the regulation of store-operated Ca(2+) influx. Biochem Pharmacol. 2012;84:1024–1035. doi: 10.1016/j.bcp.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 99.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gamble KL, Ciarleglio CM. Ryanodine receptors are regulated by the circadian clock and implicated in gating photic entrainment. J Neurosci. 2009;29:11717–11719. doi: 10.1523/JNEUROSCI.3820-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Said M, Becerra R, Valverde CA, Kaetzel MA, Dedman JR, Mundina-Weilenmann C, Wehrens XH, Vittone L, Mattiazzi A. Calcium-calmodulin dependent protein kinase II (CaMKII): a main signal responsible for early reperfusion arrhythmias. J Mol Cell Cardiol. 2011;51:936–944. doi: 10.1016/j.yjmcc.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ather S, Wang W, Wang Q, Li N, Anderson ME, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents inducible ventricular arrhythmias in mice with Duchenne muscular dystrophy. Heart Rhythm. 2013;10:592–599. doi: 10.1016/j.hrthm.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chu DK, Jordan MC, Kim JK, Couto MA, Roos KP. Comparing isoflurane with tribromoethanol anesthesia for echocardiographic phenotyping of transgenic mice. J Am Assoc Lab Anim Sci. 2006;45:8–13. [PubMed] [Google Scholar]
- 104.Constantinides C, Mean R, Janssen BJ. Effects of isoflurane anesthesia on the cardiovascular function of the C57BL/6 mouse. ILAR J. 2011;52:e21–e31. [PMC free article] [PubMed] [Google Scholar]
- 105.Santiago DJ, Curran JW, Bers DM, Lederer WJ, Stern MD, Rios E, Shannon TR. Ca sparks do not explain all ryanodine receptor-mediated SR Ca leak in mouse ventricular myocytes. Biophys J. 2010;98:2111–2120. doi: 10.1016/j.bpj.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie W, Santulli G, Guo X, Gao M, Chen BX, Marks AR. Imaging atrial arrhythmic intracellular calcium in intact heart. J Mol Cell Cardiol. 2013;64:120–123. doi: 10.1016/j.yjmcc.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCarron JG, Olson ML, Chalmers S, Girkin JM. Single cell and subcellular measurements of intracellular Ca(2)(+) concentration. Methods Mol Biol. 2013;937:239–251. doi: 10.1007/978-1-62703-086-1_15. [DOI] [PubMed] [Google Scholar]
- 108.Silvestro AM, Ashley RH. Solubilization, partial purification and functional reconstitution of a sheep brain endoplasmic reticulum anion channel. Int J Biochem. 1994;26:1129–1138. doi: 10.1016/0020-711x(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 109.Kushnir A, Betzenhauser MJ, Marks AR. Ryanodine receptor studies using genetically engineered mice. FEBS Lett. 2010;584:1956–1965. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.