Abstract
Unintentional acetaminophen-induced hepatotoxicity has been increasingly recognized as a significant problem, prompting increased scrutiny and restrictions from the US Food and Drug Administration on products combining acetaminophen with narcotics. Patterns of acetaminophen use have not previously been reported in the hospitalized patient population, which may be especially vulnerable to liver injury. We aimed to quantify the frequency at which acetaminophen dosing exceeded the recommended maximum of 4 g/day in hospitalized patients. This was a retrospective, single-center, cohort study at a large tertiary care academic hospital. We queried our inpatient electronic medical record database to identify patients admitted between 2008 and 2010 who were receiving cumulative daily acetaminophen doses exceeding 4 g on at least 1 hospital day. Of 43,761 admissions involving acetaminophen administration, the recommended maximum cumulative daily dose of 4 g was exceeded in 1119 (2.6%) cases. Patients who were administered a larger number of acetaminophen-containing medications were more likely to receive doses in excess of the recommended maximum. Alanine aminotransferase (ALT) levels were checked within 14 days following acetaminophen exposure in excess of 4 g in 35 (3.1%) cases. Excessive acetaminophen dosing of hospitalized patients, who may be at increased risk for acetaminophen-induced hepatotoxicity, occurred in a minority of patients. The use of multiple acetaminophen-containing medication formulations contributed to excessive dosing. ALT level monitoring in this group was infrequent, precluding assessment of biochemical evidence of liver injury. This cohort of patients may represent an ideal population for further prospective study with more intensive and longer-term biochemical monitoring to assess for evidence of liver injury.
Keywords: Acetaminophen, drug-induced liver injury, hepatotoxicity, hospitalized patients, drug safety
The problem of unintentional poisoning caused by acetaminophen resulting in hepatotoxicity has been increasingly recognized in recent years. The proliferation of prescription and nonprescription combination formulations containing acetaminophen with other medications is thought to contribute to this problem. This recognition has recently led the US Food and Drug Administration (FDA) to restrict the maximum dose of acetaminophen in products combined with narcotics to 325 mg per tablet.1 Further restrictions, such as complete removal of these products from the market as well as lowering the recommended maximum cumulative daily dose of acetaminophen below 4 g, are the subject of ongoing debate.2
The economic impact of these changes would be significant, with annual sales of acetaminophen products in the United States exceeding 1 billion dollars.3 This debate is relevant not only because of the magnitude of its potential economic impact, but also because it represents a paradigm shift in the FDA’s approach to the issue of acetaminophen, which had previously focused on promoting patient education and mandating clear labeling rather than restricting the availability of acetaminophen products in the market.4 The approach to this problem in other countries has been even more restrictive, with recent legislation in the United Kingdom banning the sale of more than 32 acetaminophen tablets in a single transaction in pharmacies or more than 16 tablets per transaction at other types of retail stores.5
Despite the popularity of acetaminophen and the absence of any documented life-threatening liver injury in prospective studies evaluating its safety, the threshold dose of acetaminophen at which clinically significant hepatotoxicity occurs remains poorly characterized. Previous prospective studies have repeatedly demonstrated that elevations in alanine aminotransferase (ALT) levels develop in a significant proportion of healthy volunteers who are given 4 g of acetaminophen daily for 7 to 10 days.6-8 The long-term clinical significance of these biochemical abnormalities is unknown, limited by the short duration of these prospective studies, the longest of which involved administration of acetaminophen for 14 days.
Factors contributing to unintentional acetamino-phen-induced hepatotoxicity may include malnutrition. This factor is more prevalent in a hospitalized population than in the general population9-16; therefore, hospitalized patients may be particularly vulnerable to acetamino-phen-induced hepatotoxicity. Among risk factors for acetaminophen-induced hepatotoxicity, the most readily measurable and modifiable is the cumulative daily acetaminophen dose administered. Therefore, we aimed to quantify the frequency at which the recommended maximum dose of 4 g of acetaminophen per day was exceeded in a retrospective cohort of inpatients at a large tertiary care academic hospital. We further aimed to quantify the number of acetaminophen-containing medications administered and the frequency of ALT level monitoring in this group.
Methods
This was a retrospective cohort study. Approval was obtained from the Institutional Review Board of Thomas Jefferson University. Thomas Jefferson University Hospital is a 957-bed, acute, tertiary care hospital located in the Center City District of Philadelphia, Pennsylvania. There were 108,435 emergency department visits and 45,503 admissions at Thomas Jefferson University Hospital in fiscal year 2010.
An electronic database contains records of every dose of every medication administered to the inpatient population and also serves as a repository for all laboratory data. Because the system records doses that were actually administered, we were able to capture whether patients actually received all doses of standing medication orders, refused a dose, or were unable to receive a scheduled dose because of nil per os status, for example. In cases in which doses of standing medication orders were not dispensed, the nurse would enter a free-text comment into the database (eg, “patient refused”). Similarly, for as-needed doses, only doses that were actually administered were counted. Cumulative daily doses of acetaminophen were calculated as follows: for each distinct medication formulation containing acetaminophen, the number of tablets actually dispensed to the patient was multiplied by the number of milligrams of acetaminophen contained per tablet of that formulation. It is possible that some doses of acetaminophen-containing medications could have been dispensed to individual patients by nurses but not consumed.
We performed a database query to ascertain how many patients received more than 4 g of acetaminophen on at least 1 hospital day during their stays, taking into account all sources of acetaminophen. The database query was performed by an information technologist who was employed by the Department of Pharmacy and whose duties included maintenance of this database. The database query was conducted using Microsoft Access. We defined a “hospital day” as a calendar day starting and ending at midnight (ie, from 12:00:00 AM until 11:59:59 PM on a given date).
We restricted our query to hospital admissions for adult patients with a discharge date between January 1, 2008 and December 31, 2010. We selected this particular time period because it encompassed the 2009 FDA advisory panel recommendations calling for increased attention to the problem of acetaminophen-induced hepatotoxicity and to the possible contributing role of acetaminophen-narcotic combination formulations. We included admissions for all indications to all services at our institution. We were able to track only the admitting service for each hospitalization; it is possible that some patients may have been admitted to one service but transferred to another service at a later point during their hospital course. Patients who were evaluated and treated in the emergency department and then discharged directly from there were not included in the analysis.
For each admission, we calculated the number of distinct acetaminophen-containing formulations administered during the course of the hospitalization. Formulations were considered distinct if they were different medications (acetaminophen vs acetaminophen/oxycodone) or involved different modes of administration of an identical medication (acetaminophen oral capsule vs rectal suppository). Orders that were discontinued and later reordered at the original dose or at a different dose were not considered distinct. If a medication was ordered both at a standing dose and concurrently as an as-needed dose, these orders were considered distinct.
We then queried the database to ascertain whether any ALT measurements were performed within 14 days following each exposure in excess of 4 g per calendar day. This time period was selected because prior studies detecting elevations in ALT levels in healthy volunteers found that these elevations generally began to manifest within 7 days of initiating challenge with 4 g daily.6-8 For patients who received more than 4 g of acetaminophen on at least 1 hospital day and who had ALT level measurements performed on at least 2 hospital days, we performed a chart review to verify the sequence of events (timing of ALT measurements and acetaminophen dose administration) and to assess whether a more likely reason for the laboratory abnormality could be identified. Due to the large number of patients included in the initial database query, it was not practical to perform a detailed chart review for the entire study population, and, therefore, we were not able to report the frequency of known chronic liver disease or cirrhosis.
Any ALT measurement higher than the upper limit of the reference range of our laboratory was considered elevated (normal range, 1-45 IU/L for men, 1-30 IU/L for women). Only ALT measurements performed during the hospital admission were considered.
We performed univariate analyses to detect significant associations between clinical attributes of hospital admission and whether acetaminophen was administered at doses in excess of 4 g on at least 1 calendar day during the hospitalization. We also performed univariate analyses to detect associations between clinical attributes of hospital admissions and the frequency of ALT level monitoring in this group. The t test was used to calculate P values for continuous variables, and the Fisher exact test was used to calculate P values for categoric variables. This univariate analysis was performed using Microsoft Excel 2007.
Results
Acetaminophen Dosing
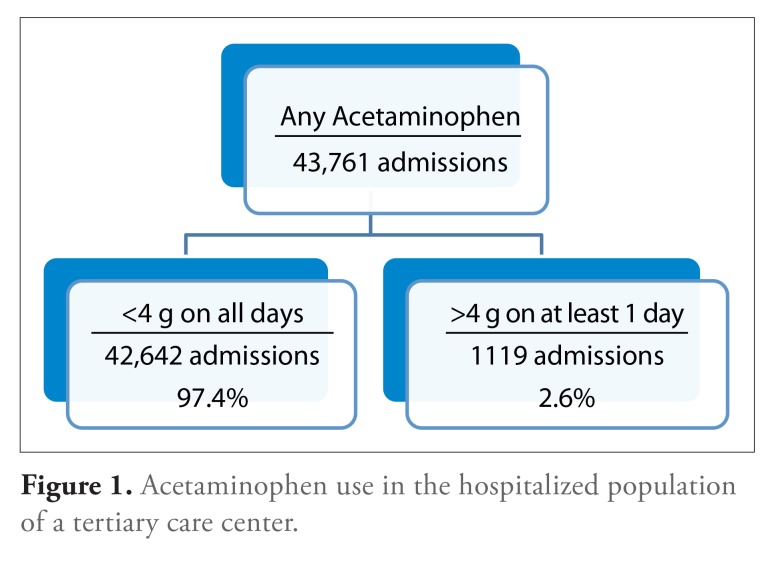
There were 43,761 hospital admissions with a discharge date between January 1, 2008 and December 31, 2010 at Thomas Jefferson University Hospital involving the administration of at least 1 dose of an acetaminophen-containing medication. The cumulative dose of acetaminophen exceeded 4 g on at least 1 day in the course of 1119 (2.6%) of these admissions (Figure 1). We found that admissions involving administration of acetaminophen in excess of 4 g on at least 1 day were statistically significantly more likely to involve patients who were slightly older, were white, had shorter lengths of stay, were admitted to a surgical service (especially orthopedic surgery), and had received a larger number of acetaminophen-containing medication formulations (Table 1).
Figure 1.
Acetaminophen use in the hospitalized population of a tertiary care center.
Table 1.
Comparison of Clinical Characteristics of Admissions Involving Administration of Acetaminophen Exceeding or Not Exceeding the Recommended Maximum Dose of 4 g Daily
| Patients Receiving >4 g on at Least 1 Hospital Day | Patients Receiving ≤4 g on Any Hospital Day | P value | ||
|---|---|---|---|---|
| Number of subjects | 1119 | 42,642 | ||
| Average age | 57.0 ± 13.4 yrs | 54.3 ± 18.0 yrs | <.001 | |
| Gender (%) | Male | 476 (42.5) | 18,037 (42.3) | .90 |
| Female | 643 (57.6) | 24,605 (57.7) | ||
| Race (%) | White | 896 (80.1) | 27,305 (64.0) | <.001 |
| Black | 166 (14.8) | 11,248 (26.4) | ||
| Other | 57(5.1) | 4,089 (9.6) | ||
| Length of stay (average ± standard deviation) | 6.2 ± 4.8 days | 7.0 ± 9.4 days | <.001 | |
| Number of acetaminophen-containing medication orders | 2.27 ± 1.03 | 1.50 ±0.78 | <.001 | |
| Admitting service (%) | Orthopedic surgery | 904 (80.8) | 7355 (17.3) | <.001 |
| General medicine | 22 (2.0) | 5423 (12.7) | ||
| General surgery | 32 (2.9) | 4767(11.2) | ||
| Obstetrics/gynecology | 5 (0.5) | 3384 (7.9) | ||
| Neuroscience | 32 (2.9) | 2656 (6.2) | ||
| Clinical decision unit | 7 (0.6) | 2311 (5.4) | ||
| Other | 117(10.5) | 15,144(35.5) | ||
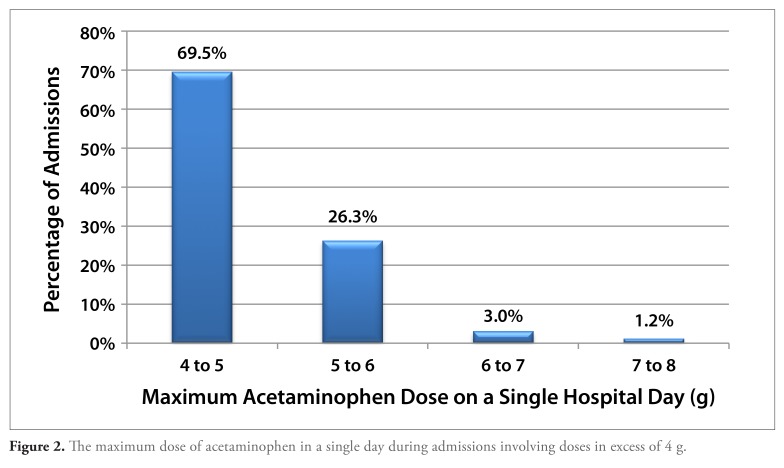
Of the 1119 admissions involving the administration of more than 4 g of acetaminophen on at least 1 day, in the majority of cases, the maximum dose on any day during the admission was between 4 and 5 g. However, the maximum cumulative dose on a single hospital day exceeded 5 g in 30% of cases and exceeded 6 g in 4% of cases (Figure 2). There was a single admission involving administration of 10 g of acetaminophen during a single day, and in no cases were larger amounts of acetaminophen administered in a single day.
Figure 2.
The maximum dose of acetaminophen in a single day during admissions involving doses in excess of 4 g.
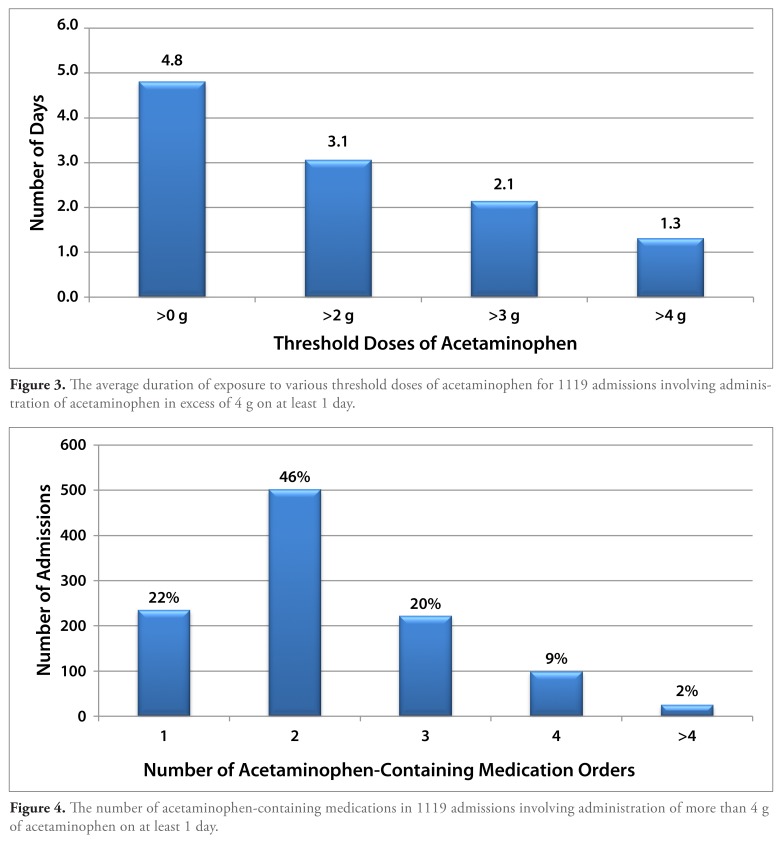
Most admissions involving the administration of more than 4 g of acetaminophen did so for a short duration of time. The average duration of daily exposure to acetaminophen at doses in excess of 4 g per day was 1.3 days (range, 1-10 days) in this cohort, which had an average length of stay of 6.2 days. At least 1 dose of acetaminophen was administered daily during the majority of hospital days for this group (Figure 3).
Figure 3.
The average duration of exposure to various threshold doses of acetaminophen for 1119 admissions involving administration of acetaminophen in excess of 4 g on at least 1 day.
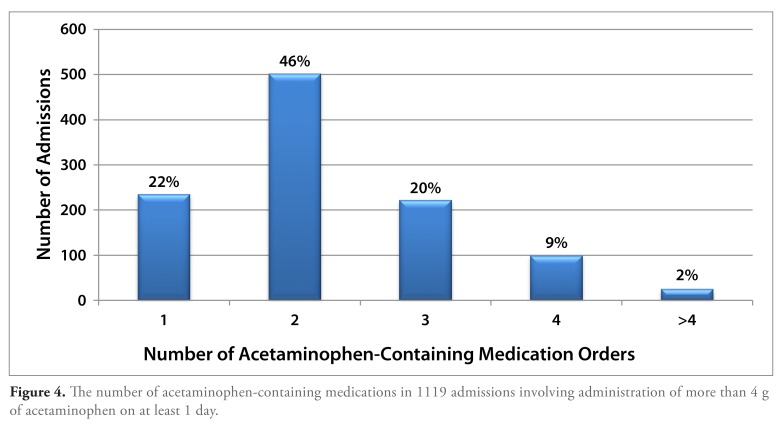
Of the admissions involving the administration of more than 4 g of acetaminophen on at least 1 day, acetaminophen use was restricted to a single formulation in 22% of cases. Two distinct acetaminophen-containing formulations were administered during the course of these admissions in 46% of cases, 3 distinct formulations in 20% of cases, and 4 or more distinct formulations in 11% of cases (Figure 4). In addition to acetaminophen alone, acetaminophen-containing combination medications also most commonly contained oxycodone, hydrocodone, codeine, propoxyphene, or butalbital. The highest dose of acetaminophen contained per tablet in these combination formulations was 500 mg.
Figure 4.
The number of acetaminophen-containing medications in 1119 admissions involving administration of more than 4 g of acetaminophen on at least 1 day.
Alanine Aminotransferase Level Monitoring
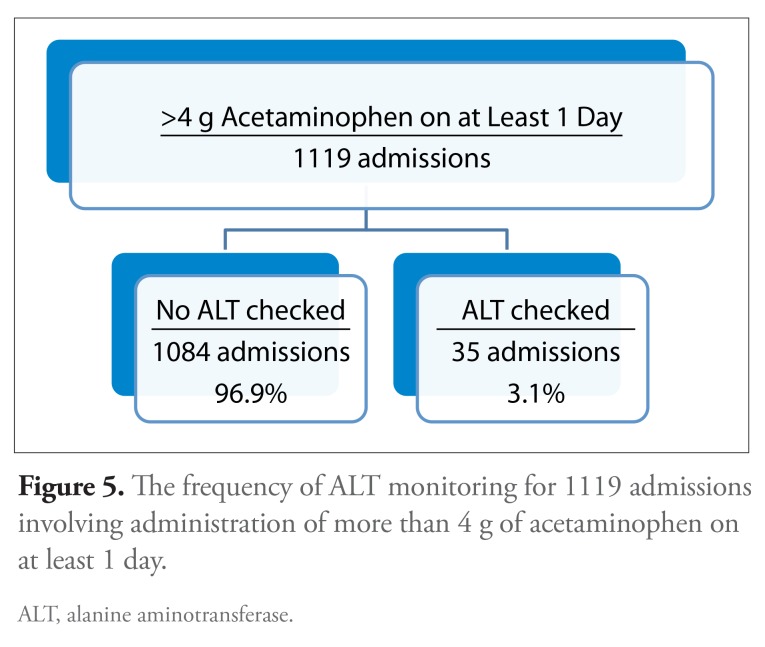
Of the 1119 admissions involving administration of more than 4 g of acetaminophen on at least 1 day, ALT levels were checked within 14 days following this exposure in 35 (3.1%) cases (Figure 5). Of the 47 patients who were administered more than 6000 mg of acetaminophen on a given hospital day, ALT levels were measured within this time frame for only 1 individual. ALT level was statistically significantly more likely to be checked during admissions with a longer length of stay and less likely to be checked during admissions to a surgical service, especially orthopedic surgery (Table 2).
Figure 5.
The frequency of ALT monitoring for 1119 admissions involving administration of more than 4 g of acetaminophen on at least 1 day.
ALT, alanine aminotransferase.
Table 2.
Comparison of Clinical Characteristics of Admissions Involving Administration of Acetaminophen in Excess of 4 g on at Least 1 Day with or without ALT Measurement Checked within 14 Days of Exposure
| Subjects with ALT Measurement(s) | Subjects without ALT Measurement | P value | ||
|---|---|---|---|---|
| Number of subjects | 35 | 1084 | ||
| Average age | 58.3 ± 15.2 yrs | 57.0 ± 13.3 yrs | .79 | |
| Gender (%) | Male | 17 (48.6) | 458 (42.2) | .49 |
| Female | 18(51.4) | 625 (57.8) | ||
| Race (%) | White | 26 (74.3) | 869 (80.2) | .39 |
| Black | 4(11.4) | 162(15.0) | ||
| Other | 5 (14.3) | 52 (4.8) | ||
| Length of stay (average ± standard deviation) | 16.7 ± 10.9 days | 5.9 ± 4.1 days | <.001 | |
| Number of acetaminophen-containing medication orders | 2.17 | 2.25 | .39 | |
| Admitting service (%) | Orthopedic surgery | 14 (40) | 888 (82.0) | <.001 |
| Neurosurgery | 3 (8.5) | 31 (2.9) | ||
| Neuroscience | 3 (8.5) | 29 (2.7) | ||
| General surgery | 4(11.4) | 28 (2.6) | ||
| Trauma surgery | 1 (2.9) | 23 (2.1) | ||
| General medicine | 2 (5.7) | 20 (1.8) | ||
| Other | 8 (22.9) | 62 (5.7) | ||
ALT, alanine aminotransferase.
Of admissions during which ALT levels were checked within 14 days following exposure to acetaminophen doses in excess of 4 g, a preexposure ALT level measurement was also available in 18 cases. Of the 18 cases in which both pre- and postexposure ALT level measurements were available, the initial measurement was outside the normal range in the majority of cases, with a median initial ALT level value of 40 IU/L (95% CI, 27-67 IU/L). The ALT level increased in 9 cases after exposure (median increment, 4 IU/L; 95% CI, 1-34 IU/L) and decreased in 9 cases (median decrement, 18 IU/L; 95% CI, 1-51 IU/L). None of these 18 patients for whom both pre- and postexposure ALT level values were available received more than 6000 mg of acetaminophen on any given hospital day.
The paucity of ALT level monitoring, combined with incomplete information available in hospital charts for retrospective review (notably quantification of chronic alcohol use), precluded conducting a formal causal analysis to determine the association between acetaminophen exposure and elevations in ALT levels.
Discussion
In this study, we found that the recommended maximum cumulative dose of 4 g/day was exceeded in 2.6% of cases in which acetaminophen was administered to an inpatient population and that ALT level monitoring was infrequent in this group. Although this was a single-center study, we suspect that similar patterns of acetaminophen use and infrequent liver test monitoring can occur within any healthcare institution. The impact of our findings and supposition is that, although exposure of hospitalized patients to excessive acetaminophen doses occurred in only a minority of patients, because of the widespread use of this medication, a large number of vulnerable patients may be potentially at risk for liver injury. Furthermore, because controversy continues to exist regarding the minimum dose at which clinically relevant toxicity can occur, we have identified a patient cohort that may represent an ideal study population for further longer-term and more intensive prospective biochemical monitoring for evidence of liver injury.
Previous prospective studies have documented a 25% to 40% incidence of ALT level elevations to at least twice the upper limit of normal in healthy volunteers who were administered acetaminophen at a dose of 4 g daily; these elevations generally begin to manifest after 7 to 10 days of acetaminophen exposure.6-8 Although these prospective studies did not report any cases of clinically severe hepatotoxicity, the duration of biochemical monitoring was short, involving administration of acetaminophen at 4 g daily for up to 14 days. Although there have been numerous case reports describing significant liver toxicity in association with acetaminophen use at doses of up to 4 g daily17-34 critics have questioned whether the true exposure may have been in excess of that reported. Overall, the interpretation of these case reports, as well as the interpretation of both retrospective and additional prospective studies35-37 of hepatotoxicity associated with acetaminophen at therapeutic doses, has been a matter of some debate.3,4,38-43
Whether ALT elevations might develop in hospitalized patients dosed with acetaminophen at a higher incidence sooner than or at a greater magnitude than in healthy volunteers is unknown. Theoretically, risk factors for acetaminophen-induced injury are more common among hospitalized patients, supporting the hypothesis that the incidence of therapeutic misadventure may be significantly higher in this group than in the general population. A specific example of this enhanced risk includes nil per os status, resulting in glutathione depletion.44,45 Although evidence in the literature suggests that necrosis rather than apoptosis may be the predominant mechanism of cell death in acetaminophen-induced liver injury in general,46 we speculate that this may be even more pronounced in a hospitalized patient population. In support of this speculation, there is some evidence from animal models suggesting that adenosine triphosphate depletion associated with a fasting state may predominantly result in necrosis rather than apoptosis in cells undergoing N-acetyl-p-benzoquinone imine—mediated injury, triggering innate immune system activation and resulting in more serious liver injury.47 These considerations comprise the underpinnings of our contention that hospitalized patients are at increased risk for development of acetaminophen-induced hepatotoxicity compared with the general population.
In our study, we found that only 3.1% of those patients administered doses of acetaminophen in excess of 4 g on at least 1 day had an ALT level measurement performed within 14 days of this exposure. Therefore, we are unable to quantify the incidence of ALT level elevations in our study population, let alone establish a causal relationship between acetaminophen exposure and any such biochemical abnormalities or determine the long-term clinical significance of this phenomenon. Because previous studies have documented ALT level elevations in healthy volunteers generally only after 7 to 10 days of acetaminophen exposure, it should not be surprising that we did not witness this phenomenon in our study population with an average length of stay of approximately 6 days, even if ALT level monitoring had been performed more frequently. Nonetheless, our findings demonstrate that there exists a sizeable population of patients who may be vulnerable to acetaminophen hepatotoxicity and in whom dosing beyond the recommended maximum occurs.
Our data show that patients administered a larger number of acetaminophen-containing medication formulations were more likely to be receiving cumulative doses exceeding the recommended maximum of 4 g daily. This finding calls into question the use of medications combining acetaminophen with other medications in the inpatient setting. There are compelling arguments in favor of the use of these products in the outpatient setting when patients are responsible for the administration of their own medications. Theoretically, the use of acetaminophen-narcotic combinations compared with narcotics alone might result in lower cumulative doses of the narcotic used and, perhaps, thereby lower rates of narcotic-induced adverse effects. Also, use of these combination products might result in decreased concomitant use of nonsteroidal anti-inflammatory medications, thereby reducing the associated risks of gastrointestinal bleeding and nephrotoxicity. However, in an inpatient population, ordering physicians control the administration of these medications; therefore, the benefit to ordering combination formulations of acetaminophen and narcotics, as opposed to ordering the component medications separately, is purely a matter of convenience. Our data suggest that the incidence of unintentional excessive cumulative dosing of acetaminophen may offset this concern, favoring more limited use of these combination formulations in the inpatient setting.
In conclusion, our data demonstrate that, although the great majority ofpatients receive acetaminophen in safe doses, patient safety could be even further improved with additional safeguards to prevent excessive dosing. One such safeguard is the addition of automated warnings in electronic order entry systems to alert ordering physicians if new orders for acetaminophen-containing medications could result in exceeding the recommended maximum daily cumulative dose. Perhaps more importantly, we suggest that hospitalized patients may represent an especially vulnerable population for acetaminophen-induced hepatotoxicity, and our data suggest that further prospective study involving longer-term biochemical monitoring after discharge of such patients will yield further insight regarding the threshold at which acetaminophen-induced hepatotoxicity can occur.
Footnotes
Dr Civan serves as the guarantor of the submission and takes responsibility for the submission as a whole from inception to completion and contributed to all aspects of the research. Dr Navarro contributed to the design of the study and to the writing of the paper. Dr Herrine contributed to the design of the study. Dr Riggio and Dr Adams contributed to the collection and analysis of the data. Dr Rossi contributed to the overall study hypothesis, aims, and design in addition to contributing to the writing of the paper.
The authors have no relevant conflicts of interest to disclose.
References
- 1. [September 13, 2013]. http://www.fda.gov/Drugs/DrugSafety/ucm239821.htm FDA drug safety communication: prescription acetaminophen products to be limited to 325 mg per dosage unit; boxed warning will highlight potential for severe liver failure. FDA.
- 2. [September 13, 2013]. http://www.fda.gov/downloads/AdvisoryCommit-tees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvi-soryCommittee/UCM174697.pdf Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) joint meeting of the Drug Safety and Risk Management Advisory Committee, Nonprescription Drugs Advisory Committee and the Anesthetic and Life Support Drugs Advisory Committee meeting to address the public health problem of liver injury related to the use of acetaminophen in both over-the-counter and prescription products. FDA.
- 3.Amar PJ, Schiff ER. Acetaminophen safety and hepatotoxicity—where do we go from here? Expert Opin Drug Saf. 2007;6(4):34l–355. doi: 10.1517/14740338.6.4.341. [DOI] [PubMed] [Google Scholar]
- 4.Schilling A, Corey R, Leonard M, Eghtesad B. Acetaminophen: old drug, new warnings. Cleve Clin J Med. 2010;77(1):19–27. doi: 10.3949/ccjm.77a.09084. [DOI] [PubMed] [Google Scholar]
- 5.Hawton K, Simkin S, Deeks J, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ. 2004;329(7474):1076. doi: 10.1136/bmj.38253.572581.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26(2):283–290. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 7.Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 8.Winnike JH, Li Z, Wright FA, Macdonald JM, O’Connell TM, Watkins PB. Use of pharmaco-metabonomics for early prediction of acetaminophen-induced hepatotoxicity in humans. Clin Pharmacol Ther. 2010;88(1):45–51. doi: 10.1038/clpt.2009.240. [DOI] [PubMed] [Google Scholar]
- 9.Hvidtfeldt UA, Rasmussen S, Gronbaek M, Becker U, Tolstrup JS. Influence of smoking and alcohol consumption on admissions and duration of hospitalization. Eur J Public Health. 2010;20(4):376–382. doi: 10.1093/eurpub/ckp153. [DOI] [PubMed] [Google Scholar]
- 10.Smothers BA, Yahr HT, Sinclair MD. Prevalence of current DSM-IV alcohol use disorders in short-stay, general hospital admissions, United States, 1994. Arch Intern Med. 2003;163(6):713–719. doi: 10.1001/archinte.163.6.713. [DOI] [PubMed] [Google Scholar]
- 11.Williams EC, Palfai T, Cheng DM, et al. Physical health and drinking among medical inpatients with unhealthy alcohol use: a prospective study. Alcohol Clin Exp Res. 2010;34(7):1257–1265. doi: 10.1111/j.1530-0277.2010.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyle UG, Pirlich M, Schuetz T, Luebke HJ, Lochs H, Pichard C. Prevalence of malnutrition in 1760 patients at hospital admission: a controlled population study of body composition. Clin Nutr. 2003;22(5):473–481. doi: 10.1016/s0261-5614(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 13.Leistra E, Willeboordse F, van Bokhorst-de van der Schueren MA, et al. Predictors for achieving protein and energy requirements in undernourished hospital patients. Clin Nutr. 2011;30(4):484–489. doi: 10.1016/j.clnu.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Naber TH, Schermer T, de Bree A, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997;66(5):1232–1239. doi: 10.1093/ajcn/66.5.1232. [DOI] [PubMed] [Google Scholar]
- 15.Pirlich M, Schutz T, Norman K, et al. The German hospital malnutrition study. Clin Nutr. 2006;25(4):563–572. doi: 10.1016/j.clnu.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Soguel L, Revelly JP, Schaller MD, Longchamp C, Berger MM. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: the intensive care unit dietitian can make the difference. Crit Care Med. 2012;40(2):412–419. doi: 10.1097/CCM.0b013e31822f0ad7. [DOI] [PubMed] [Google Scholar]
- 17.Licht H, Seeff LB, Zimmerman HJ. Apparent potentiation of acetaminophen hepatotoxicity by alcohol. Ann Intern Med. 1980;92(4):511. doi: 10.7326/0003-4819-92-4-511_1. [DOI] [PubMed] [Google Scholar]
- 18.Pirotte JH. Apparent potentiation by phenobarbital of hepatotoxicity from small doses of acetaminophen. Ann Intern Med. 1984;101(3):403. doi: 10.7326/0003-4819-101-3-403_1. [DOI] [PubMed] [Google Scholar]
- 19.Seeff LB, Cuccherini BA, Zimmerman HJ, Adler E, Benjamin SB. Acetaminophen hepatotoxicity in alcoholics a therapeutic misadventure. Ann Intern Med. 1986;104(3):399–404. doi: 10.7326/0003-4819-104-3-399. [DOI] [PubMed] [Google Scholar]
- 20.Florén CH, Thesleff P, Nilsson A. Severe liver damage caused by therapeutic doses of acetaminophen. Acta Med Scand. 1987;222(3):285–288. doi: 10.1111/j.0954-6820.1987.tb10672.x. [DOI] [PubMed] [Google Scholar]
- 21.Wootton FTM, Lee WMM. Acetaminophen hepatotoxicity in the alcoholic. South Med J. 1990;83(9):1047–1049. doi: 10.1097/00007611-199009000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson LS, Broomé U, Kalin M, Lindholm M. Hepatotoxicity due to repeated intake of low doses of paracetamol. J Intern Med. 1992;231(5):567–570. doi: 10.1111/j.1365-2796.1992.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 23.Bonkovsky HL, Kane RE, Jones DP, Galinsky RE, Banner B. Acute hepatic and renal toxicity from low doses of acetaminophen in the absence of alcohol abuse or malnutrition: evidence for increased susceptibility to drug toxicity due to cardiopulmonary and renal insufficiency. Hepatology. 1994;19(5):1141–1148. [PubMed] [Google Scholar]
- 24.Kwan D, Bartle W, Walker S. Abnormal serum transaminases following therapeutic doses of acetaminophen in the absence of known risk factors. Dig Dis Sci. 1995;40(9):1951–1955. doi: 10.1007/BF02208663. [DOI] [PubMed] [Google Scholar]
- 25.Andrade RJ, Lucena MI, García-Escano MD, Camargo R. Severe idiosyncratic acute hepatic injury caused by paracetamol. J Hepatol. 1998;28(6):1078. doi: 10.1016/s0168-8278(98)80361-9. [DOI] [PubMed] [Google Scholar]
- 26.Haviv YS. Low-dose paracetamol probably associated with hepatic injury in a patient with chronic renal failure. Clin Drug Invest. 2000;19(5):389–391. [Google Scholar]
- 27.Fabris P, Dalla Palma M, de Lalla F. Idiosyncratic acute hepatitis caused by paracetamol in two patients with melanoma treated with high-dose interferon-alpha. Ann Intern Med. 2001;134(4):345. doi: 10.7326/0003-4819-134-4-200102200-00028. [DOI] [PubMed] [Google Scholar]
- 28.Kurtovic J, Riordan SM. Paracetamol-induced hepatotoxicity at recommended dosage. J Intern Med. 2003;253(2):240–243. doi: 10.1046/j.1365-2796.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- 29.Parikh S, Dillon LC, Scharf SL. Hepatotoxicity possibly due to paracetamol with carbamazepine. J Intern Med. 2004;34(7):441–442. doi: 10.1111/j.1444-0903.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 30.Moling O, Cairon E, Rimenti G, Rizza F, Pristera R, Mian P. Severe hepatotoxicity after therapeutic doses of acetaminophen. Clin Ther. 2006;28(5):755–760. doi: 10.1016/j.clinthera.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Watelet J, Laurent V, Bressenot A, Bronowicki JP, Larrey D, Peyrin-Biroulet L. Toxicity of chronic paracetamol ingestion. Aliment Pharmacol Ther. 2007;26(11-12):1543–1544. doi: 10.1111/j.1365-2036.2007.03503.x. author reply 1545-1546. [DOI] [PubMed] [Google Scholar]
- 32.Grieco A, Miele L, Forgione A, Ragazzoni E, Vecchio FM, Gasbarrini G. Mild hepatitis at recommended doses of acetaminophen in patients with evidence of constitutionally enhanced cytochrome P450 system activity. J Clin Pharm Ther. 2008;33(3):315–320. doi: 10.1111/j.1365-2710.2008.00918.x. [DOI] [PubMed] [Google Scholar]
- 33.Pearce B, Grant IS. Acute liver failure following therapeutic paracetamol administration in patients with muscular dystrophies. Anaesthesia. 2008;63(1):89–91. doi: 10.1111/j.1365-2044.2007.05340.x. [DOI] [PubMed] [Google Scholar]
- 34.Forget P, Wittebole X, Laterre PF. Therapeutic dose of acetaminophen may induce fulminant hepatitis in the presence of risk factors: a report of two cases. Br J Anaesth. 2009;103(6):899–900. doi: 10.1093/bja/aep322. [DOI] [PubMed] [Google Scholar]
- 35.Heard KJ, Green JL, James LP, et al. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20. doi: 10.1186/1471-230X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) administration in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26(2):283–290. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 37.Kuffner EK, Green JL, Bogdan GM, et al. The effect of acetaminophen (four grams a day for three consecutive days) on hepatic tests in alcoholic patients—a multicenter randomized study. BMC Med. 2007;5:13. doi: 10.1186/1741-7015-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction? Am J Ther. 2000;7(2):99–H4. doi: 10.1097/00045391-200007020-00007. [DOI] [PubMed] [Google Scholar]
- 39.Prescott LF. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 2000;49(4):291–301. doi: 10.1046/j.1365-2125.2000.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dart RC, Bailey E. Does therapeutic use of acetaminophen cause acute liver failure? Pharmacotherapy. 2007;27(9):1219–1230. doi: 10.1592/phco.27.9.1219. [DOI] [PubMed] [Google Scholar]
- 41.Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. Am J Ther. 2000;7(2):123–134. doi: 10.1097/00045391-200007020-00009. [DOI] [PubMed] [Google Scholar]
- 42.Jalan R, Williams R, Bernuau J. Paracetamol: are therapeutic doses entirely safe? Lancet. 2006;368(9554):2195–2196. doi: 10.1016/S0140-6736(06)69874-7. [DOI] [PubMed] [Google Scholar]
- 43.Vitols S. Paracetamol hepatotoxicity at therapeutic doses. . J Intern Med. 2003;253(2):95–98. doi: 10.1046/j.1365-2796.2003.01107.x. [DOI] [PubMed] [Google Scholar]
- 44.Lucas D, Menez C, Girre C, Bodenez P, Hispard E, Menez JF. Decrease in cytochrome P4502E1 as assessed by the rate of chlorzoxazone hydroxylation in alcoholics during the withdrawal phase. Alcohol Clin Exp Res. 1995;19(2):362–366. doi: 10.1111/j.1530-0277.1995.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 45.Lauterburg BH, Velez ME. Glutathione deficiency in alcoholics: risk factor for paracetamol hepatotoxicity. Gut. 1988;29(9):1153–1157. doi: 10.1136/gut.29.9.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44(1):88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16(11-12):479–490. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]