Abstract
Type 2 diabetes has been shown to occur in response to environmental and genetic influences, among them nutrition, food intake patterns, sedentary lifestyle, body mass index (BMI), and exposure to persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs). Nutrition is essential in the prevention and management of type 2 diabetes and has been shown to modulate the toxicity of PCBs. Serum carotenoid concentrations, considered a reliable biomarker of fruit and vegetable intake, are associated with the reduced probability of chronic diseases, such as type 2 diabetes and cardiovascular disease. Our hypothesis is that fruit and vegetable intake, reflected by serum carotenoid concentrations, is associated with the reduced probability of developing type 2 diabetes in US adults with elevated serum concentrations of PCBs 118, 126, and 153. This cross-sectional study utilized the CDC database, National Health and Nutrition Examination Survey (NHANES) 2003–2004 in logistic regression analyses. Overall prevalence of type 2 diabetes was approximately 11.6% depending on the specific PCB. All three PCBs were positively associated with the probability of type 2 diabetes. For participants at higher PCB percentiles (e.g., 75th and 90th) for PCB 118 and 126, increasing serum carotenoid concentrations were associated with a smaller probability of type 2 diabetes. Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicted notably reduced probability of dioxin-like PCB-associated risk for type 2 diabetes.
Keywords: nutrition, polychlorinated biphenyls, PCBs, serum carotenoids, NHANES, type 2 diabetes, environmental health
1. Introduction
Recent decades have seen increased rates of type 2 diabetes, and it is now estimated to affect 25.8 million Americans and 346 million people worldwide [1,2]. Type 2 diabetes has been shown to occur in response to environmental and genetic influences [3–9], among them food intake patterns, sedentary lifestyle, body mass index (BMI) and exposure to persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs) [10–15]. Though not produced in the U.S. since 1977, PCBs persist in the environment and concentrate in adipose tissue of organisms. They remain detectable in soil, air, water, and sediment, where they enter the food chain [16]. The primary route of PCB exposure today is through dietary intake of contaminated foods and through inhalation of airborne pollutants [17]. Increasing evidence from animal [10,11,12,18,19] and epidemiological research suggests that background exposure to PCBs is associated with type 2 diabetes, including studies examining National Health and Nutrition Examination Survey (NHANES) data [13], low dose PCB exposure [14,15], five-year prospective data from an elderly population in Sweden [20], the PCB-exposed population of Anniston, AL [21], a review of epidemiological studies from a National Toxicology Program Workshop [22], and prospective data from the Nurses’ Health Study as part of a meta-analysis [23].
Nutrition is essential in the prevention and management of type 2 diabetes [6–9] and has been shown to modulate the toxicity of PCBs [19,24–29]. Consistent intake of fruits and vegetables has been associated with a healthy weight, positive antioxidant status, and a reduced risk of chronic diseases. Dietary antioxidants, such as vitamin C and the carotenoids, when consumed at adequate levels, can provide a balanced defense against the harmful effects of reactive oxygen species (ROS) [30]. Serum carotenoids, a family of lipophilic plant pigments with potent antioxidant activity, are considered a reliable biomarker of fruit and vegetable intake [31]. Serum responses to carotenoids have been reported to depend on a variety of factors, including the amount consumed, food matrix, and half-life variability of individual carotenoids [32]. The predominant carotenoids in human sera are α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin [33]. Observational studies of carotenoids reveal inverse associations with type 2 diabetes [34], CVD [35,36], and all-cause mortality [37,38], although individual carotenoid effects have been inconsistent.
In the present study, we tested the hypothesis that fruit and vegetable intake, reflected by serum carotenoid concentrations, is associated with the reduced probability of developing type 2 diabetes in U.S. adults with elevated serum concentrations of PCBs 118, 126, and 153. The objective was to use the CDC database, National Health and Nutrition Examination Survey (NHANES) 2003–2004 to establish whether serum carotenoid concentrations are associated with a reduced risk of developing type 2 diabetes in adult participants with elevated serum concentrations of PCB118, PCB126, and PCB 153.
2. Methods and materials
2.1. Procedure and study population
NHANES is a series of nationally representative, cross-sectional surveys of the civilian, non-institutionalized U.S. population. NHANES 2003–2004 survey procedures, laboratory assays, and ethics review board approval are published in detail [39]. Approval for our analysis of the NHANES data was granted by the University of Kentucky Institutional Review Board.
In NHANES 2003–2004, a random, ½ subsample was selected for fasting plasma glucose measurements, and a separate random, but overlapping, 1/3 subsample was selected for serum PCB measurements. Measurement of serum carotenoids was a fixed component of the mobile examination center (MEC) examination without dedicated subsample status.
For our study, we used the following inclusion criteria for a larger subpopulation (n = 5,041, Table 1): adults: ≥ 20 years of age examined at the MEC, having measurements for serum carotenoid concentrations, and either a fasting glycosylated hemoglobin value or history of type 2 diabetes diagnosis. A prototype individual represents this group. Mean values were drawn from this larger subpopulation. The prototype was a fifty year old non-Hispanic, white male, at the 50th percentile of PIR (2.23), the 50th percentile of body mass index (BMI) (27), and the 50th percentile serum carotenoid concentrations (1.7 μmol/L). Approximately 1200 of these subjects had at least one PCB concentrations measured.
Table 1.
Characteristics of study participants from National Health and Nutrition Examination Survey (NHANES), 2003–2004, Age ≥ 20 years of age (n = 5041).
| Characteristic | Valuea |
|---|---|
| Male (%) | 2418 (47.97) |
| Age (y), mean | 50.85 (± 9.70) |
| Race/ethnicity (%) | |
| Non-Hispanic white | 2689 (53.34) |
| Non-Hispanic black | 994 (19.72) |
| Mexican American | 985 (19.54) |
| Other race/ethnicity | 373 (7.40) |
| PIRb, mean | 2.57 (± 1.59) |
| BMI (kg/m2) (%) | |
| <18.5 | 170 (1.39) |
| 18.5–24.9 | 1408 (27.93) |
| 25.0–29.9 | 1631 (32.35) |
| 30.0–39.9 | 1299 (25.77) |
| ≥40.0 | 239 (4.74) |
| Total carotenoids (umol/L), mean | 1.83 (± 0.87) |
| Use dietary supplements (%) | 2658 (52.80) |
| Current cigarette smoker (%) | 1131 (22.48) |
| Serum cotinine (%) | |
| ≥0.015 ng/mL | 3632 (81.14) |
| <0.015 ng/mL | 844 (18.86) |
| Physical activity (%) | |
| Sedentary | 1379 (47.97) |
| Low activity | 498 (17.32) |
| Moderate to vigorously active | 995 (34.61) |
| Alcohol consumption (%) | |
| Non-drinker | 980 (19.44) |
| Non-excessive drinker | 1664 (33.01) |
| Excessive drinker | 1032 (20.47) |
Values shown are mean ± SD or n (%).
Abbreviations: PIR: poverty income ratio, BMI: body mass index
The BMI of the prototype individual was varied to help understand the changing relationship between the variables in our models. The National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) defines BMI, as weight (kg) divided by height squared (m2) and its use, as a measure of body fat based on height and weight in adults [40]. The Center for Disease Control and Prevention (CDC) interprets BMI using standard weight status categories: : <18.5 = underweight; 18.5–24.9 = normal weight; 25.0–29.9 = overweight; 30.0–39.9 = obese; ≥ 40.0 = extremely obese [43].
Socio-demographic covariates included gender, age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and other ethnicities), PIR, and BMI. PIR was determined by the ratio of total family income to poverty, as determined by the U.S. Department of Health and Human Services annual poverty guidelines. Women and non-Hispanic whites comprised over 50% of the sample (Table 1). Approximately 22% of participants reported being current cigarette smokers although 79–81% had serum cotinine levels ≥ 0.015 ng/mL, considered a positive indicator of passive or active smoking, thus we did not include smoking in our models.
2.2. Exposure variables
2.2.1 Serum carotenoids
Individual carotenoids were pooled and assessed as total carotenoids. The carotenoids of interest in this study were α-carotene, β-carotene, α-cryptoxanthin, β-cryptoxanthin, lycopene, lutein, and zeaxanthin. Lutein and zeaxanthin measures were combined within the same peak and consequently analyzed together in the NHANES analysis. Carotenoids were measured by absorbance at 450 nm using high performance liquid chromatography (HPLC) with multi-wavelength photodiode-array absorbance detection.
2.2.2. PCBs
PCBs were measured in serum samples and were lipid-standardized, representing the quotient of PCB concentration and total serum lipid content (mg/dL). This has been considered a better reflection of body burden in epidemiological studies. Three PCBs were selected for analysis representing one from each subclass: PCB118 mono-ortho-substituted, PCB126 non-ortho-substituted (coplanar or dioxin-like), and PCB153 di-ortho-substituted (non-coplanar or non-dioxin-like). To avoid bias at lower concentrations, PCBs were selected with ≥60% of participants over the limit of detection (LOD) (see Online Supplementary Materials, Table S1). PCB118 and PCB153 had zero observations below the LOD. PCBs were measured in 5–10 ml serum by high-resolution gas chromatography/isotope-dilution high-resolution mass spectrometry.
2.3 Response variable
2.3.1 Type 2 Diabetes
Participants were evaluated for type 2 diabetes from the examination and questionnaire data files of NHANES. A positive finding of type 2 diabetes was found if one of two criteria was met: (1) a participant’s glycosylated hemoglobin result was ≥ 6.5%; or (2) they answered “yes” to the question, “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes”. Annually, the American Diabetes Association publishes the most current clinical guidelines for the diagnosis of diabetes, which include a glycosylated hemoglobin (A1C) ≥ 6.5% [42].
2.4. Statistical analyses
All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC). In the individual analysis, participants were categorized linearly by quantile for each PCB congener. In this manner, the slope of each PCB quantile represented the probability of chronic disease per total serum carotenoid concentration. Quantiles were categorized at 25th, 50th, 75th, and 90th percentiles; however, all analyses were set at the lower LOD of 60%, and were variable by compound and extractable volume. Variables in Table 1 were considered for inclusion in our models. If a variable was significant in one PCB model, it was included in all three models. Interactions as well as quadratic terms found to be significant (p < 0.05) were added to the models.
Logistic regression models included covariates for age, race/ethnicity, PIR, BMI, and gender, rather than NHANES subsample weights. This method of covariate modeling has been regarded as a good alternative in subpopulation methodology and a suitable compromise between efficiency and bias [43,44]. A p-value of < 0.05 was used to indicate statistical significance.
Survey logistic regression models were also run using the NHANES subsample weights Online Supplementary Materials, Tables S2,S3,S4). The standard errors were calculated using Taylor series approximations, which rely upon all of the cases in the data file to arrive at appropriate estimates. Therefore, in order to use the full sample, the subpopulation options were activated with the survey logistic commands. The variable that identifies the subpopulation is coded 0 for cases that are excluded and 1 for cases that are included.
3. Results
Age was directly associated with the probability of type 2 diabetes in all analyses (p<0.01). BMI was associated with an increased probability of disease with congener-specific effects observed for the obese in diabetes modeling.
Prevalence of type 2 diabetes was specific for each PCB: PCB 118 was 11.6%, PCB 126 was 11.5% and PCB 153 was 11.6%. All three PCBs were significantly associated with an increased probability of type 2 diabetes (p<0.01) (Tables 2,3,4). The mean (± SD) serum carotenoid concentration was 1.83 ± 0.87 μmol/L. Statistically significant interactions were identified between PCB 118 and serum carotenoids and PCB 126 and serum carotenoids (p<0.05).
Table 2.
Logistic model for adult NHANES 2003–2004 participants with serum PCB118 concentrations.
| Type 2 Diabetes Mellitus (n = 1195) | ||
|---|---|---|
| Parameter | Estimateb | P-value |
| Male | 0.2573 | 0.0127 |
| Age | 0.0360 | <0.0001 |
| Race/Ethnicity | ||
| Mexican American | 1.1257 | 0.0829 |
| Other Hispanic | 0.7815 | 0.3398 |
| Non-Hispanic White | 0.3419 | 0.5862 |
| Non-Hispanic Black | 0.5503 | 0.4049 |
| PIRa | −0.0375 | 0.5780 |
| PCB118 | 0.1222 | 0.0008 |
| PCB118*118 | −0.00257 | 0.0012 |
| BMI | 0.0421 | 0.0127 |
| Serum Carotenoids | 0.0677 | 0.7338 |
| PCB118*Serum Carotenoids | −0.0306 | 0.0161 |
| PCB118*PCB118*Serum Carotenoids | 0.000538 | 0.0023 |
| PCB118*PCB118*BMI | 0.000024 | 0.0118 |
Abbreviations: PIR: poverty income ratio, BMI: body mass index
Logistic regression coefficient estimates including covariates for age, race/ethnicity, PIR, BMI, and gender
Table 3.
Logistic model for adult NHANES 2003–2004 participants with serum PCB126 concentrations.
| Type 2 Diabetes Mellitus (n = 1188) | ||
|---|---|---|
| Parameter | Estimate | P-value |
| Male | 0.5922 | 0.0041 |
| Age | 0.0322 | <0.0001 |
| Race/Ethnicity | ||
| Mexican American | 1.2236 | 0.0617 |
| Other Hispanic | 1.0374 | 0.2087 |
| Non-Hispanic White | 0.6019 | 0.3465 |
| Non-Hispanic Black | 0.7885 | 0.2391 |
| PIRa | −0.0405 | 0.5486 |
| PCB126 | 0.0664 | 0.0011 |
| PCB126*126 | −0.00045 | 0.0013 |
| BMI | 0.0496 | 0.0022 |
| Serum Carotenoids | 0.0695 | 0.7807 |
| PCB126*Serum Carotenoids | −0.0155 | 0.0746 |
| PCB126*PCB126*Serum Carotenoids | 0.000139 | 0.0124 |
Abbreviations: PIR: poverty income ratio, BMI: body mass index
Logistic regression coefficient estimates including covariates for age, race/ethnicity, PIR, BMI, and gender
Table 4.
Logistic model for adult NHANES 2003–2004 participants with serum PCB153 concentrations
| Type 2 Diabetes Mellitus (n = 1204) | ||
|---|---|---|
| Parameter | Estimateb | P-value |
| Male | 0.3542 | 0.0708 |
| Age | 0.0348 | <0.0001 |
| Race/Ethnicity | ||
| Mexican American | 1.1303 | 0.0809 |
| Other Hispanic | 0.6129 | 0.4542 |
| Non-Hispanic White | 0.2691 | 0.6673 |
| Non-Hispanic Black | 0.3593 | 0.5876 |
| PIRa | −0.0301 | 0.6496 |
| PCB153 | 0.0158 | 0.0051 |
| PCB153*153 | −0.00005 | 0.0266 |
| BMI | 0.0647 | <0.0001 |
| Serum Carotenoids | −0.1597 | 0.1815 |
Abbreviations: PIR: poverty income ratio, BMI: body mass index
Logistic regression coefficient estimates including covariates for age, race/ethnicity, PIR, BMI, and gender
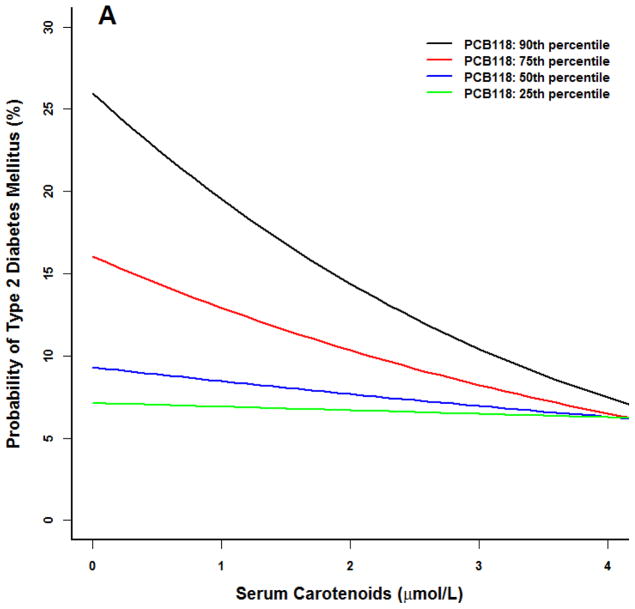
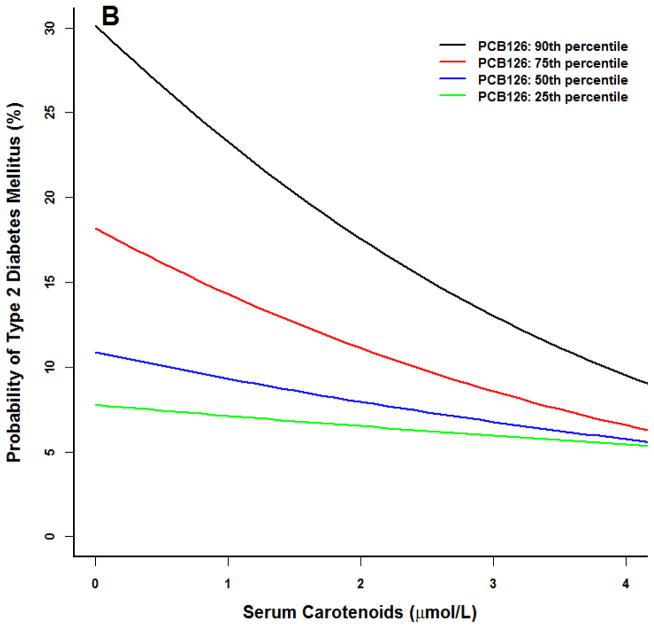
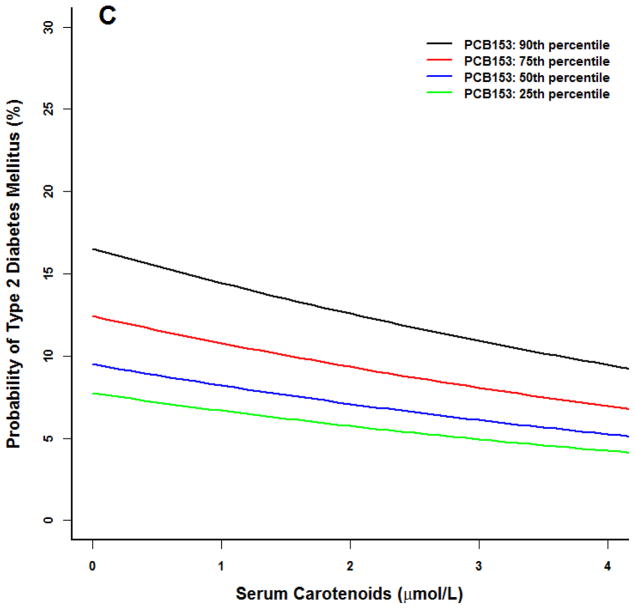
For models including PCB118, there was a lack of observable effect at any serum carotenoid concentration at the 25th PCB percentile (Figure 1) for the prototype individual at the 50th percentile BMI (27). A similar lack of effect was noted in the 25th first percentile of PCB126. Conversely, at higher concentrations of these two PCBs, the probability of type 2 diabetes declined with increasing serum carotenoid concentrations to levels similar to that observed at the 25th and 50th PCB percentiles.
Figure 1.
Logistic model for adult NHANES 2003–2004 participants for the prototype individual: 50 year old white male at the 50th percentile poverty income ratio (2.23), 50th percentile body mass index (BMI 27), 50th percentile serum carotenoid concentrations (1.7 μmol/L). All three PCBs were positively associated with an increased probability of type 2 diabetes (p<0.01). (A) PCB118. (B) PCB126. (C) PCB 153. At higher PCB percentiles for PCB 118 and 126, increasing serum carotenoid concentrations were associated with a smaller probability of type 2 diabetes (p<0.05). No serum carotenoid benefit and no statistical interaction effects were observed for PCB 153.
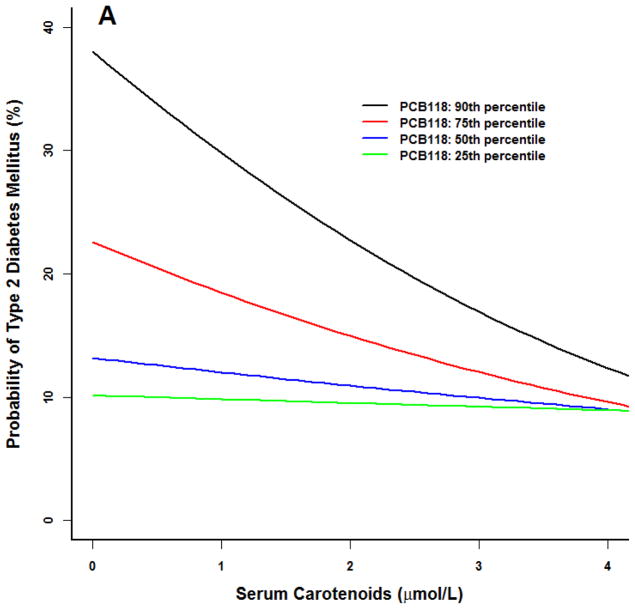
Similar trends were noted for our prototype individual at the 90th percentile of BMI (Figure 2). For PCBs 118 and 126, diabetes probability was higher at the 75th and 90th percentiles. As serum carotenoid concentrations increased, diabetes risk diminished to levels similar to participants in the 25th and 50th PCB percentiles.
Figure 2.
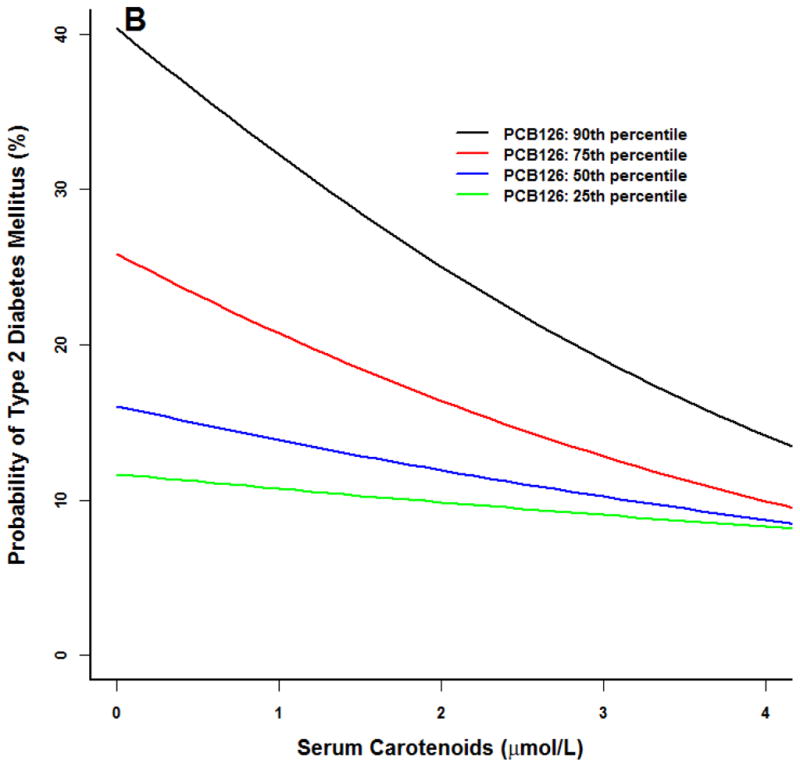
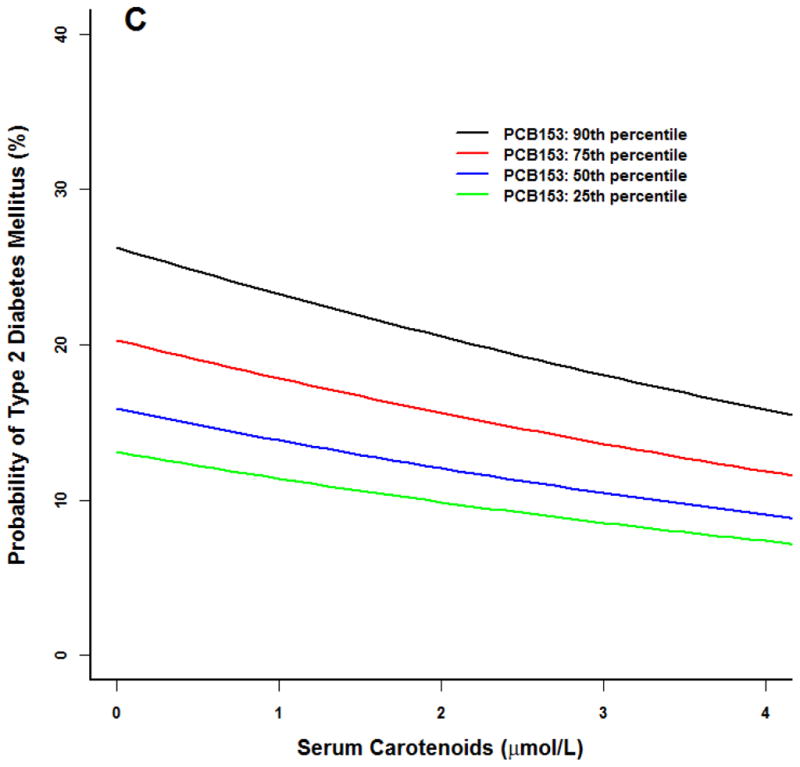
Logistic model for adult NHANES 2003–2004 participants for the prototype individual: 50 year old white male at the 50th percentile poverty income ratio (2.23), 90th percentile body mass index (BMI 37), 50th percentile serum carotenoid concentrations (1.7 umol/L). All three PCBs were associated with an increased probability of type 2 diabetes (p<0.01). (A) PCB118. (B) PCB126. (C) PCB 153. Similar results at higher PCB percentiles for PCB 118 and 126, increasing serum carotenoid concentrations were associated with a smaller probability of type 2 diabetes (p<0.05). No serum carotenoid effects were observed for PCB 153.
As an alternative analysis, subsample weights for subjects with PCB measurements and then NHANES subsample weights were used in survey logistic regression models (Online Supplementary Materials, Tables S2,S3,S4). These models found similar results.
While PCB153 was associated with an increased probability of T2DM, serum carotenoids did not correlate with any variable in this model nor were statistical interaction effects observed.
4. Discussion
These data support our hypothesis that increased serum carotenoids exhibited a protective effect in the probability of developing type 2 diabetes for this adult population with elevated serum concentrations of PCBs 118 and 126. PCB153, also considered relatively resistant to metabolism [45], however, showed no significant benefit of serum carotenoids at any concentration. To our knowledge, this is the first study to investigate associations among serum carotenoids, a nutrition biomarker indicative of fruit and vegetable intake; serum concentrations of PCBs; and the probability of developing type 2 diabetes in a representative sample of U.S. adults.
In our analysis, increased serum carotenoids provided benefits in reducing the risk of type 2 diabetes for models at higher serum concentrations of PCBs 118 and 126 at both BMI percentiles. For those at the 90th percentile BMI (BMI 37) for both PCB 118 and 126, our models showed a greater decrease in probability of type 2 diabetes than the decrease observed at the 50th percentile (BMI 27). It is notable that the purpose of BMI is to assign morbidity and mortality risk status to individuals based on their weight (kg) to height (m2) ratio [46].
Carotenoid absorption and utilization is complex and known to be influenced by many factors, one of which is cytochrome P450 (CYP) induction. Carotenoids and their derivatives influence the activity of several nuclear families [47,48], and through gene regulation [49] exhibit modulating effects in diabetes [50]. Cross-sectional studies have reported an inverse association between serum carotenoids and the mean blood glucose status of participants with normal glucose tolerance and those with type 2 diabetes [36] and between total serum carotenoids and T2DM in nonsmokers [51]. Studies have also found a fairly consistent inverse correlation between BMI and carotenoid status [52,53]. In general, effects of individual carotenoids have been inconsistent, although nutrients seldom appear in the diet in isolation.
In supplement form, a pro-oxidant effect has been found and is not recommended [54,55]. The two studies cited most often for these effects, randomized controlled trials which supplemented the diet of smokers with β-carotene and either retinol (Beta-Carotene and Retinol Efficacy Trial (CARET)) [54] or α-tocopherol (Alpha-Tocopherol, Beta-Carotene Cohort (ATBC) Study) [55], were terminated early due to increased incidence of disease and deaths. Follow-up dietary analysis, however, revealed significantly lower disease risk in both studies, with reported high weekly fruit and vegetable intake and/or elevated dietary lycopene, lutein/zeaxanthin, beta-cryptoxanthin, and total carotenoids [56,57].
Of all the carotenoids, lycopene is the most effective antioxidant (58). Studies have shown protection of lycopene against PCB-induced reproductive dysfunction [59] and in glucose homeostasis. Lycopene supplementation when administered to rats concurrent with Aroclor 1254, a highly toxic mixture of 54% PCBs, prevented decreased expression of the glucose transporter protein, GLUT4, which normalized glucose uptake in skeletal muscle [60].
No one etiologic mechanism or pattern has been able to explain the association of PCBs with diabetes. Various studies have examined their capacity to disrupt normal hormone regulation with the pathogenesis of obesity [10–12,18, 61], insulin resistance [12,13,14], and nonalcoholic fatty liver disease (NAFLD) [62]. A synergistic interaction was observed between PCB153 and high-fat diet, increasing obesity and NAFLD, beyond any effect seen with high-fat diet alone. PCB153 with control diet had no effect on these parameters, suggesting the influence of PCBs on obesity and NAFLD may depend more on nutritional interactions with PCB congener than on either factor alone [62]. Other factors have been shown to complicate studies; obesity alone is a risk factor for type 2 diabetes and increased body fat may suggest increased storage of and, therefore, exposure to PCBs. While cross-sectional surveys have established associations between POPs and diabetes [14], insulin resistance [15], and NAFLD [63], the influence of diet was not addressed in these studies.
While increasing evidence suggests that environmental pollutants increase susceptibility to type 2 diabetes, little research overall has been conducted on the effects of nutrition in mitigating this risk. PCBs and isoforms of β-carotene derived from foods and supplements were significant for type 2 diabetes among 543 environmental factor and genetic markers [3]. Endothelial cells treated with quercetin [64] or green tea catechins [65] were protected against coplanar PCB-induced inflammation. In vitro and in vivo supplementation of resveratrol diminished PCB77-induced oxidative stress in cells and adipose tissue by enhancing antioxidant signaling pathways associated with insulin and glucose tolerance [19]. Modifying the composition of dietary fatty acids from primarily omega-6 (linoleic acid) to omega-3 (alpha-linolenic acid) inhibited pro-inflammatory signaling pathways and completely blocked PCB-induced effects when pretreated with alpha-linolenic acid [24]. These and related findings may be best confirmed in longitudinal studies, which could prove critical in determining the magnitude of nutrition in preventing disease in pollutant-exposed populations.
Environmental PCB levels have been declining in the decades since PCB production was banned in the U.S., yet rates of obesity and type 2 diabetes have increased. It has been shown that these effects may still occur at low-dose, persistent exposures [14,15]. Dietary intake is a major route for PCB exposure [17], with one market study estimating daily PCB intake from typical American foods at 33 ng per day [66]. The foods highest in PCBs tend to be those with a lipid compartment; marine, mammal, and dairy foods [17]. Regular consumption of fruits and vegetables, which are naturally low in lipid content and high in antioxidants, would reduce exposure and protect against toxic insults. Reporter gene assays have identified fruits, vegetables, and herbs that may modulate PCB transformation and toxicity through CYP enzyme induction [67,68], or as aryl hydrocarbon receptor (AhR) agonists and antagonists [69]. More research is needed to understand how various ligands activate the AhR, and how this can result in clinically relevant outcomes [29].
While nutritional supplementation of carotenoids is not advised, the benefits of whole food nutrition at normal physiological levels should be promoted. Diet has been repeatedly shown to mitigate the effects of progressive chronic diseases, especially type 2 diabetes [70, 71], on persons at elevated risk. Studies with participants consuming diets that are high in vegetables and comprised of a variety of fruits and vegetables [72], increased servings of fruits, particularly blueberries, grapes, and apples [73], and Mediterranean diets, also known to be high in fruits, vegetables, olive oil, nuts, and grains [74,75], are associated with a reduced risk of type 2 diabetes. Two studies of the Mediterranean diet supplemented with extra-virgin olive oil or nuts in a Spanish cohort showed reduced CVD risk factors in persons with type 2 diabetes [74] and reduced risk of diabetes in older individuals (55 to 80 yrs) at high CVD risk [75]. Women with history of gestational diabetes, a condition placing them at greatly increased diabetes risk subsequent to pregnancy (76), were placed on one of three healthy meal plans [77], and experienced a a 40% (alternate Mediterranean), 46% (Dietary Approaches to Stop Hypertension), or 57% (alternate Healthy Eating Index) lower risk of type 2 diabetes. Current recommendations for an 1800–2000 calorie meal plan are to consume eight to nine servings of fruits and vegetables per day for optimal health [78].
There are limitations with the current study, and results should be interpreted with caution. Due to the cross-sectional nature of the study, it was not possible to determine time or extent of the original chemical exposures. Direction of causation cannot be determined, allowing for the possibility that perturbations of metabolic disease could affect serum concentrations. Neither was it possible at this time to measure all potential confounders that could influence results. Last, other POPs, including PCBs not investigated in this study, may contribute to the compromised health of these participants.
This study has several strengths as well. NHANES 2003–2004 is representative of the U.S. population for those two years. Results are based on actual clinical parameters and nutritional examination to assess health status, whereas most research studies have examined one pollutant and one nutrient at concentrations that may not reflect true environmental risk ratios.
Nutrition is an ideal modulator of PCB toxicity. PCBs are widely known to cause oxidative stress [10–12,18,19] and exposure to them continues, primarily through dietary intake of contaminated foods and through inhalation of airborne pollutants. Chronic PCB exposure is associated with a persistent inflammatory state associated with diseases, such as type 2 diabetes [13–15,20–23]. Daily exposure to foods rich in beneficial nutrients, such as carotenoid-containing fruits and vegetables, may provide a dynamic barrier against the chemical, physical, and biological stressors that are a part of today’s life. Further studies are needed to advance these findings.
Supplementary Material
Acknowledgments
The authors would like to thank the volunteers and the research staff of the National Health and Nutrition Examination Survey (NHANES) 2003–2004. This work was supported by grant P42ES007380 from the National Institutes of Environmental Health Sciences, National Institutes of Health. NIEHS/NIH had no role in the design, analysis or writing of this article.
Abbreviations
- A1C
glycosylated hemoglobin
- AhR
Aryl hydrocarbon receptor
- ATBC
Alpha-Tocopherol, Beta-Carotene Cancer Prevention Trial
- BMI
Body mass index
- CARET
Beta-Carotene and Retinol Efficacy Trial
- CDC
Center for Disease Control and Prevention
- CVD
Cardiovascular disease
- CYP
Cytochrome P450
- HDL
High density lipoprotein
- LOD
Limit of detection
- MEC
Mobile examination center
- NAFLD
nonalcoholic fatty liver disease
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey
- PCBs
Polychlorinated biphenyls
- PIR
Poverty income ratio
- POPs
Persistent organic pollutants
- T2DM
Type 2 diabetes mellitus
Footnotes
The authors declare that there are no known conflicts of interest associated with this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. [Accessed January 3, 2014];Diabetes Programme Facts and Figures. 2011 http://www.who.int/diabetes/facts/en/
- 2.Center for Disease Control and Prevention. National Diabetes Fact Sheet. National Estimates on Diabetes; 2011. [Accessed January 3, 2014]. http://www.cdc.gov/diabetes/pubs/estimates11.htm. [Google Scholar]
- 3.Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One. 2010;5:e10746. doi: 10.1371/journal.pone.0010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel CJ, Chen R, Kodama K, Ioannidis JP, Butte AJ. Systematic identification of interaction effects between genome- and environment-wide associations in type 2 diabetes mellitus. Hum Genet. 2013;132:495–508. doi: 10.1007/s00439-012-1258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everett CJ, Frithsen I, Player M. Relationship of polychlorinated biphenyls with type 2 diabetes and hypertension. J Environ Monit. 2011;3:241–51. doi: 10.1039/c0em00400f. [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169:798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–9. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 9.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–9. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 10.Baker N, Karounos M, English V, Fang J, Wei Y, Stromberg A, et al. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121:105–10. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray SL, Shaw AC, Gagne AX, Chan HM. Chronic exposure to PCBs (Aroclor 1254) exacerbates obesity-induced insulin resistance and hyperinsulinemia in mice. J Toxicol Environ Health A. 2013;76:701–15. doi: 10.1080/15287394.2013.796503. [DOI] [PubMed] [Google Scholar]
- 12.Ruzzin J, Petersen R, Meugnier E, Madsen L, Lock EJ, Lillefosse H, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–71. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–44. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR. Low dose of some persistent organic pollutants predicts type 2 diabetes: A nested case-control study. Environ Health Perspect. 2010;118:1235–42. doi: 10.1289/ehp.0901480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, Jacobs DR. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson MD, Kaley RG. Applications of polychlorinated biphenyls. Environ Sci Pollut Res. 2011;18:135–51. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- 17.ATSDR (Agency for Toxic Substances and Disease Registry) Toxicological Profiles. Atlanta, GA: ATSDR; 2000. [Accessed March 27, 2011]. Toxicological Profile for Polychlorinated Biphenyls (PCBs) http://www.atsdr.cdc.gov/ToxProfiles/tp17.pdf. [PubMed] [Google Scholar]
- 18.Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–8. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker NA, English V, Sunkara M, Morris AJ, Pearson KJ, Cassis LA. Resveratrol protects against polychlorinated biphenyl-mediated impairment of glucose homeostasis in adipocytes. J Nutr Biochem. 2013;24:2168–74. doi: 10.1016/j.jnutbio.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DH, Lind PM, Jacobs DR, Jr, Salihovic S, van Bavel B, Lind L. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care. 2011;34:1778–84. doi: 10.2337/dc10-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Herman R, Foushee, Shelton Christie, et al. Polychlorinated Biphenyl (PCB) Exposure and Diabetes: Results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120:727–32. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–83. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the nurses’ health study and meta-analysis. Environ Health Perspect. 2013;121:153–61. doi: 10.1289/ehp.1205248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem-Biol Interact. 2008;172:27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majkova Z, Oesterling E, Toborek M, Hennig B. Impact of nutrition on PCB toxicity. Environ Toxicol Pharmacol. 2008;25:192–6. doi: 10.1016/j.etap.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Watkins BA, Hannon K, Ferruzzi M, Li Y. Dietary PUFA and flavonoids as deterrents for environmental pollutants. J Nutr Biochem. 2007;18:196–205. doi: 10.1016/j.jnutbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Ravoori S, Srinivasan C, Pereg D, Robertson LW, Ayotte P, Gupta RC. Protective effects of selenium against DNA adduct formation in Inuit environmentally exposed to PCBs. Environ Int. 2010;36:980–6. doi: 10.1016/j.envint.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, et al. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect. 2007;115:493–5. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennig B, Ormsbee L, McClain CJ, Watkins BA, Blumberg B, Bachas LG, et al. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect. 2012;120:771–4. doi: 10.1289/ehp.1104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliwell B, Murcia MA, Chirico S, Aruoma OI. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit Rev Food Sci Nutr. 1995;35:7–20. doi: 10.1080/10408399509527682. [DOI] [PubMed] [Google Scholar]
- 31.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids: a report of the Panel on Dietary Antioxidants and Related Compounds. Washington, D.C: National Academy Press; 2000. Beta-carotene and other carotenoids; pp. 325–82. [Google Scholar]
- 32.Martínez-Tomás R, Larqué E, González-Silvera D, Sánchez-Campillo M, Burgos MI, Wellner A, et al. Effect of the consumption of a fruit and vegetable soup with high in vitro carotenoid bioaccessibility on serum carotenoid concentrations and markers of oxidative stress in young men. Eur J Nutr. 2012;51:231–9. doi: 10.1007/s00394-011-0211-6. [DOI] [PubMed] [Google Scholar]
- 33.Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007;55:207–16. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, et al. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–93. doi: 10.1093/ajcn.82.3.685. [DOI] [PubMed] [Google Scholar]
- 35.Karppi J, Laukkanen JA, Makikallio TH, Ronkainen K, Kur S. Serum beta-carotene and the risk of sudden cardiac death in men: A population-based follow-up study. Atherosclerosis. 2013;226:172–7. doi: 10.1016/j.atherosclerosis.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 36.Xu XR, Zou ZY, Huang YM, Xiao X, Ma L, Lin XM. Serum carotenoids in relation to risk factors for development of atherosclerosis. Clin Biochem. 2012;45:1357–61. doi: 10.1016/j.clinbiochem.2012.07.101. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Ford ES, Zhao G, Balluz LS, Giles WH, Liu S. Serum alpha-carotene concentrations and risk of death among US adults: the Third National Health and Nutrition Examination Survey Follow-up Study. Arch Intern Med. 2011;171:507–15. doi: 10.1001/archinternmed.2010.440. [DOI] [PubMed] [Google Scholar]
- 38.Shardell MD, Alley DE, Hicks GE, El-Kamary SS, Miller RR, Semba RD, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in US adults: the Third National Health and Nutrition Examination Survey. Nutr Res. 2011;31:178–89. doi: 10.1016/j.nutres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NCHS (National Center for Health Statistics) National Health and Nutrition Examination Survey Data, 2003–2004. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed May 14, 2011]. http://www.cdc.gov/nchs/nhanes/nhanes2003-2004/nhanes03_04.htm. [Google Scholar]
- 40.NIH (National Institutes of Health), NHLBI (National Heart, Lung, and Blood Institute) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults, the evidence report. No. 98-4083. U.S. Department of Health and Human Services; 1998. [Accessed December 3, 2012]. Available at: http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf. [Google Scholar]
- 41.Center for Disease Control and Prevention, CDC. 24/7: Saving Lives. Protecting People. [Accessed December 26, 2013];Healthy Weight, Body Mass Index. http://www.cdc.gov/healthyweight/assessing/bmi/
- 42.American Diabetes Association (ADA) Executive Summary: Standards of Medical Care in Diabetes – 2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 43.Graubard BL, Korn EL. Analyzing health surveys for cancer-related objectives. J Natl Cancer Inst. 1999;91:1005–16. doi: 10.1093/jnci/91.12.1005. [DOI] [PubMed] [Google Scholar]
- 44.Korn EL, Graubard BI. Epidemiologic Studies Utilizing Surveys - Accounting for the Sampling Design. Am J Public Health. 1991;81:1166–73. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muhlebach S, Wyss PA, Bickel MH. The use of 2,4,5,2′,4′,5′-hexachlorobiphenyl (6-CB) as an unmetabolizable lipophilic model-compound. Pharmacol Toxicol. 1991;69:410–15. doi: 10.1111/j.1600-0773.1991.tb01322.x. [DOI] [PubMed] [Google Scholar]
- 46.WHO (World Health Organization) Overweight and obesity (high body mass index) In: Ezzati MLA, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks. Vol. 1. Geneva: World Health Organization; 2004. pp. 497–596. [Google Scholar]
- 47.Sharoni Y, Linnewiel-Hermoni K, Khanin M, Salman H, Veprik A, Danilenko M, et al. Carotenoids and apocarotenoids in cellular signaling related to cancer: a review. Mol Nutr Food Res. 2012;56:259–69. doi: 10.1002/mnfr.201100311. [DOI] [PubMed] [Google Scholar]
- 48.Shirakura Y, Takayanagi K, Mukai K, Tanabe H, Inoue M. Beta-cryptoxanthin suppresses the adipogenesis of 3T3-L1 cells via RAR activation. J Nutr Sci Vitaminol. 2011;57:426–31. doi: 10.3177/jnsv.57.426. [DOI] [PubMed] [Google Scholar]
- 49.Harrison EH, dela Sena C, Eroglu A, Fleshman MK. The formation, occurrence, and function of β-apocarotenoids: β-carotene metabolites that may modulate nuclear receptor signaling. Am J Clin Nutr. 2012;96(Suppl):1189S–1192S. doi: 10.3945/ajcn.112.034843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigiura M, Ohshima M, Ogawa K, Yano M. Chronic administration of Satsuma mandarin fruit (Citrus unshiuMarc) improves oxidative stress in Streptozotocin-induced diabetic rat liver. Biol Pharm Bull. 2006;29:588–591. doi: 10.1248/bpb.29.588. [DOI] [PubMed] [Google Scholar]
- 51.Hozawa A, Jacobs DR, Steffes MW, Gross MD, Steffen LM, Lee DH. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: Interaction with smoking - The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2006;163:929–37. doi: 10.1093/aje/kwj136. [DOI] [PubMed] [Google Scholar]
- 52.Al-Delaimy WK, van Kappe AL, Ferrari P, Slimani N, Steghens JP, Bingham S, et al. Plasma levels of six carotenoids in nine European countries: report from the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2004;7:713–22. doi: 10.1079/phn2004598. [DOI] [PubMed] [Google Scholar]
- 53.Andersen LF, Jacobs DR, Gross MD, Schreiner PJ, Williams OD, Lee DH. Longitudinal associations between body mass index and serum carotenoids: the CARDIA study. Br J Nutr. 2006;95:358–65. doi: 10.1079/bjn20051638. [DOI] [PubMed] [Google Scholar]
- 54.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;34:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 55.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62(Suppl 6):S1427–30. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 56.Neuhouser ML, Patterson RE, Thornquis MD, Omenn GS, King IB, Goodman GE. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the Beta-Carotene and Retinol Efficacy Trial (CARET) Cancer Epidemiol Biomarkers Prev. 2003;12:350–8. [PubMed] [Google Scholar]
- 57.Holick CN, Michaud DS, Stolzenberg-Solomon R, Mayne ST, Pietinen P, Taylor PR, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the Alpha-Tocopherol, Beta-Carotene Cohort Study. Am J Epidemiol. 2002;156:536–47. doi: 10.1093/aje/kwf072. [DOI] [PubMed] [Google Scholar]
- 58.Moreiraa EM, Fagundes RM, Filho DW, Neves D, Sell F, Bellisle F, et al. Effects of diet energy level and tomato powder consumption on antioxidant status in rats. Clin Nutr. 2005;24:1038–46. doi: 10.1016/j.clnu.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Krishna Moorthy G, Selvakumar K, Venkataraman P, Elumalai P, Arunakaran J. Lycopene supplementation prevents reactive oxygen species mediated apoptosis in Sertoli cells of adult albino rats exposed to polychlorinated biphenyls. Interdiscip Toxicol. 2013;6:83–92. doi: 10.2478/intox-2013-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams AA, Selvaraj J, Srinivasan C, Sathish S, Rajesh P, Balaji V, et al. Protective role of lycopene against Aroclor 1254-induced changes on GLUT4 in the skeletal muscles of adult male rat. Drug & Chem Toxicol. 2013;36:320–328. doi: 10.3109/01480545.2012.720991. [DOI] [PubMed] [Google Scholar]
- 61.Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol Cell Endocrinol. 2012;361:106–15. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, et al. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. J Nutr Biochem. 2013;24:1587–95. doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cave M, Appana S, Pate M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ Health Perspect. 2010;118:1735–42. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2010;36:931–34. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol Appl Pharmacol. 2012;261:181–8. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schecter A, Colacino J, Haffner D, Patel K, Opel M, Päpke O. Perfluorinated compounds, polychorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amakura Y, Tsutsumi T, Nakamura M, Handa H, Yoshimura M, Matsuda R, et al. Aryl hydrocarbon receptor ligand activity of commercial health foods. Food Chem. 2011;126:1515–20. doi: 10.1016/j.foodchem.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 68.James MO, Sacco JC, Faux LR. Effects of food natural products on the biotransformation of PCBs. Environ Toxicol Pharmacol. 2008;25:211–7. doi: 10.1016/j.etap.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeuken A, Keser BJG, Khan E, Brouwer A, Koeman J, Denison MS. Activation of the Ah receptor by extracts of dietary herbal supplements, vegetables, and fruits. J Agric Food Chem. 2003;51:5478–87. doi: 10.1021/jf030252u. [DOI] [PubMed] [Google Scholar]
- 70.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–42. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97:505–16. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 72.Cooper AJ, Sharp SJ, Lentjes MA, Luben RN, Khaw KT, Wareham NJ, et al. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care. 2012;35:1293–300. doi: 10.2337/dc11-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 75.Salas-Salvado J, Bullo M, Estruch R, Ros E, Covas MI, Ibarrola-Jurado N, et al. Prevention of diabetes with Mediterranean diets: A Subgroup Analysis of a Randomized Trial. Ann Intern Med. 2014;160:1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 76.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes, a systematic review. Diabetes Care. 2002;25:1862–68. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 77.Tobias DK, Hu FB, Chavarro J, Rosner B, Mozffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. 2012;20:1566–72. doi: 10.1001/archinternmed.2012.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. [Assessed January 10, 2014];Your Guide to lowering your blood pressure with DASH. http://www.nhlbi.nih.gov/health/public/heart/hbp/dash/new_dash.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.