Abstract
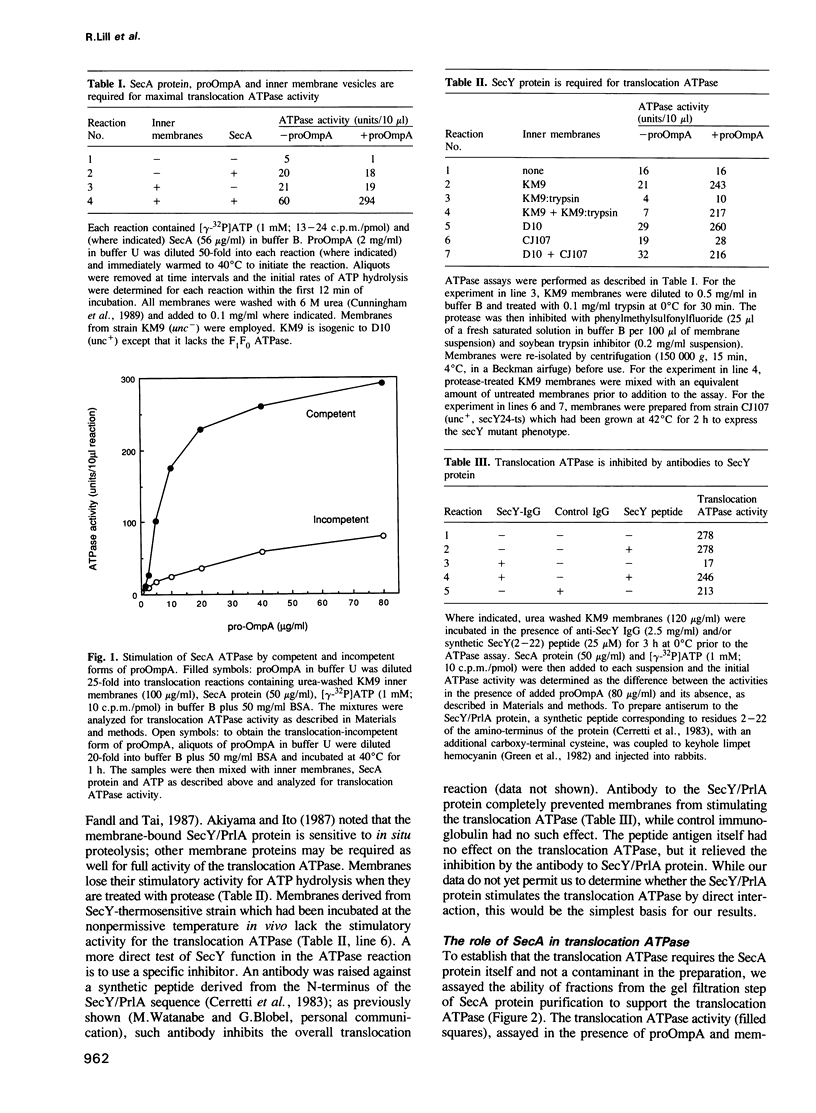
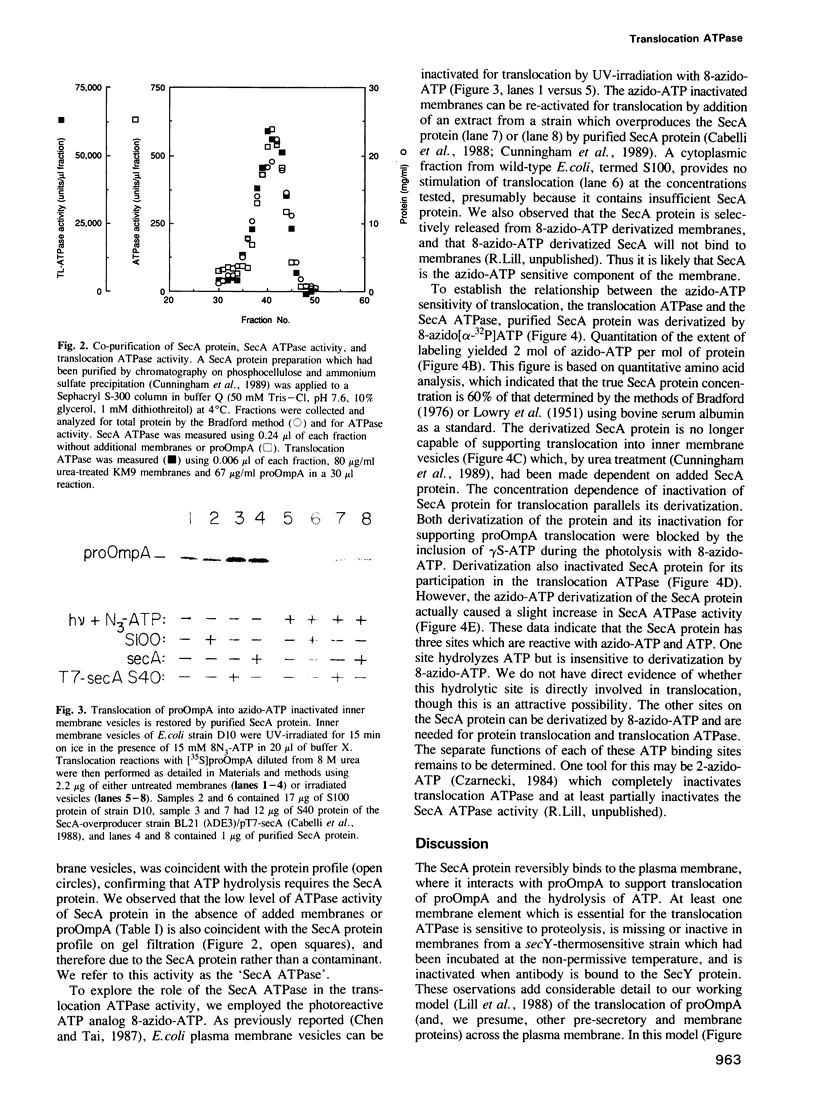
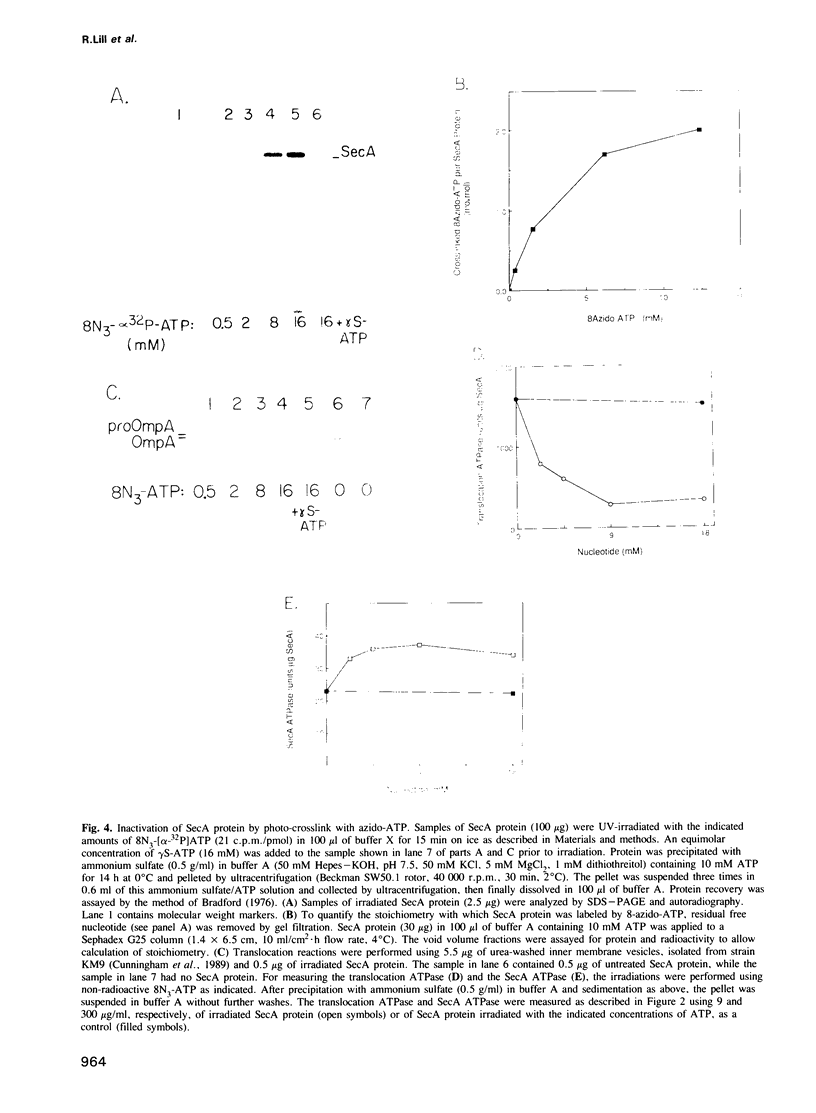
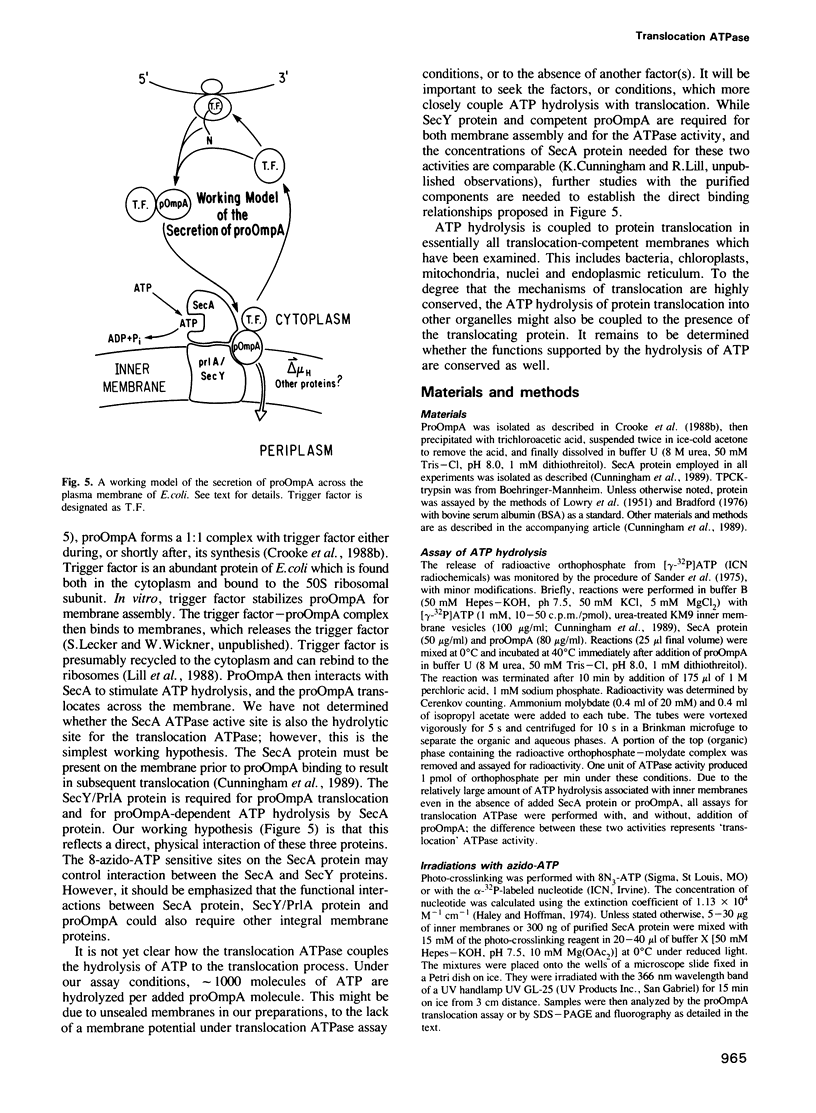
Bacterial protein export requires two forms of energy input, ATP and the membrane electrochemical potential. Using an in vitro reaction reconstituted with purified soluble and peripheral membrane components, we can now directly measure the translocation-coupled hydrolysis of ATP. This translocation ATPase requires inner membrane vesicles, SecA protein and translocation-competent proOmpA. The stimulatory activity of membrane vesicles can be blocked by either antibody to the SecY protein or by preparing the membranes from a secY-thermosensitive strain which had been incubated at the non-permissive temperature in vivo. The SecA protein itself has more than one ATP binding site. 8-azido-ATP inactivates SecA for proOmpA translocation and for translocation ATPase, yet does not inhibit a low level of ATP hydrolysis inherent in the isolated SecA protein. These data show that the SecA protein has a central role in coupling the hydrolysis of ATP to the transfer of pre-secretory proteins across the membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Ito K. Topology analysis of the SecY protein, an integral membrane protein involved in protein export in Escherichia coli. EMBO J. 1987 Nov;6(11):3465–3470. doi: 10.1002/j.1460-2075.1987.tb02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao R., Crooke E., Shiba K., Wickner W., Ito K. The secY protein can act post-translationally to promote bacterial protein export. J Biol Chem. 1986 Sep 25;261(27):12907–12910. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabelli R. J., Chen L., Tai P. C., Oliver D. B. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988 Nov 18;55(4):683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. L., Tai P. C. Evidence for the involvement of ATP in co-translational protein translocation. Nature. 1987 Jul 9;328(6126):164–166. doi: 10.1038/328164a0. [DOI] [PubMed] [Google Scholar]
- Chen L., Tai P. C. ATP is essential for protein translocation into Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4384–4388. doi: 10.1073/pnas.82.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Crooke E., Brundage L., Rice M., Wickner W. ProOmpA spontaneously folds in a membrane assembly competent state which trigger factor stabilizes. EMBO J. 1988 Jun;7(6):1831–1835. doi: 10.1002/j.1460-2075.1988.tb03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke E., Guthrie B., Lecker S., Lill R., Wickner W. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell. 1988 Sep 23;54(7):1003–1011. doi: 10.1016/0092-8674(88)90115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham K., Lill R., Crooke E., Rice M., Moore K., Wickner W., Oliver D. SecA protein, a peripheral protein of the Escherichia coli plasma membrane, is essential for the functional binding and translocation of proOmpA. EMBO J. 1989 Mar;8(3):955–959. doi: 10.1002/j.1460-2075.1989.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki J. J. Tautomerism of 2-azidoadenine nucleotides. Effects on enzyme kinetics and photoaffinity labeling. Biochim Biophys Acta. 1984 Jul 16;800(1):41–51. doi: 10.1016/0304-4165(84)90092-8. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Oppliger W., Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987 Apr;6(4):1073–1077. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandl J. P., Tai P. C. Biochemical evidence for the secY24 defect in Escherichia coli protein translocation and its suppression by soluble cytoplasmic factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7448–7452. doi: 10.1073/pnas.84.21.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Hinz G. Energy dependence of protein translocation into chloroplasts. Eur J Biochem. 1986 Nov 3;160(3):563–570. doi: 10.1111/j.1432-1033.1986.tb10075.x. [DOI] [PubMed] [Google Scholar]
- Geller B. L., Movva N. R., Wickner W. Both ATP and the electrochemical potential are required for optimal assembly of pro-OmpA into Escherichia coli inner membrane vesicles. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4219–4222. doi: 10.1073/pnas.83.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Haley B. E., Hoffman J. F. Interactions of a photo-affinity ATP analog with cation-stimulated adenosine triphosphatases of human red cell membranes. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3367–3371. doi: 10.1073/pnas.71.9.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Garcia P. D., Walter P. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell. 1986 May 9;45(3):397–406. doi: 10.1016/0092-8674(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Small G. M., Lazarow P. B. Translocation of acyl-CoA oxidase into peroxisomes requires ATP hydrolysis but not a membrane potential. J Cell Biol. 1987 Dec;105(6 Pt 2):2915–2922. doi: 10.1083/jcb.105.6.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lill R., Crooke E., Guthrie B., Wickner W. The "trigger factor cycle" includes ribosomes, presecretory proteins, and the plasma membrane. Cell. 1988 Sep 23;54(7):1013–1018. doi: 10.1016/0092-8674(88)90116-x. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Lucocq J. M., Bürglin T. R., De Robertis E. M. Assembly in vitro of nuclei active in nuclear protein transport: ATP is required for nucleoplasmin accumulation. EMBO J. 1986 Mar;5(3):501–510. doi: 10.1002/j.1460-2075.1986.tb04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of F1-ATPase subunit beta into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986 Dec 15;209(2):152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: translocation and glycosylation of prepro-alpha-factor in vitro can occur via an ATP-dependent post-translational mechanism. EMBO J. 1986 May;5(5):1031–1036. doi: 10.1002/j.1460-2075.1986.tb04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Kornberg R. D. Cell biology. An unfolding story of protein translocation. Nature. 1986 Jul 17;322(6076):209–210. doi: 10.1038/322209a0. [DOI] [PubMed] [Google Scholar]
- Sander G., Marsh R. C., Voigt J., Parmeggiani A. A comparative study of the 50S ribosomal subunit and several 50S subparticles in EF-T-and EF-G-dependent activities. Biochemistry. 1975 May 6;14(9):1805–1814. doi: 10.1021/bi00680a001. [DOI] [PubMed] [Google Scholar]
- Waters M. G., Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986 May;102(5):1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech H., Sagstetter M., Müller G., Zimmermann R. The ATP requiring step in assembly of M13 procoat protein into microsomes is related to preservation of transport competence of the precursor protein. EMBO J. 1987 Apr;6(4):1011–1016. doi: 10.1002/j.1460-2075.1987.tb04853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]





