Abstract
It is commonly accepted that the presence of high amounts of maternal T cells excludes Omenn syndrome (OS) in severe combined immunodeficiency (SCID). We report a SCID patient with a novel mutation in the recombination activating gene (RAG)1 gene (4-BP DEL.1406 TTGC) who presented with immunodeficiency and OS. Several assays, including representatives of specific T cell receptors (TCR), Vβ families and TCR-γ rearrangements, were performed in order to understand more clearly the nature and origin of the patient's T cells. The patient had oligoclonal T cells which, based on the patient–mother human leucocyte antigen (HLA)-B50 mismatch, were either autologous or of maternal origin. These cell populations were different in their numbers of regulatory T cells (Treg) and the diversity of TCR repertoires. This is the first description of the co-existence of large amounts of clonal expanded autologous and transplacental-acquired maternal T cells in RAG1-deficient SCID.
Keywords: maternal engraftment, Omenn syndrome, RAG1, SCID, T cell receptor repertoire
Introduction
Severe combined immunodeficiency (SCID) encompasses a heterogeneous group of genetic disorders often characterized by the arrest of T lymphocyte maturation and severe susceptibility to infections. Some SCID patients may, however, present with autologous T cells, which may clonally expand in the peripheral blood, infiltrate lymphoid tissues and other organs and produce an autoimmune phenotype clinically defined as Omenn syndrome (OS) [1]. OS was reported in patients with hypomorphic mutations in the recombination activating gene (RAG) 1 or 2 [2], including frameshift mutations within the 5′ coding region of RAG1, resulting in partial activity of the encoded enzyme [3]. OS has also been identified recently in a growing list of other leaky SCIDs. It is characterized by generalized scaly exudative erythrodermia, enlarged lymph nodes, hepatosplenomegaly, severe susceptibility to infections, activation of T helper type 2 lymphocytes, eosinophilia and hyperimmunoglobulin E (IgE) and peripheral expansion of self-reactive oligoclonal T cells [4]. The suggested mechanism for this self-reactivity is the possible inability of the thymus to delete abnormal clones due to profoundly reduced thymic AIRE mRNA and protein levels [5]. A distinctive feature of SCID patients, which can sometimes clinically resemble OS, is the presence of alloreactive cells originating from transplacental-acquired maternal T cells. OS and maternal T lymphocyte engraftment have many clinical and laboratory features in common, although maternally engrafted T lymphocytes most often result in a milder clinical presentation [6,7]. These phenotypes can be distinguished by different patterns of cell activity, enumeration and function of regulatory T cells (Tregs) and diversity of the T cell receptor (TCR) repertoire [7]. OS and transplacental-acquired maternal T cells have rarely been reported to co-exist, and it was commonly accepted that the presence of large amounts of maternal T cells excluded the OS phenotype [8], suggesting that maternal T cells can only occur in the complete absence of host T cells. This concept was refuted by a recent publication of long-term co-existence of autologous non-reactive T cells and high levels of engraftment of haploidentical maternal T cells in a B+SCID patient with a Janus kinase (JAK) 3 mutation [9]. Here, we report an unusual case of an immunodeficient SCID patient with OS harbouring a novel homozygous mutation in RAG1 and the concomitant presence of autologous and maternal T cells.
Methods
Patient
The patient was diagnosed and treated at the ‘Edmond and Lily Safra’ Children's Hospital. The Institutional Review Board (Sheba Medical Center, Tel Hashomer) approved this study and written informed consent was obtained from the patient's parents.
Immune-workup and TCR repertoire analysis
Cell surface markers of peripheral blood mononuclear cells (PBMCs), Tregs [CD4+CD25+forkhead box protein 3 (FoxP3)+], lymphocyte proliferative response to mitogens and the amount of signal joint (sj) T cell receptor excision circles (TRECs) were determined as described previously [7]. Representatives of specific T cell receptors (TCRs) Vβ families and TCR-γ rearrangements were detected and quantified using flow cytometry or amplified by polymerase chain reaction (PCR) [1].
Visualization of patient's cells
The patients' lymphocytes were visualized by a multi-parametric cell-scanning system (Duet; BioView Ltd, Rehovot, Israel) for detecting the presence of transplacentally acquired maternal T lymphocytes, as described previously [7]. The system combines morphological and fluorescence in-situ hybridization (FISH) analyses of the same cell, thereby enhancing the specificity of pathological cell detection.
Human leucocyte antigen (HLA) typing
The peripheral blood of the patient and his mother were tested for HLA-A,-B and-DR typing using either standard serological methods or DNA hybridization with sequence-specific oligonucleotide probes in order to define tissue typing.
Results
Patient characterization
Our patient was a male of Palestinian descent who was born after a normal pregnancy and delivery to parents who are first-degree cousins. An older sister died in infancy from infection without signs suggestive of OS. Our patient had fever, failure to thrive, prolonged diarrhoea and lip abscess and mild dermatitis. Physical examination revealed mild erythrodermia, generalized oedema, mild alopecia, lymphadenopathy and hepatosplenomegaly, all characteristics of OS. The patient responded very rapidly to low doses of steroids. The family history, clinical picture and immunological workup were suggestive of SCID, OS phenotype, and the patient underwent successful bone marrow transplantation from a matched related donor.
Immune evaluations and genetic analysis
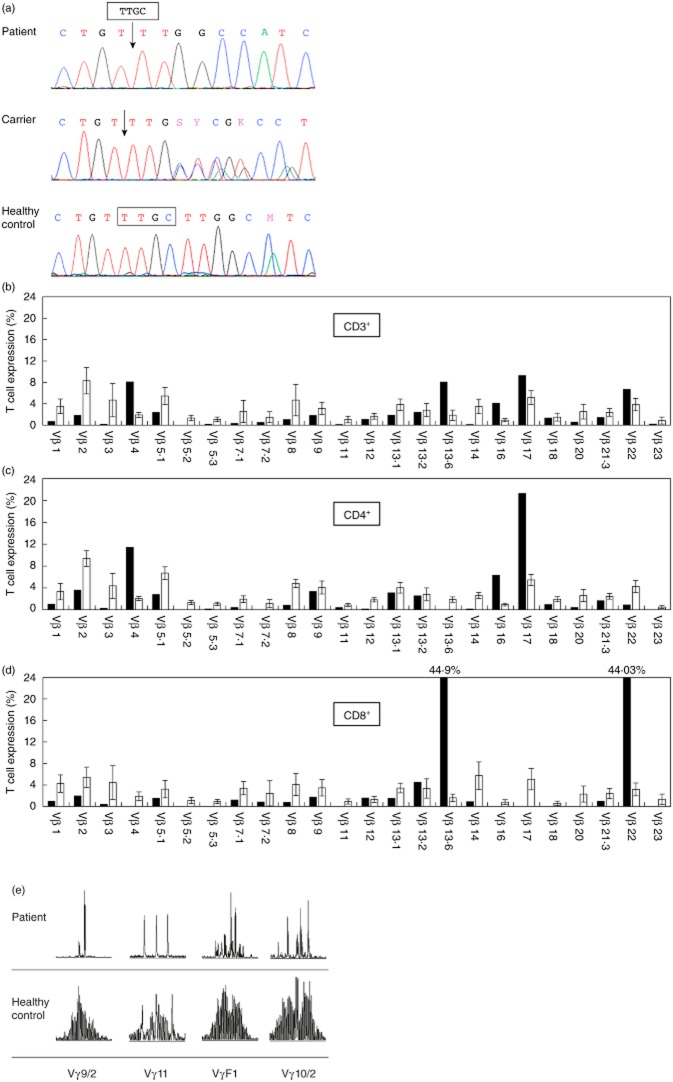
The patient had high levels of circulating lymphocytes (12 500 cells/μl) with mild eosinophilia (1780 cells/μl). The immunoglobulin G (IgG) level after intravenous immunoglobulin treatment was 2340 mg/dl (normal range for age: 350–930 mg/dl), the IgM level was 27 mg/dl (40–150 mg/dl), the IgA level was 72 mg/dl (15–90 mg/dl) and the IgE level was 5 IU/ml (0–12 IU/ml). Flow cytometric analysis showed 4442 cells/μl of CD3+ cells, 3109 CD4+ cells/μl and 1036 CD8+ cells/μl. There were 8000 natural killer cells/μl, but CD20+ B cells (30 cells/μl) were barely detectable. Response to mitogenic stimulation with phytohaemagglutinin (PHA) and anti-CD3 was significantly lower than normal (10 and 15%, respectively), and thymus output as determined by TRECs was undetectable. In order to quantify the Tregs, the patient's non-stimulated freshly isolated peripheral blood mononuclear cells (PBMCs) were stained with CD25 and FoxP3 antibodies on live CD4+ T cells. The levels of circulating TRECs were normal (6·04%). The reduced levels of circulating B cells, together with features of OS syndrome, were suggestive of RAG deficiency. This was confirmed by sequence analysis of the patient's RAG1 gene that revealed a novel four-base pair deletion homozygous mutation (4-BP DEL.1406 TTGC), which predicted a frameshift and premature stop codon. The parents were both heterozygous for this mutation and asymptomatic. Other family members were found to be either homozygous or heterozygous for the wild-type allele (Fig. 1a).
Figure 1.
Genetic and T cell receptor (TCR) analyses. (a) DNA sequences of the mutation region (4-BP DEL.1406 TTGC) of RAG1 in the studied patient, his parent and a healthy control. (b–d) Fluorescence activated cell sorter (FACS) analysis of the relative expression levels of 24 TCR Vβ families in the patient's total CD3+ cells (b, black bars), CD3+CD4+cells (c) and CD3+CD8+ cells (d) compared with the relative expression of the control (white bars). (e) Four different Vγ rearranged TCR genes (Vγ9·2, Vγ11, VγF1, Vγ10·2) of the patient (upper panel) and control (lower panel) were polymerase chain reaction (PCR) amplified followed by Gene Scan analysis.
TCR repertoire in different cell populations
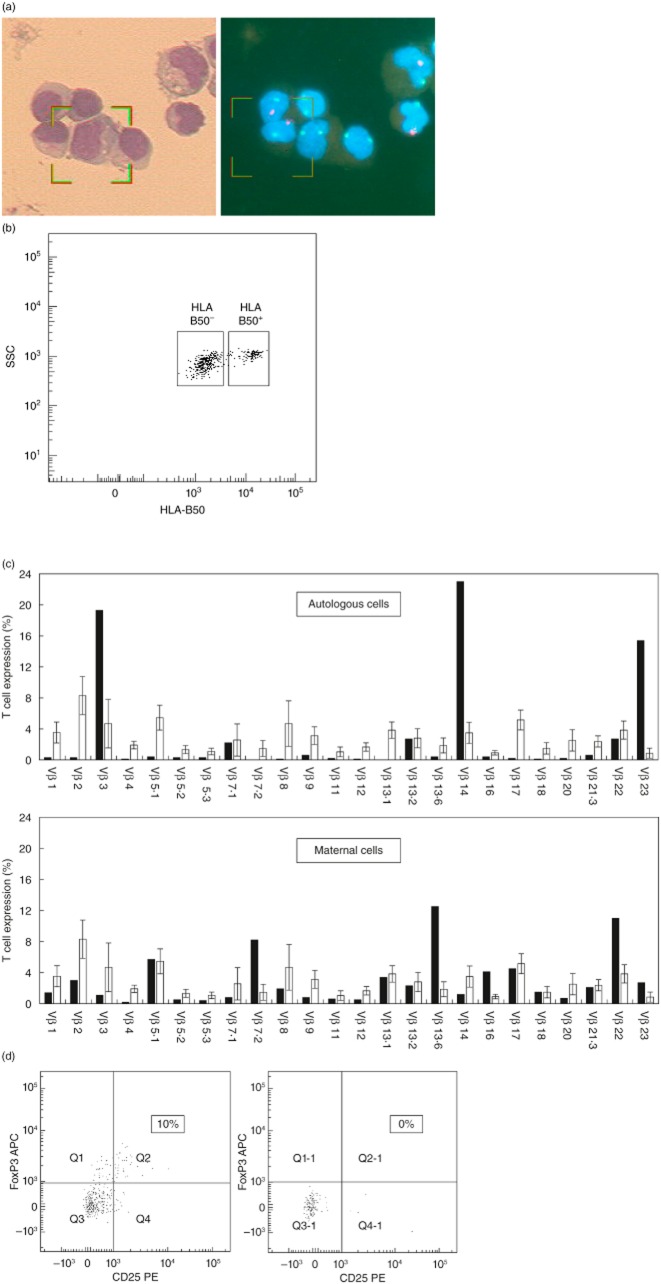
To define the patient's circulating T cells, we studied the repertoire diversity of both TCR-Vβ and TCR-Vγ (Fig. 1b–e). Flow cytometric analysis of 24 different TCRs on CD3+ lymphocytes showed oligoclonal expansion of a few Vβ families (Vβ4, Vβ13·6, Vβ16, Vβ17 and Vβ22) (Fig. 1b). There was under-representation of the Vβ families in another 11 TCRs. TCR Vβ clonality was better demonstrated when the different TCRs were examined on either CD4+ or CD8+ cells (Fig. 1c,d). Thus, the Vβ4, Vβ16 and Vβ17 were all CD4+ and the Vβ13·6 and Vβ22 were all CD8+. We analysed the TCR-γ-chain gene rearrangement to further delineate the clonality of these cells in peripheral blood. PCR analysis of the TCR-γ gene rearrangement in the DNA obtained from the patient's peripheral blood revealed mono/oligoclonal patterns in all four detected rearrangements (vγ9/2, vγ11, vγf1 and vγ10/2) compared to the normal control (Fig. 1e). Careful cytogenetic analysis showed that 38% of the patient's lymphocytes were of maternal origin (Fig. 2a). These cells could be distinguished from autologous cells due to the patient–mother HLA mismatch for HLA-B50 typing. Accordingly, the patient's autologous T cells were classified as CD3+HLA-B50+ and the maternally engrafted T lymphocytes were classified as CD3+HLA-B50– (Fig. 2b). Next, we studied the diversity of TCR-Vβ in both cell populations compared with published normal values (Fig. 2c). The patient's autologous T cells (CD3+HLA-B50+) were found to be oligoclonal with a restricted pattern (Fig. 2c, upper panel). This included three dominant clones: Vβ3 (19·3% of his CD3+ cells), Vβ14 (23%) and Vβ23 (15·4%), and markedly reduced 17 CD3+ Vβs. The maternally engrafted T cells (CD3+HLA-B50–) were less clonal and restricted (Fig. 2c, lower panel), including four over-represented TCR-Vβ families: Vβ7·2 (8·2% of the maternally engrafted CD3+ cells), Vβ13·6 (12·5%), Vβ16 (4·1%) and Vβ22 (11%), while only eight TCRs were reduced. The expression of the remaining 12 TCR-Vβ was normal. Finally, we detected greater numbers of circulating Tregs (CD4+CD25+FoxP3+) in the maternally engrafted T cell population (10%, Fig. 2d, left), whereas the patient's autologous T cells had no CD4+CD25+FoxP3+ expression whatsoever (Fig. 2d, right).
Figure 2.
Distinguishing autologous cells from maternal cells in the patient's peripheral blood. (a) Combined morphological and fluorescence in-situ hybridization (FISH) analysis using the X and Y chromosome probes confirmed the presence of maternal lymphocytes in the patient's peripheral blood. The left panel shows cells stained with Giemsa and the right panel shows the same cells with FISH using dual-colour X (green) and Y (red) DNA probes. (b) Fluorescence activated cell sorter (FACS) analyses could distinguish between autologous T cells [CD3+human leucocyte antigen (HLA)-B50+] and maternally engrafted T lymphocytes (CD3+HLA-B50–). (c) Relative expression levels of 24 T cell receptor (TCR) Vβ families in autologous T cells (CD3+HLA-B50+, upper panel) and maternal T lymphocytes (CD3+HLA-B50–, lower panel) compared with the relative expression of normal controls (white bars). (d) CD25 and forkhead box protein 3 (FoxP3) expression levels in autologous (HLA-B50+, right) and maternally engrafted (HLA-B50–, left) CD4+ T lymphocytes. Quadrants were set up based on staining with an isotype control. The boxed numbers indicate the percentage of regulatory T cells (Treg) within the CD4+ population.
Discussion
The currently accepted view is that autoreactive cells in OS and transplacental-acquired maternal T cells cannot occur concomitantly in the same patient. This point was emphasized in a recent report defining the absence of maternal engraftment as one of the OS criteria [10]. Indeed, none of 39 OS cases in which transplacental-acquired maternal T cells were investigated was positive for maternal chimerism [8]. Overall, transplacental-acquired maternal T cells have been reported in 40% of SCID patients, but in none when OS was diagnosed. Moreover, 62% of B-SCID patients (e.g. RAG deficiency) who were free of signs suggestive of OS were found to have signs of maternal T cell engraftment [i.e. typical graft-versus-host disease (GVHD) symptoms] [11]. In some rare cases, these cells either conferred immunity and protection against infection [12] or they caused allograft rejection and immune cytopenias [13]. A massive expansion of maternal T cells in response to Epstein–Barr virus infection in a patient with X-linked SCID has been also reported [14]. The first report of long-term co-existence of autologous T cells and high levels of engraftment of haploidentical maternal T cells was published recently [9]. In that study by Cattaneo et al., analysis of the autologous cells revealed a naive phenotype with a polyclonal repertoire of proliferating and activated cells in vivo. The residual function and the diversified repertoire of the autologous cells contributed to the excellent clinical status of the reported patient, who had no obvious signs suggestive of OS [9]. It is possible that the co-existence of autologous and maternal cells in patients exhibiting milder OS symptoms was simply not detected, rather than that these cells are mutually exclusive. Here, we show the co-existence of autologous and maternal oligoclonal expanded T cells in a case of an SCID patient with a milder form of OS. Our patient presented with typical clinical features and was found to have a homozygous four nucleotides deletion in RAG1, resulting in frameshift and premature stop codon. We speculate that the frameshift deletion alleles remain partially functional for recombination, thus explaining his partial rearrangement phenotype, as was shown previously for OS patients with a similar type of mutation [3]. Indeed, our patient displayed milder OS clinical and laboratory characteristics (e.g. mild skin rash, eosinophilia and alopecia and a low IgE level) and he responded very rapidly to low doses of steroids. In contrast to Cattaneo et al.'s [9] findings, our patient's autologous cells had a clonal representation, which probably contributed to the severity of his immunodeficiency. We confirmed our patient's oligoclonality by using different methods for investigating both the TCR-Vβ and TCR-γ rearrangements. Interestingly, his symptoms were less severe than would be expected from the nature of the autoreactive cells, suggesting the presence of a protective mechanism. His symptoms were, however, more severe than those seen in the GVHD that is related to the presence of maternal cells in SCID patients [15]. Indeed, both cell populations were visualized in his peripheral blood. Moreover, fluorescence activated cell sorter (FACS) analysis demonstrated that both cell populations were clonal. Also, interestingly, is the finding that the maternal cells had better representation of the TCR-Vβ families and a greater number of cells expressing CD4+CD25+FoxP3+, supporting our hypothesis that these cells provide some degree of immunity and prevent autoimmunity. We have shown previously that SCID patients with 100% maternal cells had greater amounts of CD4+CD25+FoxP3+ than SCID patients with barely detectable maternal cells. These cells were not secreting interferon-gamma or interleukin-2, suggesting that they were functional Tregs [7]. We therefore think that co-engraftment of maternal cells could end up being associated with a distinctly milder OS phenotype, which could be more prevalent if it were sought. Further functional studies and the reporting of more similar patients are needed to confirm our hypothesis that transplacental-acquired maternal T cells have such a tolerance capacity.
In summary, we describe the co-existence of significantly high amounts of both autologous and maternal cells in an SCID patient. While the oligoclonal pattern of the TCRs in both cell populations appears to contribute to the severity of the immunodeficiency observed in our patient, the presence of maternal cells may have lessened the autoimmune manifestations associated with OS.
Acknowledgments
This study was supported by the Jeffrey Modell Foundation (JMF), the Israeli Science Foundation and the Chief Scientist Office of the Ministry of Health, Israel.
Disclosure
The authors have declared that they have no conflicts of interest.
References
- 1.Somech R, Simon AJ, Lev A, et al. Reduced central tolerance in Omenn syndrome leads to immature self-reactive oligoclonal T cells. J Allergy Clin Immunol. 2009;124:793–800. doi: 10.1016/j.jaci.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 2.Villa A, Santagata S, Bozzi F, et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell. 1998;93:885–896. doi: 10.1016/s0092-8674(00)81448-8. [DOI] [PubMed] [Google Scholar]
- 3.Santagata S, Gomez CA, Sobacchi C, et al. N-terminal RAG1 frameshift mutations in Omenn's syndrome: internal methionine usage leads to partial V(D)J recombination activity and reveals a fundamental role in vivo for the N-terminal domains. Proc Natl Acad Sci USA. 2000;97:14572–14577. doi: 10.1073/pnas.97.26.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Cavadini P, Vermi W, Facchetti F, et al. AIRE deficiency in thymus of 2 patients with Omenn syndrome. J Clin Invest. 2005;115:728–732. doi: 10.1172/JCI23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plebani A, Stringa M, Prigione I, et al. Engrafted maternal T cells in human severe combined immunodeficiency: evidence for a TH2 phenotype and a potential role of apoptosis on the restriction of T-cell receptor variable beta repertoire. J Allergy Clin Immunol. 1998;101:131–134. doi: 10.1016/s0091-6749(98)70207-6. [DOI] [PubMed] [Google Scholar]
- 7.Lev A, Simon AJ, Trakhtenbrot L, et al. Characterizing T cells in SCID patients presenting with reactive or residual T lymphocytes. Clin Dev Immunol. 2012;2012:261470. doi: 10.1155/2012/261470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleman K, Noordzij JG, de Groot R, van Dongen JJ, Hartwig NG. Reviewing Omenn syndrome. Eur J Pediatr. 2001;160:718–725. doi: 10.1007/s004310100816. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo F, Recher M, Masneri S, et al. Hypomorphic Janus kinase 3 mutations result in a spectrum of immune defects, including partial maternal T-cell engraftment. J Allergy Clin Immunol. 2013;131:1136–1145. doi: 10.1016/j.jaci.2012.12.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shearer WT, Dunn E, Notarangelo LD, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.044. pii: S0091-6749 (13) 01495-4. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacentally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood. 2001;98:1847–1851. doi: 10.1182/blood.v98.6.1847. [DOI] [PubMed] [Google Scholar]
- 12.Tezcan I, Ersoy F, Sanal O, et al. Long-term survival in severe combined immune deficiency: the role of persistent maternal engraftment. J Pediatr. 2005;146:137–140. doi: 10.1016/j.jpeds.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Palmer K, Green TD, Roberts JL, et al. Unusual clinical and immunologic manifestations of transplacentally acquired maternal T cells in severe combined immunodeficiency. J Allergy Clin Immunol. 2007;120:423–428. doi: 10.1016/j.jaci.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 14.Touzot F, Dal-Cortivo L, Verkarre V, et al. Massive expansion of maternal T cells in response to EBV infection in a patient with SCID-Xl. Blood. 2012;120:1957–1959. doi: 10.1182/blood-2012-04-426833. [DOI] [PubMed] [Google Scholar]
- 15.Denianke KS, Frieden IJ, Cowan MJ, Williams ML, McCalmont TH. Cutaneous manifestations of maternal engraftment in patients with severe combined immunodeficiency: a clinicopathologic study. Bone Marrow Transplant. 2001;28:227–233. doi: 10.1038/sj.bmt.1703128. [DOI] [PubMed] [Google Scholar]