Abstract
X-linked inhibitor of apoptosis (XIAP) deficiency, caused by mutations in BIRC4, is an immunodeficiency associated with immune dysregulation and a highly variable clinical presentation. Current diagnostic screening tests such as flow cytometry for XIAP expression or lymphocyte apoptosis assays have significant limitations. Based on recent evidence that XIAP is essential for nucleotide-binding and oligomerization domains (NOD)1/2 signalling, we evaluated the use of a simple flow cytometric assay assessing tumour necrosis factor (TNF) production of monocytes in response to NOD2 stimulation by muramyl dipeptides (L18-MDP) for the functional diagnosis of XIAP deficiency. We investigated 12 patients with XIAP deficiency, six female carriers and relevant disease controls. Irrespective of the diverse clinical phenotype, the extent of residual protein expression or the nature of the mutation, the TNF response was severely reduced in all patients. On average, L18-MDP induced TNF production in 25% of monocytes from healthy donors or female carriers, while fewer than 6% of monocytes responded in affected patients. Notably, the assay clearly discriminated affected patients from disease controls with other immunodeficiencies accompanied by lymphoproliferation, hypogammaglobulinaemia or inflammatory bowel disease. Functional testing of the NOD2 signalling pathway is an easy, fast and reliable assay in the diagnostic evaluation of patients with suspected XIAP deficiency.
Keywords: Crohn's disease, MDP, NOD2, XIAP, XLP
Introduction
X-linked inhibitor of apoptosis (XIAP) deficiency, caused by mutations in BIRC4, was identified originally by Rigaud et al. in patients with X-linked lymphoproliferative disease (XLP) who had no mutations in SH2D1A [1]. These patients presented with haemophagocytic lymphohistiocytosis (HLH), pronounced splenomegaly and secondary hypogammaglobulinaemia, in most cases associated with Epstein–Barr virus (EBV) infection [1,2]. Recent reports have uncovered additional inflammatory manifestations associated with XIAP deficiency, including uveitis, recurrent fevers and inflammatory bowel disease (IBD) [3–5]. In fact, in our recently published cohort of 27 patients, IBD and severe Crohn's-like disease in particular were the most frequent disease manifestations of XIAP deficiency [3,6].
The first described in-vitro function of XIAP was the inhibition of caspases, and therefore the limitation of apoptosis [7]. Recently a second function was published, identifying a role for XIAP downstream of the intracellular pattern recognition receptor nucleotide-binding and oligomerization domains (NOD)1 and NOD2 [8–12]. Interestingly, mutations in NOD2 are associated with the development of Crohn's disease [13–16]. NOD2 is expressed primarily in monocytes and induces mitogen-activation protein (MAP) kinases and nuclear factor kappa B (NF-κB) activation, leading to proinflammatory cytokine production such as tumour necrosis factor (TNF), interleukin (IL)-1β, IL-6 and IL-8 [17]. The involvement of XIAP in the NOD-2 signalling pathway commits on its function as a ubiquitin ligase inducing ubiquitin-dependent signalling by induction of MAP kinases and IκB kinase complex activation (IKK). IKK promotes NF-κB function and cytokine production [8,18]. Disease-causing mutations that compromise the functional integrity of the XIAP Really Interesting New Gene (RING) domain or the baculoviral IAP repeat-containing protein (BIR2) domain lead to an impaired NF-κB response after NOD2 activation [8,9,19].
Currently, analysis of XIAP protein expression and apoptosis testing are used as screening assays for XIAP deficiency [20,21]. However, apoptosis tests are highly variable, and we have previously provided several examples of patients with disease-causing missense mutations in XIAP who showed normal protein expression using flow cytometry or Western blotting [3].
Based on the recent advances in the understanding of the physiological role of XIAP, we therefore evaluated the clinical value of a functional assay addressing XIAP function via NOD2 stimulation with muramyl dipeptides (MDP), a cell wall component of bacterial peptidoglycans.
Material and methods
Patients
The study was carried out after obtaining institutional review board approval (University of Freiburg ethics committee's protocol numbers 143/12 and 40/08). Informed consent was obtained from each patient and their families. Disease controls were recruited in the context of a study on patients with lymphoproliferation and autoimmunity. The studies are listed in the German Clinical Trial Registry (http://www.drks.de/DRKS00004592 and http://www.drks.de/DRKS00000298).
Genetic analysis
Genetic analysis was performed by Sanger Sequencing of the six coding exons of the gene-encoding XIAP (BIRC4) using genomic DNA from peripheral blood mononuclear cells (PBMCs) or granulocytes [3].
XIAP expression
XIAP expression was assessed by flow cytometry as described using purified anti-XIAP IgG1 (BD Bioscience, San Jose, CA, USA), that recognizes the C-terminal part of the protein [20].
L18-MDP assay
PBMC were isolated within 24 h after venipuncture and 1–1·5 × 106 cells per well were plated in Iscove's modified Eagle's medium (IMDM) 10% fetal calf serum (FCS) in a 24-well plate and rested overnight at 37°C. On the next day, non-adherent cells were gently washed off and the adherent cells were incubated in medium alone or in medium supplemented with 200 ng/ml lipopolysaccharide (LPS) (Sigma, St Louis, MO, USA) or 200 ng/ml of a lipidated muramyl dipeptide (L18-MDP; InvivoGen, San Diego, CA, USA) in the presence of GolgiPlug (BD Biosciences). After 2·5 h, the cells were scraped off using a cell scraper, put on ice and stained with monoclonal antibodies against CD14-allophycocyanin (APC) (clone: M5E2; BD Bioscience) and human leucocyte antigen D-related-phycoerythrin-cyanin 7) (HLA-DR-PC7) (clone: G46-6, BD Bioscience). The cells were fixed and permeabilized using Cytofix/Cytoperm Kit (BD Bioscience) and stained for TNF-PE (clone: MAb11; BD Bioscience). The samples were acquired with a Navios™ flow cytometer (Beckman Coulter, Brea, CA, USA) and analysed with Kaluza software (Beckman Coulter).
Statistical analysis
Statistical analysis was performed using prism software (GraphPad software, San Diego, CA, USA) using the two-tailed t-test.
Results
A flow cytometric assay to assess NOD2 responses in human monocytes
The following practical considerations guided the development of the L18-MDP assay: (i) the protocol should be based on intracellular flow cytometry allowing rapid evaluation; (ii) the assay should be sufficiently robust to yield reliable results with a sample that needs to travel for up to 24 h; and (iii) the assay should be able to differentiate patients clearly not only from healthy donors, but also from disease controls.
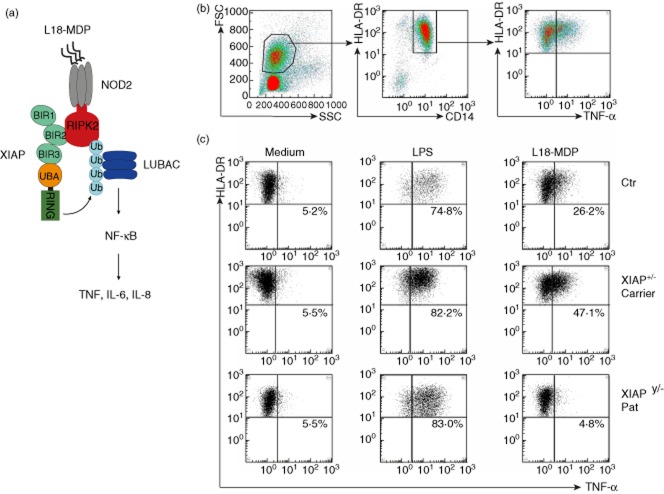
Figure 1b illustrates the gating strategy using CD14 and HLA-DR to define monocytes. Following incubation with medium alone, background staining for TNF was in the range of 2–14%, depending in part upon the extent of enrichment of the monocytes and the time of sample travel (Fig. 1c). Stimulation with LPS as a positive control induced TNF production via Toll-like receptor (TLR)-4 stimulation in more than 50% of monocytes of healthy controls. L18-MDP stimulation induced a TNF response in more than 20% of monocytes of a healthy donor and of a female carrier of a XIAP mutation, but a poor response in one patient with XIAP (Fig. 1c). Quantification of TNF in supernatants from stimulated cells by ELISA revealed similar data to intracellular flow cytometry (data not shown).
Figure 1.
(a) 6-O-stearoyl-MDP muramyldipeptide (L18-MDP) (lipidated derivative of muramyl dipeptide) is recognized by nucleotide-binding and oligomerization domain (NOD2) and induces cytokine production via recruitment of X-linked inhibitor of apoptosis (XIAP) and receptor-interacting serine–threonine kinase 2 (RIPK2). XIAP induces RIPK2 ubiquitilation leading to recruitment of the linear ubiquitin chain assembly complex (LUBAC) complex followed by nuclear factor-kappa B (NF-kB) activation [8]. (b) Gating strategy for tumour necrosis factor (TNF)-producing macrophages as defined by forward-/side-scatter, CD14 and human leucocyte antigen D-related (HLA-DR) expression. (c) TNF-producing monocytes from a healthy donor, an asymptomatic XIAP carrier and a XIAP patient cultured in the presence of medium alone, 200 ng/ml L18-MDP or 200 ng/ml lipopolysaccharide (LPS). The percentages indicate the percentage of TNF-positive cells of all HLA-DR+CD14+ monocytes.
L18-MDP stimulation of monocytes identifies patients with XIAP deficiency independent of residual protein expression
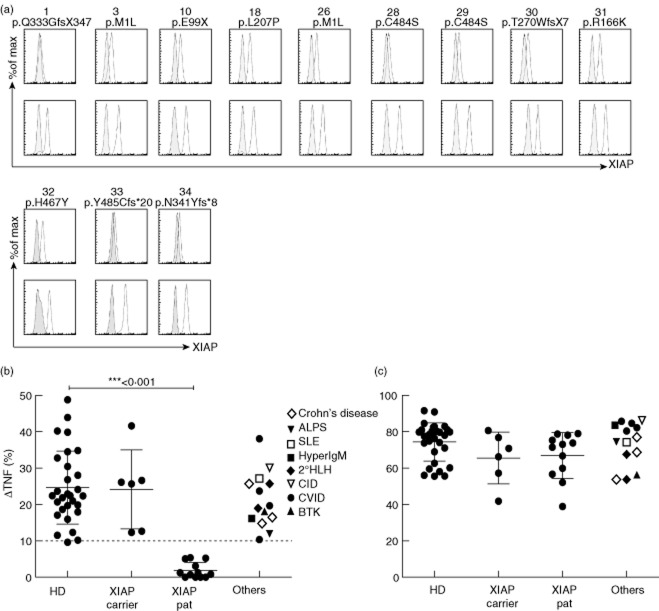
To confirm these initial results in a larger patient cohort and to evaluate the diagnostic value of the L18-MDP test in a clinical setting, we analysed samples from 12 patients with XIAP deficiency, six female carriers, 14 disease controls and 29 healthy donors. The spectrum of clinical manifestations in the XIAP-deficient patients was broad (Table 1) and included severe Crohn's-like disease (one), splenomegaly (seven), hypogammaglobulinaemia (two), recurrent fever (two), severe infectious mononucleosis (one) and partial (two) or full (seven) HLH. Patient no. 26 was 13 years of age, had never experienced any symptoms despite EBV seroconversion and was diagnosed because of an affected brother (Table 1). Details of five patients have been reported previously [3]. Most patients had inactive disease; three patients received steroids and two other patients received HLH-94 treatment at the time of investigation. XIAP expression was assessed by flow cytometry in all patients. It was significantly reduced to absent in 10 patients, but P18 and P31 expressed almost normal levels of XIAP protein (Fig. 2a). The diagnosis was confirmed in all cases by genetic sequencing. Mutations included a duplication, deletions, missense and non-sense mutations (Table 1). Disease controls included four patients with CVID and splenomegaly, one patient with Bruton's disease and splenomegaly, one patient with ALPS, two patients with 2° HLH, one induced by unknown trigger and one by EBV, three patients with Crohn's disease, one patient with hyper-IgM syndrome of unknown genetic origin and one patient with systemic lupus erythematosus (SLE). Several of these patients received steroids and other immunosuppressive agents at the time of analysis. Healthy donors were aged between 22 and 65 years.
Table 1.
Summary of patients.
| UPN | Clinical manifestation (age at onset/years) | Disease activity/treatment at investigation | BIRC4 mutation | Effect of mutation |
|---|---|---|---|---|
| 1† | SM (1), periodic fevers (2), partial HLH (3), low IgG (13) | Inactive, intermittent low-dose steroids, SCIG | c.997_1001 | p.Q333GfsX347 |
| 3† | SIM (10), SM (10) | Inactive, no treatment | Exon 2 c.1A > T | p.M1L |
| 10† | CD (1), SM (1), periodic fevers (2) | Inactive, intermittent low-dose steroids | c.295G > T | p.E99X |
| 18† | Recurrent HLH (17) | Inactive, no treatment | c.620T > C | p.L207P |
| 26† | Asymptomatic older brother (13) of patient 3 | Inactive, no treatment | Exon 2 c.1A > T | p.M1L |
| 28 | HLH, SM, arthralgia (3) | Inactive, no treatment | c.1450T > A | p.C484S |
| 29 | HLH, SM (6) | Early remission, post-steroid pulse and MTX | c.1450T > A | p.C484S |
| 30 | SM (4), recurrent HLH (13) | Inactive, no treatment | Exon 5 c.811_815del | p.T270WfsX7 |
| 31 | Recurrent partial HLH (4), SM (4), low IgG (16) | Active, steroids, IVIG | c.497G > A | p.R166K |
| 32 | HLH (5) | HLH controlled with HLH-94-SCT | c.1399C > T | p.H467Y |
| 33 | HLH (0,25) – 1× relapse, autoimmune haemolytic anaemia | HLH controlled with HLH-94-SCT | c.1449_1453dup | p.Y485Cfs*20 |
| 34 | HLH (2) | HLH-94 – steroids only | c.1021_1022del | p.N341Yfs*8 |
Clinical details reported in Speckmann et al. [3]. UPN = unique patient numbers of our XIAP cohort; CD = Crohn's disease; HLH = haemophagocytic lymphohistiocytosis, ≥ 5 HLH criteria; partial HLH = < 5 HLH criteria and self-limiting or oral steroid treatment. Periodic fevers: recurrent fever flares without signs of HLH. SM = splenomegaly; BIRC3 = baculovirus IAP repeat containing 4; SCIG/IVIG = subcutaneous/intranvenous immunoglobulin substitution; HLH-94 = treatment according to HLH-94 study protocol; SCT = stem cell transplantation.
Figure 2.
(a) Overlay of staining with anti-X-linked inhibitor of apoptosis (XIAP) and an isotype control antibody, gated on CD3+ T lymphocytes. XIAP patients (upper panel) are shown with their day control (lower panel). (b,c) Overnight rested monocytes from healthy donors, XIAP carriers (mothers of affected patients), XIAP patients and other disease controls, as indicated, were stimulated either with 6-O-stearoyl-MDP muramyldipeptide (L18-MDP) (b) or with lipopolysaccharide (LPS) (c). Intracellular tumour necrosis factor (TNF) production was measured via flow cytometry. The numbers indicate the increase in the fraction of monocytes producing TNF upon stimulation, calculated by subtracting the percentage of monocytes producing TNF after incubation in medium alone from that measured after providing the stimulus. P-values were calculated with a two-tailed t-test.
The results of the monocyte stimulation assays confirmed our initial observation. In all 29 healthy donors, L18-MDP induced an increase in TNF-producing monocytes (compared to medium) by more than 10%, in all but four by more than 15% (Fig. 2b). Similar responses were observed in healthy carrier mothers. In contrast, all 12 XIAP-deficient patients showed responses that were at or below 6% (Fig. 2b). This impaired response was observed independently of their mutation, the extent of residual protein expression or their clinical phenotype. Importantly, the monocyte response to LPS was retained, indicating that the lack of response to MDP reflected an impaired NOD2 pathway and not a general impairment of monocyte activation for cytokine production (Fig. 2c). All disease controls showed results for MDP and LPS stimulation that were in the range of healthy donors, suggesting that the assay is useful for the differential diagnosis in a relevant clinical context.
Discussion
In this study, we show that L18-MDP stimulation of TNF production by monocytes assessed by flow cytometry allows for an easy, fast and reliable diagnostic evaluation of patients with suspected XIAP deficiency.
The pathogenesis of XIAP deficiency was thought originally to be linked primarily to increased apoptosis of lymphocytes and decreased numbers of invariant natural killer T cells (iNK T cells), which are considered important for the control of EBV infections [8,18]. However, more recent studies have shown that activation-induced cell death (AICD) and also iNK T cell numbers can be normal in XIAP-deficient patients [3,22], thus limiting the diagnostic value of these parameters. Moreover, XIAP protein expression, assessed by flow cytometry or Western blotting, can be normal in symptomatic patients harbouring missense mutations or mutations not affecting the binding region of the diagnostic antibody [i.e. the BIR3 and ubiquitin-associated (UBA) domain of BIRC4] [3].
The rationale for using L18-MDP stimulation as a new screening assay for XIAP deficiency came from previous studies, which demonstrated that disease-causing XIAP mutations impair ubiquitilation of receptor-interacting serine–threonine kinase 2 (RIPK2)-and NOD2-dependent induction of NF-κB target genes such as TNF [8,18]. In the study by Damgaard et al., PBMC from two of our patients with the XLP phenotype (including patient 18 from this study) were stimulated with L18-MDP and TNF and IL-6 transcription were measured by reverse transcription–polymerase chain reaction (RT–PCR) [8]. Because this experimental set-up is not particularly suited for a routine diagnostic setting, we adapted the assay to flow cytometry.
As predicted from the previous studies, the assay identified patients with a variety of different mutations, including a point mutation in the BIR2 domain as well as those with more deleterious non-sense or frame-shift mutations or deletions. This included two patients with almost normal expression of XIAP protein. Notably, patients 28 and 29 harbour a novel mutation, c.T1450A, which causes a C484S substitution in the protein. C484 is involved in co-ordinating one of two Zn2+ ions required for folding of the RING, and the mutation probably results in severe impairment of ubiquitin ligase activity, similar to the previously described RING mutations G466X and P482R [8,18]. Moreover, the assay not only identified XIAP-deficient patients with a phenotype of inflammatory bowel disease, where a link to impaired NOD2 signalling may be more obvious, but also patients presenting with HLH, recurrent fever, splenomegaly or hypogammaglobulinaemia. A note of caution is warranted, because the assay has been evaluated so far in only 12 XIAP patients with 11 different mutations. However, in combination with the recent data on mutant cell lines [8,18], we expect that this functional test will be a more sensitive screening test than intracellular staining for XIAP protein. Furthermore, the assay is more reliable and the difference between patients and healthy donors is more robust when compared to apoptosis studies that we have reported previously in some of the patients in our cohort [3].
Importantly, the L18-MDP test also had good specificity when evaluated in a cohort of patients with disease presentations overlapping those of XIAP deficiency. It should be stated that most patients and disease controls were studied in a stable phase of their disease without significant immunosuppressive treatment. It is possible that, during active HLH, the monocyte population among PBMC will be too small for reproducible results. None the less, three XIAP patients with active HLH (two of them receiving HLH-94 treatment) were clearly recognized. Considering the wide spectrum of clinical presentations of XIAP deficiency, this diagnosis has to be considered in many clinical situations. Gene sequencing is not cost-effective as a screening method in all these situations. Moreover, the L18-MDP assay is much faster (24 h) than sequencing, which is particularly relevant in patients with HLH, where a rapid diagnosis is important, and many genes can be associated with the phenotype. Finally, the significance of previously unreported missense mutations is frequently unclear, and functional assays such as the L18-MDP assay are necessary to prove their significance in a diagnostic context.
From a pathophysiological viewpoint, this study confirms that impaired NOD2 signalling is a key feature of XIAP deficiency in primary human cells. This overlap with autoinflammatory diseases may change the view on the pathogenesis of this potentially life-threatening disorder and may indicate the pathway towards novel therapeutic interventions.
Acknowledgments
We thank our patients, their families and referring physicians (Dr Emma Morris, Dr Melchior Lauten and Dr Martina Kohl) who made this study possible. We also thank the technicians of the Center of Chronic Immunodeficiency Advanced Diagnostic Unit for excellent technical assistance. This work was supported by the German Federal Ministry of Education and Research (BMBF 01 EO 0803 grant to the Center of Chronic immunodeficiency and BMBF 01GM1111B grant to the PID-NET initiative).
Disclosures
No author has any financial or other potential conflict of interest to disclose.
References
- 1.Rigaud S, Fondaneche MC, Lambert N, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 2.Marsh RA, Madden L, Kitchen BJ, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speckmann C, Lehmberg K, Albert MH, et al. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol. 2013;149:133–141. doi: 10.1016/j.clim.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Pachlopnik Schmid J, Canioni D, Moshous D, et al. Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency) Blood. 2011;117:1522–1529. doi: 10.1182/blood-2010-07-298372. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Kanegane H, Nishida N, et al. Clinical and genetic characteristics of XIAP deficiency in Japan. J Clin Immunol. 2012;32:411–420. doi: 10.1007/s10875-011-9638-z. [DOI] [PubMed] [Google Scholar]
- 6.Speckmann C, Ehl S. XIAP deficiency is a mendelian cause of late-onset IBD. Gut. 2013 doi: 10.1136/gutjnl-2013-306474. gutjnl-2013-306474. doi: 10.1136/gutjnl-2013-306474. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 8.Damgaard RB, Fiil BK, Speckmann C, et al. Disease-causing mutations in the XIAP BIR2 domain impair NOD2-dependent immune signalling. EMBO Mol Med. 2013;5:1278–1295. doi: 10.1002/emmm.201303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damgaard RB, Nachbur U, Yabal M, et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell. 2012;46:746–758. doi: 10.1016/j.molcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Krieg A, Correa RG, Garrison JB, et al. XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci USA. 2009;106:14524–14529. doi: 10.1073/pnas.0907131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauler LD, Duckett CS, O'Riordan MX. XIAP regulates cytosol-specific innate immunity to Listeria infection. PLOS Pathog. 2008;4:e1000142. doi: 10.1371/journal.ppat.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipinski S, Grabe N, Jacobs G, et al. RNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognition. Proc Natl Acad Sci USA. 2012;109:21426–21431. doi: 10.1073/pnas.1209673109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 14.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 15.Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn's disease. Annu Rev Genomics Hum Genet. 2009;10:89–116. doi: 10.1146/annurev-genom-082908-150013. [DOI] [PubMed] [Google Scholar]
- 16.van Heel DA, Ghosh S, Butler M, et al. Muramyl dipeptide and Toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet. 2005;365:1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 17.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 18.Beug ST, Cheung HH, LaCasse EC, Korneluk RG. Modulation of immune signalling by inhibitors of apoptosis. Trends Immunol. 2012;33:535–545. doi: 10.1016/j.it.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Worthey EA, Mayer AN, Syverson GD, et al. Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med. 2011;13:255–262. doi: 10.1097/GIM.0b013e3182088158. [DOI] [PubMed] [Google Scholar]
- 20.Bryceson YT, Pende D, Maul-Pavicic A, et al. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood. 2012;119:2754–2763. doi: 10.1182/blood-2011-08-374199. [DOI] [PubMed] [Google Scholar]
- 21.Marsh RA, Bleesing JJ, Filipovich AH. Flow cytometric measurement of SLAM-associated protein and X-linked inhibitor of apoptosis. Methods Mol Biol. 2013;979:189–197. doi: 10.1007/978-1-62703-290-2_15. [DOI] [PubMed] [Google Scholar]
- 22.Marsh RA, Villanueva J, Kim MO, et al. Patients with X-linked lymphoproliferative disease due to BIRC4 mutation have normal invariant natural killer T-cell populations. Clin Immunol. 2009;132:116–123. doi: 10.1016/j.clim.2009.03.517. [DOI] [PMC free article] [PubMed] [Google Scholar]