Abstract
Although monitoring tuberculosis (TB) infection during long-term treatment with tumour necrosis factor (TNF) antagonists is of great importance, no monitoring strategy has yet proved successful. Indeed, even the newly proposed interferon-gamma release assays (IGRAs) are known to produce dynamic changes in IFN-γ plasma levels, making them unreliable indicators of patients' pathological/clinical status. We used intracellular cytokine flow cytometry (ICCFC) to investigate the performance of multi-functional CD4+ T cells producing IFN-γ, interleukin (IL)-2 and/or TNF in response to Mycobacterium tuberculosis-specific antigens in subjects treated with TNF antagonists. Patients were classified into three groups based on their TB status before commencement of treatment and on IFN-γ level fluctuations evaluated by IGRA during a 36-month follow-up period. The cytokine profile of M. tuberculosis-specific CD4+ T cells showed that latent tuberculosis infection (LTBI) subjects had a higher frequency of double-positive IFN-γ+ IL-2+ CD4+ T cells and triple-positive IFN-γ+ IL-2+ TNF+ CD4+ T cells compared to those without LTBI, who showed IFN-γ-level fluctuations over time. In contrast, this latter group of patients showed similar proportions of cells producing IFN-γ alone, IL-2 alone and IL-2 in combination with TNF in response to M. tuberculosis-specific antigens. It therefore appears that patients with and without LTBI infection are characterized by different intracellular cytokine profiles. This is the first study evaluating ICCFC in patients treated with TNF antagonists, and suggests that multi-functional analysis of CD4+ T cells could be useful for ruling out TB infection in patients classified at screening as LTBI-negative but who show IGRA fluctuations under long-term TNF antagonist treatment.
Keywords: flow cytometry, latent infection, polyfunctional T cells, TNF antagonists, tuberculosis
Introduction
There is a continuing need to diagnose tuberculosis (TB) in high-risk patients, particularly those scheduled for biological therapy, in order to prevent the spread of the disease [1–3]. Therefore, screening for latent TB infection (LTBI) in cases of immune suppression has become common practice. However, the limitations of the long-standing tuberculin skin test (TST) are well known. Even the more recent interferon-gamma release assays (IGRAs) [4] are characterized by a high rate of false negatives and indeterminate results in immune-compromised subjects, including those with HIV [5,6] and those undergoing biological therapy [7–10]. In addition, IGRA conversions and reversions have been observed when repeated over time in the same individuals [10–15]. These fluctuations may limit the reliability of IGRA in detecting TB infection in patients taking immunosuppressive drugs, including biological agents such as tumour necrosis factor (TNF) antagonists [10,12–15]. Indeed, the majority of studies have shown little correlation between interferon (IFN)-γ plasma levels and clinical change, and in only a few exceptions did IGRA conversion predict the emergence of active TB [16–18].
In a previous study we measured IFN-γ level fluctuations with serial QuantiFERON-TB Gold In-Tube (QFT-GIT), one of the commercially available IGRA tests, in patients with chronic inflammatory rheumatic diseases during long-term systemic anti-TNF treatment, irrespective of baseline LTBI status [10]. Because these changes (conversions and reversions) were not correlated with clinical outcome they could lead to confusion, and new tools are therefore required to clarify their significance.
Recent studies have shown that multi-functional analysis of CD4+ T cells producing a range of cytokines [IFN-γ, interleukin (IL)-2 and TNF] in response to Mycobacterium tuberculosis-specific antigens may be a sensitive means of discriminating between active and latent TB infection [19,20] and, indeed, superior to IGRAs in terms of TB detection [21].
We therefore used, for the first time, multi-functional flow cytometry to analyse CD4+ T cells in patients treated with TNF antagonists. Our objectives were as follows: (i) to analyse the ability of M. tuberculosis antigen-specific T cells to produce IL-2, TNF and IFN-γ in such patients; (ii) to investigate the pattern of cytokine production as a biomarker of TB status during long-term biological therapy; and (iii) to assess whether fluctuations in IFN-γ, measured by QFT-GIT, in patients not classified as LTBI before the onset of biological therapy, could be related to a specific multi-functional profile of CD4+ T cells suggestive of TB infection.
Materials and methods
Study subjects
The population studied included 33 patients with chronic inflammatory rheumatic diseases who were followed-up for 36 months during long-term systemic anti-TNF treatment. Before initiating biological therapy, all patients were given a posterior–anterior chest radiograph, each of which was assessed by a radiologist aware that anti-TNF therapy was being considered with a view to detecting any signs of LTBI [22].
Patients also underwent TST and QFT-GIT. The former (Biocine Test PPD; Chiron, Siena, Italy) was performed according to the Mantoux method by the same experienced operator, considering an induration of ≥5 mm as positive. All QFT-GIT (Cellestis Limited, Carnegie, Victoria, Australia) tests were carried out and interpreted by the same trained technician, as per the manufacturer's instructions. Both operators were blind to the clinical status of the patients.
Patients with evidence of TB infection based on any of QFT-GIT, TST, chest radiograph results (apical pleural thickening, pulmonary nodules, upper lobe bronchiectasis, interstitial granulomatous calcification, cavitation and lymph node or pericardial calcification) or other risk factors (a history of exposure to a case of active TB or originating from an area with a high prevalence of TB infection) were considered to be affected by LTBI after excluding active TB. All patients received a 9-month course of isoniazid (INH) prophylaxis, and biological therapy was introduced 4 weeks after the start of this regimen.
Thirty-six months after commencement of biological therapy, blood was taken from all patients so that QFT-GIT and intracellular cytokine flow cytometry (ICCFC) assays could be performed. Measurement of IFN-γ plasma levels and multi-functional analysis of CD4+ T cells were performed on the same blood samples. Subjects were classified into the following three groups, based on both their TB status before biological therapy and the fluctuations in their IFN-γ levels observed during follow-up: group A, 12 patients with LTBI who showed IFN-γ level fluctuations during follow-up; group B, 11 patients with no evidence of LTBI at baseline showing IFN-γ level fluctuations during follow-up; and group C, 10 patients with no evidence of LTBI at baseline and persistently negative IFN-γ levels during follow-up. When samples were taken, five patients from group A and one patient from group B had previously been taken off biological therapy (four after the 18th month and two after the 24th month), due to either efficacy loss or non-TB-related side effects. The remaining patients did not develop any symptoms suggestive of TB during the follow-up period, and are still undergoing biological therapy. Table 1 summarizes the demographic and clinical characteristics of the three patient groups. The study received approval from the local Ethics Committee, and informed written consent was provided by all patients.
Table 1.
Baseline demographics and clinical characteristics of the three groups of patients at the time of blood collection for multi-functional analysis of T cells.
| Characteristics | Group A (n = 12) |
Group B (n = 11) |
Group C (n = 10) |
|---|---|---|---|
| M/F (n) | 5/7 | 7/4 | 4/6 |
| Age (years; median/range) | 63·5/21–76 | 45/36–71 | 58/30–75 |
| Underlying disease (n/%) | |||
| Rheumatoid arthritis | 9/75 | 2/18·2 | 4/40 |
| Psoriatic arthritis | 1/8·3 | 6/54·5 | 5/50 |
| Ankylosing spondylitis | 2/16·7 | 3/27·3 | 1/10 |
| BCG-vaccinated (n/%) | 0 | 0 | 0 |
| Risk factors for LTBI (n/%) | |||
| Birth in a TB-endemic area† | 1/8·3 | 0 | 1/10 |
| History of household contact | 0 | 1/9·1 | 0 |
| Chest X-ray suggestive of LTBI | 4/33·3 | 0 | 0 |
| Previous diagnosis of TB | 0 | 0 | 0 |
| Concomitant treatment regimen (n/%) | |||
| Glucocorticoids | 0 | 0 | 0 |
| DMARDs | 0 | 0 | 0 |
| Biologicals | 3/25 | 8/72·7 | 4/40 |
| DMARDs + glucocorticoids | 3/25 | 0 | 0 |
| Biologicals + glucocorticoids | 1/8·3 | 1/9·1 | 1/10 |
| Biologicals + DMARDs | 2/16·7 | 1/9·1 | 3/30 |
| Biologicals + DMARDs + glucocorticoids | 1/8·3 | 0 | 2/20 |
| No immunosuppressants | 2/16·7 | 1/9·1 | 0 |
Includes Albania (group A) and Argentina (group C). Group A: patients with latent tuberculosis infection (LTBI) (before the onset of biological therapy) showing fluctuations (conversions/reversions) in released interferon (IFN)-γ levels in the QuantiFERON-TB Gold In-Tube (QFT-GIT) during long-term treatment with biological agents. Group B: patients with no evidence of LTBI at baseline showing IFN-γ levels fluctuations during follow-up. Group C: patients with no evidence of LTBI at baseline showing persistently negative IFN-γ levels released in the QFT-GIT during follow-up. BCG = bacille Calmette-Guérin; DMARDs = disease modifying anti-rheumatic drugs; TB = tuberculosis; M/F = male/female. DMARDs include methotrexate, leflunomide, cyclosporin A, sulphasalazine, azathioprine and hydroxychloroquine.
Intracellular cytokine flow cytometry (ICCFC)
Heparinized peripheral blood was collected, and 0·5 ml of whole blood was added to three test tubes containing, respectively, saline (negative control), phytohaemagglutinin (PHA, positive control) and TB antigens [early secreted antigenic target of 6 (ESAT-6), 10-kDa culture filtrate protein (CFP-10) and TB 7·7] following an identical procedure to the QFT-GIT assay. Whole blood was co-stimulated with anti-CD28 plus anti-CD49d (5 μg/ml; BD Bioscience, Pharmingen, Italy) and brefeldin A (50 μg/ml; Sigma-Aldrich, St Louis, MO, USA) was added immediately to each tube. After 18 h of incubation, the red cells were lysed with fluorescence activated cell sorter (FACS) lysing solution (BD Bioscience), and cell surface staining was performed with the following markers: anti-CD45-VioBlue and anti-CD4 phycoerythrin (PE)-Vio770 (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were then permeabilized with 0·5 ml FACS permeabilizing solution (BD Bioscience), and intracellularly stained with anti-IFN-γ fluorescein isothiocyanate (FITC), anti-TNF allophycocyanin (APC) and anti-IL-2 PE (Miltenyi Biotec). Cells were fixed in 1% paraformaldehyde and analysed within 1 h using a MACSQuant Analyzer flow cytometer (Miltenyi Biotec) after calibration and automatic compensation. We acquired at least 100 000 cells in the lymphocyte gate.
FlowJo Software version 7·6.5 was used to perform Boolean gate analysis of the frequency of the different combinations of IFN-γ, IL-2 and TNF produced by CD4+ T cells. Background cytokine production from the negative control was subtracted from each stimulated condition. Figure 1 shows the gating strategy used to identify CD4+ T cells producing single, double or multiple cytokines. We classed CD4+ T cells producing any of the three cytokines (IFN-γ or IL-2 or TNF) as ‘activated CD4+ T cells’, those producing IFN-γ alone or in combination with IL-2 and/or TNF as ‘total IFN-γ+ CD4+ T cells’, those producing IL-2 alone or in combination with IFN-γ and/or TNF as ‘total IL-2+ CD4+ T cells’ and those producing TNF alone or in combination with IL-2 and/or IFN-γ as ‘total TNF+ CD4+ T cells’. Similarly, we classed double-positive (those simultaneously producing IFN-γ and IL-2) and triple-positive T cells (those producing all three cytokines) as ‘IFN-γ+ IL-2+ CD4+ T cells’ and ‘IFN-γ+ IL-2+ TNF+ CD4+ T cells’, respectively.
Figure 1.
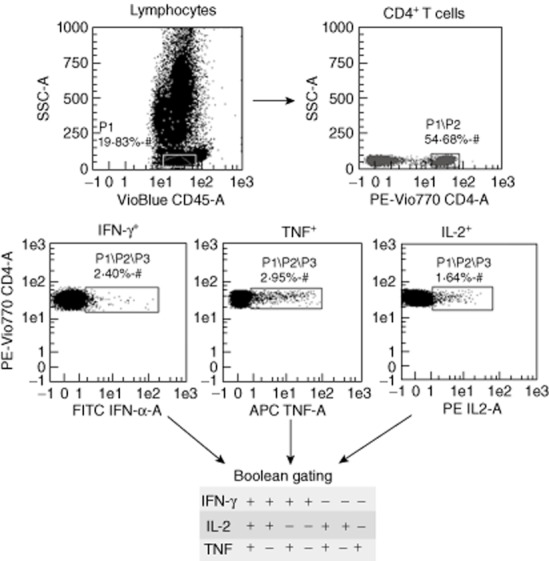
Characterization of distinct population of T cell responses using flow cytometry. Representative flow cytometry plot of cytokine production of CD4+ T cells from patients with latent tuberculosis infection (LTBI). Stimulated CD4+ T cells were categorized into cells expressing interferon (IFN)-γ, interleukin (IL)-2 and tumour necrosis factor (TNF). Boolean gate analysis was used to study the frequency of the seven CD4+ T cell subsets, each expressing one of the possible combinations of IFN-γ, IL-2 and TNF.
Statistical analysis
Non-parametric Kruskal–Wallis analysis of variance (anova) with Dunn's post-test comparison was applied to compare T cell frequencies and the percentage of cytokine-secreting cells between the three groups of patients. All statistical analyses were two-sided, performed using GraphPad Prism Software version 5 (Software MacKiev, Boston, MA, USA), and considered significant at P-values <0·05.
Results
Specific response to M. tuberculosis antigens in QFT-GIT
QFT-GIT was positive in two of the 12 (16·6%) LTBI patients (group A), and in one of the 11 (9·09%) patients with no evidence of LTBI but IFN-γ level fluctuations during follow-up (group B). A negative test was obtained in all 10 patients without LTBI who had persistently negative IFN-γ levels in the follow-up (group C). No QFT-GIT assay yielded indeterminate results.
Specific response to M. tuberculosis antigens in intracellular cytokine flow cytometry
CD4+ T cells producing any of the three cytokines (IFN-γ, IL-2 or TNF)
The first cytokine analysis was performed to detect any activated CD4+ T cells (those producing any of the three cytokines IFN-γ or IL-2 or TNF). These activated CD4+ T cells were detected more frequently in LTBI patients (group A). Indeed, when we compared LTBI patients and those with no evidence of LTBI, we found a significantly higher frequency of activated CD4+ T cells in group A (median 0·78%, range 0–5·36%) than in group C (0·09%, 0–0·82%, P = 0·0421). No significant differences were found between groups A and B. Hence, measuring the frequency of activated CD4+ T cells in response to M. tuberculosis-specific antigens enabled us to distinguish between LTBI patients and non-LTBI patients who did not show IFN-γ fluctuations over time (Fig. 2).
Figure 2.
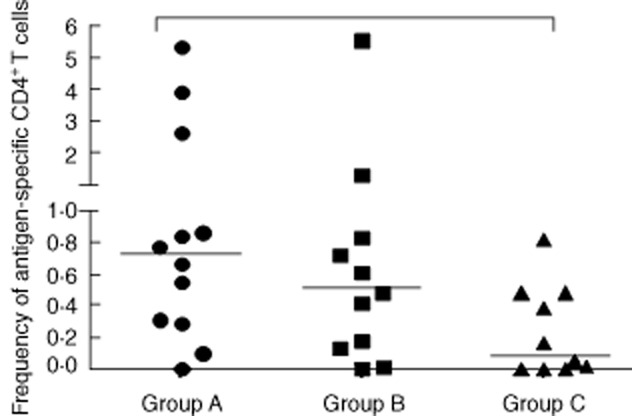
Antigen-specific CD4+ T cells producing any of the cytokines [interferon (IFN)-γ, interleukin (IL)-2 or tumour necrosis factor (TNF)] in 33 patients treated with TNF antagonists. Group A: 12 patients with latent tuberculosis infection (LTBI) who showed IFN-γ-level fluctuations during follow-up. Group B: 11 patients with no evidence of LTBI at baseline but showing IFN-γ-level fluctuations during follow-up. Group C: 10 patients with no evidence of LTBI at baseline and persistently negative IFN-γ levels throughout follow-up. Horizontal bars represent the median values. Statistical analysis was performed using Kruskal–Wallis analysis of variance (anova) with Dunn's post-test.
Analysis of cytokine production by CD4+ T cells at the single-cell level
In another set of experiments we compared the frequency of ‘total IFN-γ+ CD4+ T cells’, ‘total IL-2 +CD4+ T cells’ and ‘total TNF+ CD4+ T cells’ (as defined in Materials and methods) after M. tuberculosis-antigen specific stimulation in our three groups of chronic inflammatory rheumatic disease patients (Fig. 3). This analysis revealed a greater frequency of ‘total IFN-γ+ CD4+ T cells’ in the LTBI group compared to both groups of patients, with no evidence of LTBI at baseline (groups B and C) (P = 0·0149, Kruskal–Wallis test) (Fig. 3a).
Figure 3.
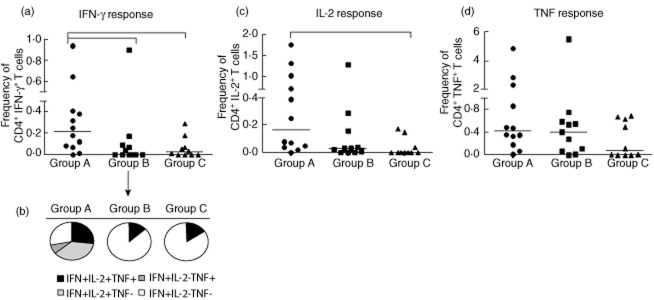
Analysis of production patterns of each cytokine by CD4+ T cells in 33 patients treated with tumour necrosis factor (TNF) antagonists. Group A: 12 patients with latent tuberculosis infection (LTBI) who showed interferon (IFN)-γ level fluctuations during follow-up. Group B: 11 patients with no evidence of LTBI at baseline but showing IFN-γ-level fluctuations during follow-up. Group C: 10 patients with no evidence of LTBI at baseline and persistently negative IFN-γ levels throughout follow-up. (a) Frequency of ‘total IFN-γ+ CD4+ T cells’, i.e. those producing IFN-γ alone or in combination with interleukin (IL)-2 and/or TNF, in the three different groups. (b) Proportion of four combinations of cytokine-producing T cell subsets that contributed to the overall IFN-γ production in response to M. tuberculosis-specific antigens. (c) Frequency of ‘total IL-2+ CD4+ T cells’, i.e. those that produced IL-2 alone or in combination with IFN-γ and/or TNF. (d) Frequency of ‘total TNF+ CD4+ T cells’, those producing TNF alone or in combination with IL-2 and/or IFN-γ. Horizontal bars represent the median values. Statistical analysis was performed using Kruskal–Wallis analysis of variance (anova) with Dunn's post-test.
The cytokine profile illustrated in Fig. 3b reveals that the total IFN-γ production in the LTBI group was yielded mainly by CD4+ T cells that produced two or three cytokines simultaneously, with a predominant IFN-γ+ IL-2+ CD4+ T cell subset (>30%). In contrast, in patients with no LTBI, whether or not their IFN-γ level fluctuated over time (groups B and C), the proportion of cells producing the three cytokines simultaneously was very low, with a strong shift towards cells producing IFN-γ alone (>80% in both of these two groups). Similarly, the frequency of ‘total IL-2+ CD4+ T cells’ was higher in LTBI patients compared to those in groups B and C, although this difference attained statistical significance only versus the latter (P = 0·0076; Fig. 3c).
There were no significant differences in the frequency of ‘total TNF+ CD4+ T cells’ between the three groups of individuals (P = 0·2367; Kruskal–Wallis test), suggesting that this T cell subset was unable to indicate LTBI status (Fig. 3d). However, combined analysis of ‘total IFN-γ+ CD4+ T’ and ‘total IL-2+ CD4+ T cells’ seemed strongly indicative of LTBI status, providing a clear distinction between LTBI and no LTBI at baseline (groups B and C).
Multi-functional cytokine analysis of M. tuberculosis-specific CD4+ T cells
We analysed our samples for all possible combinations of intracellular expression of IFN-γ, IL-2 and TNF (seven distinct functional subsets) in cytokine-producing CD4+ T cells in the three groups of patients. This revealed a significantly greater frequency of the triple-positive IFN-γ+ IL-2+ TNF+ CD4+ T cell subset in LTBI patients (group A) than in LTBI-negative patients showing IFN-γ level fluctuations (group B) (P = 0·0156; Kruskal–Wallis test; Fig. 4a), but no difference with respect to patients with no LTBI and persistently negative IFN-γ levels during follow-up (group C). Most notably, the frequency of the double-positive IFN-γ+ IL-2+ CD4+ T cell subset was significantly higher in LTBI patients compared to the other two groups of patients with no evidence of LTBI at baseline (groups B and C) (P = 0·0065; Kruskal–Wallis test). However, no difference in the frequency of any other double or single cytokine-secreting CD4+ T cell subset was detected between the three groups after antigen stimulation. In particular, we observed that the frequency of TNF single-positive T cells was very high and comparable between all three groups.
Figure 4.

Multi-functional cytokine analysis of Mycobacterium tuberculosis-specific CD4+ T cells in 33 patients treated with tumour necrosis factor (TNF) antagonists. Group A: 12 patients with latent tuberculosis infection (LTBI) who showed interferon (IFN)-γ-level fluctuations during follow-up. Group B: 11 patients with no evidence of LTBI at baseline but showing IFN-γ-level fluctuations during follow-up. Group C: 10 patients with no evidence of LTBI at baseline and persistently negative IFN-γ levels throughout follow-up. (a) Frequency of antigen-specific CD4+ T cells producing all combinations of IFN-γ, IL-2 and TNF in the three different groups. Horizontal bars represent the median values. Statistical analysis was performed using Kruskal–Wallis analysis of variance (anova) with Dunn's post-test. (b) Pie charts representing the relative proportions of cytokine-producing T cell subsets in each group after M. tuberculosis-specific antigen stimulation. A key to colours used in the pie charts is shown at the bottom of (a).
Observing the cytokine profile shown in the pie charts (Fig. 4b), the proportions of the CD4+ T cells producing two or three cytokines simultaneously were most common in the LTBI group than in the other two groups. In particular, in LTBI patients the proportion of IFN-γ+ IL-2+ CD4+ T cells was greater than the other double and triple combinations. In contrast, the subjects without LTBI at baseline but fluctuating IFN-γ levels showed similar proportions of cells producing IFN-γ alone, IL-2 alone and IL-2 in combination with TNF in response to M. tuberculosis-specific antigens (Fig. 4b).
Taking into consideration these ICCFC results, it appears that the CD4+ T cell subsets producing IFN-γ/IL-2/TNF and IFN-γ/IL-2 are the most frequent multiple cytokine-producing cells identified in LTBI patients treated with INH. This intracellular combination of cytokines may enable distinction between LTBI patients and those with no TB infection.
Discussion
The risk of TB reactivation in patients undergoing long-term systemic anti-TNF treatment has been widely documented [1,2], and screening for TB in patients with rheumatic diseases both prior to and during biological therapy is therefore strongly recommended. Indeed, both false negatives and de-novo infection may occur, making surveillance during treatment necessary because, combined with proper management, this can substantially reduce the incidence of active TB [23].
Unfortunately TST, the standard test for TB, has many drawbacks in the monitoring of immunosuppressed patients. Although many of these problems have been effectively overcome by the new class of immunological diagnostic tests, the IGRAs, some pressing issues still remain to be addressed. Indeed, although IGRAs do not affect the immune response of subjects in repeated testing, conversions and reversions of IGRA results have been observed upon repeated tests performed in the same individuals [10–15]. As variability in IFN-γ plasma levels not paralleled by significant clinical outcome in patients undergoing long-term systemic anti-TNF treatment may cause diagnostic confusion, new approaches for investigating the significance of IFN-γ fluctuations during anti-TNF therapy are vital.
Other studies have previously investigated the ability of antigen-specific T cells to produce simultaneously a range of cytokines in human TB [24–33], but contrasting data regarding the distribution of cytokine-producing CD4+ T cell subsets in different stages of TB have been reported. However, it has been suggested that combined analyses of different cytokines produced by multi-functional T cells may aid distinction between active TB patients and subjects with LTBI [30,32].
In this study we used multi-parameter flow cytometry analysis to investigate the functional cytokine profile of specific CD4+ T cells in patients with rheumatic diseases who showed IFN-γ fluctuations during treatment with TNF antagonists. This is the first study to evaluate the performance of ICCFC in such patients. We found that measurement of the frequency of activated CD4+ T cells that produced any of the three cytokines (IFN-γ or IL-2 or TNF) in response to M. tuberculosis-specific antigens did indeed enable distinction between patients with LTBI and those with no evidence of LTBI and persistently negative IFN-γ levels over time. By analysing the frequency of ‘total IFN-γ+ CD4+ T cells’ and the entire functional subset of cytokine-producing CD4+ T cells, we showed that latent infection is associated with an increased frequency of CD4+ T cells producing two or three cytokines simultaneously, with a predominance of those producing IFN-γ in combination with IL-2. Interestingly, LTBI appears to be associated with a paucity of cells producing IFN-γ alone, IL-2 alone and IL-2 in combination with TNF, which were the dominant profiles in patients without LTBI. We found no differences in the frequency of CD4+ T cells producing TNF alone after M. tuberculosis stimulation between the three groups considered.
Our results are similar to those reported by Caccamo et al. [19], in which specific double-positive IFN-γ+ IL-2+ CD4+ T cells were found in individuals with LTBI, but partially in contrast with findings from two studies reporting that a higher proportion of multi-functional CD4+ T lymphocytes which simultaneously produced IFN-γ, IL-2 and TNF was associated with LTBI status [32,34]. However, we considered only a very specific clinical scenario, i.e. patients with chronic inflammatory rheumatic diseases being treated with anti-TNF agents, which may favour the reactivation of LTBI in previously exposed patients [35]. Nevertheless, although our LTBI patients were treated with a 9-month course of INH, their CD4+ T cells were found to produce multiple cytokines (predominantly IFN-γ and IL-2), unlike patients with no LTBI. In other words, our ICCFC approach enabled us to determine the TB status of our immunosuppressed patients.
Seder et al. [36] proposed a linear model of differentiation for CD4+ T cells, in which cells progressively gain functionality with further differentiation until they reach the stage optimized for their effector function (such as the production of IFN-γ, IL-2 and TNF). Continued antigenic stimulation may lead to the progressive loss of memory potential as well as cytokine production (effector memory cells producing IFN-γ and IL-2), resulting in short-lived terminally differentiated CD4+ T cells producing IFN-γ alone. This model may help to explain why our patients with LTBI who received 9 months of INH prophylaxis had a significantly greater proportion of double-positive IFN-γ+ IL-2+ CD4+ T cells compared with other triple, double and single combinations.
Nevertheless, overall, our findings indicate that levels of double-positive IFN-γ+ IL-2+ and triple-positive IFN-γ+ IL-2+ TNF+ CD4+ T cells, as revealed by multi-functional ICCFC analysis, may enable a clear distinction between LTBI patients and those with no evidence of LTBI. According to this approach, patients without LTBI at baseline but with QFT-GIT conversion during follow-up were judged TB-negative, as they showed a similar cytokine profile to patients with no LTBI and persistently negative IFN-γ levels. Indeed, despite high specificity for TB infection, fluctuations of IGRAs not associated with clinical changes have also been shown in other studies involving patients treated with biological agents [10–15]. Although longitudinal studies are still needed to determine the prognostic value of positive response to antigens in the development of active disease, on the basis of our study ICCFC may enable clearer interpretation of the dynamic pattern of IGRAs in these situations. Multi-functional analysis may therefore be a useful means of clarifying TB status when the available diagnostic tests (TST and IGRA) produce discordant findings, or when IFN-γ levels fluctuate with repeated IGRA testing, even in patients undergoing biological therapy.
Acknowledgments
The authors gratefully acknowledge the contributions to this research made by the study participants and staff. In particular, they acknowledge Jessica Brandt and Anna Forster for editing the manuscript. This study was supported in part by grants from the Sapienza University of Rome.
Disclosure
The authors declare no conflicts of interest.
Author contributions
I. S. and R. S. designed the research study. I. S., F. M. and A. E. performed the research and analysed the data. R. S. and G. V. enrolled the subjects. V. V. contributed essential reagents and tools. I. S., R. S.and C. M M. wrote the paper, which was revised by G. V., V. V. and C. M. M.
References
- 1.Gómez-Reino JJ, Carmona L, Rodríguez V, et al. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk. Arthritis Rheum. 2003;48:2122–2127. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 2.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 3.Wallis RS, Broder MS, Wong JY, et al. Granulomatous infection diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261–1265. doi: 10.1086/383317. [DOI] [PubMed] [Google Scholar]
- 4.Winthrop KL, Weinblatt ME, Daley CL. You can't always get what you want, but if you try sometimes (with two tests – TST and IGRA – for tuberculosis) you get what you need. Ann Rheum Dis. 2012;71:1757–1760. doi: 10.1136/annrheumdis-2012-201979. [DOI] [PubMed] [Google Scholar]
- 5.Sauzullo I, Mengoni F, Scrivo R, et al. Evaluation of QuantiFERON-TB Gold In-Tube in human immunodeficiency virus infection and in patient candidates for anti-tumour necrosis factor-alpha treatment. Int J Tuberc Lung Dis. 2010;14:834–840. [PubMed] [Google Scholar]
- 6.Santin M, Muñoz L, Rigau D. Interferon-γ release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLOS ONE. 2012;7:e32482. doi: 10.1371/journal.pone.0032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamdi H, Mariette X, Godot V, et al. Inhibition of anti-tuberculosis T-lymphocyte function with tumour necrosis factor antagonists. Arthritis Res Ther. 2006;8:R114. doi: 10.1186/ar1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DY, Shen GH, Hsieh TY, et al. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum. 2008;59:800–806. doi: 10.1002/art.23705. [DOI] [PubMed] [Google Scholar]
- 9.Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases performance of a Mycobacterium tuberculosis antigen specific IFN-gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 10.Scrivo R, Sauzullo I, Mengoni F, et al. Mycobacterial interferon-γ release variations during longterm treatment with tumor necrosis factor blockers: lack of correlation with clinical outcome. J Rheumatol. 2013;40:157–165. doi: 10.3899/jrheum.120688. [DOI] [PubMed] [Google Scholar]
- 11.Zwerling A, van den Hof S, Scholten J, et al. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 12.Sauzullo I, Mengoni F, Marocco R, et al. IFN-γ release assay for tuberculosis in psoriasis patients treated with TNF antagonists: in vivo and in vitro analysis. Br J Dermatol. 2013;169:1133–1140. doi: 10.1111/bjd.12544. [DOI] [PubMed] [Google Scholar]
- 13.Ringrose JS, Sanche SE, Taylor-Gjevre RM. Detecting latent tuberculosis infection during anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2011;29:790–794. [PubMed] [Google Scholar]
- 14.Garcovich S, Ruggeri A, D'Agostino M, et al. Clinical applicability of Quantiferon-TB-Gold testing in psoriasis patients during long-term anti-TNF-alpha treatment: a prospective, observational study. J Eur Acad Dermatol Venereol. 2012;26:1572–1576. doi: 10.1111/j.1468-3083.2011.04220.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Lee SW, Chung WT, et al. Serial interferon-gamma release assays for the diagnosis of latent tuberculosis infection in patients treated with immunosuppressive agents. Korean J Lab Med. 2011;31:271–278. doi: 10.3343/kjlm.2011.31.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen DY, Shen GH, Chen YM, et al. Biphasic emergence of active tuberculosis in rheumatoid arthritis patients receiving TNF (alpha) inhibitors: the utility of IFN (gamma) assay. Ann Rheum Dis. 2012;71:231–237. doi: 10.1136/annrheumdis-2011-200489. [DOI] [PubMed] [Google Scholar]
- 17.Xie X, Chen JW, Li F, Tian J, Gao JS, Zhang D. A T-cell-based enzyme-linked immunospot assay for tuberculosis screening in Chinese patients with rheumatic diseases receiving infliximab therapy. Clin Exp Med. 2011;11:155–161. doi: 10.1007/s10238-010-0123-4. [DOI] [PubMed] [Google Scholar]
- 18.Klein M, Jarosová K, Forejtová S, et al. Quantiferon TB Gold and tuberculin skin tests for the detection of latent tuberculosis infection in patients treated with tumour necrosis factor alpha blocking agents. Clin Exp Rheumatol. 2013;31:111–117. [PubMed] [Google Scholar]
- 19.Caccamo N, Guggino G, Joosten SA, et al. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 20.Sester U, Fousse M, Dirks J, et al. Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLOS ONE. 2011;6:e17813. doi: 10.1371/journal.pone.0017813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Lee SY, Won DI, et al. Comparison of whole-blood interferon-γ assay and flow cytometry for the detection of tuberculosis infection. J Infect. 2013;66:338–345. doi: 10.1016/j.jinf.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 23.Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–1772. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- 24.Tesfa L, Koch FW, Pankow W, et al. Confirmation of Mycobacterium tuberculosis infection by flow cytometry after ex vivo incubation of peripheral blood T cells with an ESAT-6-derived peptide pool. Cytometry B Clin Cytom. 2004;60:47–53. doi: 10.1002/cyto.b.20007. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AJ, Hutchinson P, Gooding T, et al. Diagnosis of Mycobacterium tuberculosis infection using ESAT-6 and intracellular cytokine cytometry. Clin Exp Immunol. 2005;142:132–139. doi: 10.1111/j.1365-2249.2005.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosmi L, Maggi L, Santarlasci V, et al. Detection by flow cytometry of ESAT-6-and PPD-specific circulating CD4+ T lymphocytes as a diagnostic tool for tuberculosis. Int Arch Allergy Immunol. 2007;143:1–9. doi: 10.1159/000098220. [DOI] [PubMed] [Google Scholar]
- 27.Leung WL, Law KL, Leung VS, et al. Comparison of intracellular cytokine flow cytometry and an enzyme immunoassay for evaluation of cellular immune response to active tuberculosis. Clin Vaccine Immunol. 2009;16:344–351. doi: 10.1128/CVI.00159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won DI, Park JR. Flow cytometric measurements of TB-specific T cells comparing with QuantiFERON-TB gold. Cytometry B Clin Cytom. 2010;78:71–80. doi: 10.1002/cyto.b.20503. [DOI] [PubMed] [Google Scholar]
- 29.Sargentini V, Mariotti S, Carrara S, et al. Cytometric detection of antigen-specific IFNgamma/IL-2 secreting cells in the diagnosis of tuberculosis. BMC Infect Dis. 2009;9:99–109. doi: 10.1186/1471-2334-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streitz M, Fuhrmann S, Powell F, et al. Tuberculin-specific T cells are reduced in active pulmonary tuberculosis compared to LTBI or status post BCG vaccination. J Infect Dis. 2011;203:378–382. doi: 10.1093/infdis/jiq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petruccioli E, Petrone L, Vanini V, et al. IFNγ/TNFα specific-cells and effector memory phenotype associate with active tuberculosis. J Infect. 2013;66:475–486. doi: 10.1016/j.jinf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Harari A, Rozot V, Enders FB, et al. Dominant TNF-alpha Mycobacterium tuberculosis specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Commandeur S, Lin MY, van Meijgaarden KE, et al. Double-and monofunctional CD4+ and CD8+ T-cell responses to Mycobacterium tuberculosis DosR antigens and peptides in long-term latently infected individuals. Eur J Immunol. 2011;41:2925–2936. doi: 10.1002/eji.201141602. [DOI] [PubMed] [Google Scholar]
- 34.Day CL, Mkhwanazi N, Reddy S, et al. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. J Infect Dis. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardam MA, Keystone EC, Menzies R, et al. Anti-tumor necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis. 2003;3:148–155. doi: 10.1016/s1473-3099(03)00545-0. [DOI] [PubMed] [Google Scholar]
- 36.Seder RA, Darra PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]


