Abstract
Transient receptor potential (TRP) channels are important mediators of sensory signals with marked effects on cellular functions and signalling pathways. Indeed, mutations in genes encoding TRP channels are the cause of several inherited diseases in humans (the so-called ‘TRP channelopathies’) that affect the cardiovascular, renal, skeletal and nervous systems. TRP channels are also promising targets for drug discovery. The initial focus of research was on TRP channels that are expressed on nociceptive neurons. Indeed, a number of potent, small-molecule TRPV1, TRPV3 and TRPA1 antagonists have already entered clinical trials as novel analgesic agents. There has been a recent upsurge in the amount of work that expands TRP channel drug discovery efforts into new disease areas such as asthma, cancer, anxiety, cardiac hypertrophy, as well as obesity and metabolic disorders. A better understanding of TRP channel functions in health and disease should lead to the discovery of first-in-class drugs for these intractable diseases. With this review, we hope to capture the current state of this rapidly expanding and changing field.
LINKED ARTICLES
This article is part of a themed section on the pharmacology of TRP channels. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-10
Keywords: TRP channels, TRPV1, TRPV3, TRPV4, TRPA1, TRPM8, TRPCs, pain, respiratory disorders, bladder disorders, cancer
Introduction
Regulated transport of ions via ion channels underpins a number of fundamental physiological functions (Bagal et al., 2013). Conversely, inherited (‘channelopathy’) or acquired dysfunction of these channels disrupts physiological processes, leading to a broad array of disorders (Bagal et al., 2013). Ion channels are important targets for many currently prescribed drugs, second only to GPCRs (Clare, 2010). Indeed, the worldwide sales of ion channel drugs are estimated to be in excess of $12 billion annually. Although ion channels have been successful drug targets, achieving subtype-selectivity has been a major challenge, particularly with voltage-gated sodium and calcium channels (Clare, 2010).
Recently, a number of novel, ‘druggable’ ion channels have been identified. Of these newly discovered channels, those of the transient receptor potential family [TRP; Figure 1 (Nilius and Owsianik, 2011); channel and receptor nomenclature follows Alexander et al., 2013] are arguably the most appealing therapeutic targets (see Moran et al., 2011; Fernandes et al., 2012; Vay et al., 2012; Kaneko and Szallasi, 2013). Generally speaking, TRP channels are cellular sensors involved in nociception (Patapoutian et al., 2009), taste perception (Nilius and Appendino, 2013), thermosensation (Tominaga, 2007), mechano-and osmolarity sensing (Pedersen and Nilius, 2007; Guilak et al., 2010). TRP channels also play a crucial role in normal physiological processes such as signal transmission (Minke, 2010; Wu et al., 2010). Dysfunction of TRP channels has been implicated in various disease states (summarized in Figure 2) ranging from chronic pain and overactive bladder (TRPV1) through obesity (TRPV4 and TRPM5), diabetes (TRPV1, TRPM4), chronic cough (TRPA1, TRPV1), and chronic obstructive pulmonary disease (COPD; TRPV4) to cardiac hypertrophy (TRPC6), familial Alzheimer's disease (TRPM7), dermatological disorders (TRPV3 in Olmsted syndrome) and cancer (TRPC6, TRPV2 and TRPM8). Gain-of-function mutations in genes encoding TRP channels have been linked to human diseases as exemplified by familial episodic pain syndrome (TRPA1; Kremeyer et al., 2010). Taming these hyperactive TRP channels by antagonists may prove clinically beneficial. Loss-of-function mutations (e.g. loss of TRPML1 function in type-IV mucolipidosis) are also pathogenic, but their correction is more problematic (Dong et al., 2008).
Figure 1.

Simplified topographical structure of TRP channels (A). Please note the similarities and differences between TRP channel subfamilies (B). Reprinted, with permission, from Nilius and Owsianik (2011).
Figure 2.
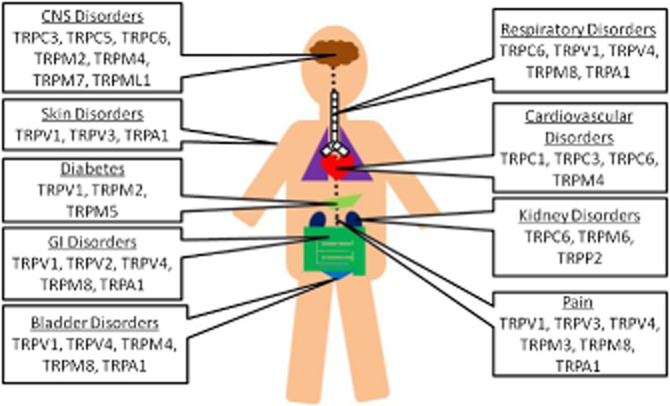
Schematic illustration of the tissue-distribution of TRP channels and their putative roles in the pathogenesis of human disease.
TRP channels are also primary targets for a number of natural products with therapeutic potential (summarized in Table 1). For instance, TRPV1 is highly expressed in a distinct population of sensory neurons where it mediates excitation and subsequent desensitization to capsaicin (see Figure 3 for structure) and its ultrapotent analogue, resiniferatoxin (Szallasi and Blumberg, 1999). At present, resiniferatoxin (Figure 3) is undergoing clinical trials (NCT00804154) as a ‘molecular scalpel’ to achieve permanent analgesia in patients with intractable cancer pain (Iadarola and Mannes, 2011; Iadarola and Gonnella, 2013). TRPM8 is the ‘menthol receptor’ (Knowlton and McKemy, 2011) and TRPC6 is believed to mediate the mood-improving effect of hyperforin, the main ingredient in St. John's Wort (Leuner et al., 2007).
Table 1.
Endogenous and exogenous ligands of TRP channels
| TRP channels | Ligands | References |
|---|---|---|
| TRPV1 | Endogenous agonists: | |
| Anandamide*2009 | Zygmunt et al. (1999) | |
| N-arachidonoyldopamine | Huang et al. (2002) | |
| N-oleoyldopamine | Chu et al. (2003) | |
| 12-and 15-hydroperoxyeicosatetraenoic acid, 5-and 15-hydroxyeicosatetraenoic acid, Leukotriene B4 | Hwang et al. (2000) | |
| 9-and 13-hydroxy-octadecadienoic acid(ODE), 9 and 13-oxoODE | Patwardhan et al. (2009) | |
| Oleoylethanolamide | Ahern (2003) | |
| Palmitoylethanolamide*2009 | Ambrosino et al. (2013) | |
| Lysophosphatidic acid | Nieto-Posadas et al. (2011b) | |
| Endogenous antagonists: | ||
| Resolvin D2 | Park et al. (2011a) | |
| Exogenous agonists | ||
| 2-Aminoethoxydiphenyl borate (2-APB) | Hu et al. (2004) | |
| Ornithoctonus huwena toxin [‘double-knot’ toxin (DkTx)] | Bohlen et al. (2010) | |
| Capsaicin*2009 | Caterina et al. (1997) | |
| Piperine | McNamara et al. (2005) | |
| Resiniferatoxin*2009 | Szallasi and Blumberg (1989) | |
| Gingerol | Liu et al. (2000) | |
| Evodiamine | Pearce et al. (2004) | |
| Cannabidiol*2009 | Bisogno et al. (2001) | |
| Cannabigerol | De Petrocellis et al. (2011) | |
| Polygodial | Andrè et al. (2006) | |
| Vanillotoxin | Siemens et al. (2006) | |
| Exogenous antagonists | ||
| Capsazepine | Dickenson and Dray (1991) | |
| Iodo-resiniferatoxin | Seabrook et al. (2002) | |
| BCTC | Valenzano et al. (2003) | |
| Thapsigargin | Toth et al. (2002) | |
| Yohimbine | Dessaint et al. (2004) | |
| AG489, AG505 | Kitaguchi and Swartz (2005) | |
| ABT-102*2009, AMG-517*2009, AZD-1386*2009, DWP-05195, GRC-6211*2009, JTS-653*2009, MK-2295, PHE377, SB-705498*2009 | Moran et al. (2011); Brederson et al. (2013) | |
| TRPV2 | Endogenous agonists: | |
| Lysophosphatidylcholine, Lysophosphatidylinositol | Monet et al. (2009) | |
| Exogenous agonists | ||
| Cannabidiol*2009, Δ9-tetrahydrocannabinol cannabinol*2009 | Qin et al. (2008) | |
| 2-APB | Hu et al. (2004) | |
| Probenecid | Bang et al. (2007) | |
| Exogenous antagonists | ||
| Tranilast | Hisanaga et al. (2009) | |
| TRPV3 | Endogenous agonist: | |
| Farnesyl pyrophosphate | Bang et al. (2010a) | |
| Endogenous antagonists: | ||
| Isopentenyl pyrophosphate | Bang et al. (2011) | |
| Resolvin D1 | Bang et al. (2010b) | |
| Exogenous agonists | ||
| Camphor | Moqrich et al. 2005) | |
| Menthol | Macpherson et al. 2006) | |
| Eugenol, thymol, carvacrol | Xu et al. (2006a) | |
| 6-t-butyl-m-cresol, dihydrocarveol, (+)-borneol | Vogt-Eisele et al. (2007) | |
| 2-APB | Hu et al. (2004) | |
| Incensole acetate | Moussaieff et al. (2008) | |
| Exogenous antagonist: | ||
| GRC15300 | Khairatkar-Joshi et al. (2010) | |
| TRPV4 | Endogenous agonists: | |
| Citric acid | Suzuki et al. (2003) | |
| 5,6-and 8,9-epoxyeicosatrienoic acid | Watanabe et al. (2003) | |
| Dimethylallyl pyrophosphate | Bang et al. (2012) | |
| Endogenous antagonist: | ||
| Resolvin D1 | Bang et al. (2010b) | |
| Exogenous agonists: | ||
| 4α-Phorbol 12, 13-dedecanoate | Klausen et al. (2009) | |
| Bisandrographolide | Smith et al. (2006) | |
| Apigenin | Ma et al. (2012a) | |
| GSK1016790A*2009 | Thorneloe et al. (2008) | |
| RN-1747 | Vincent et al. (2009) | |
| Exogenous antagonists: | ||
| HC-067047*2009 | Everaerts et al. (2010a) | |
| RN-1734 | Vincent et al. (2009) | |
| GSK2193874 | Huh et al. (2012; Thorneloe et al. (2012) | |
| TRPV6 | Exogenous antagonist: | |
| 2-APB | Kovacs et al. (2012) | |
| TRPC3 | Exogenous antagonists: | |
| Pyr3*2009 | Kiyonaka et al. (2009) | |
| Pyr10 | Schleifer et al. (2012) | |
| TRPC4 | Exogenous antagonist: | |
| ML204 | Miller et al. (2011) | |
| TRPC5 | Endogenous agonist: | |
| Lysophosphatidylcholine | Flemming et al. (2006) | |
| Sphingosine-1-phosphate | Xu et al. (2006b) | |
| Exogenous agonist: | ||
| Rosiglitazone | Majeed et al. (2011a) | |
| Progesterone and neurosteroids | Majeed et al. (2011b) | |
| TRPC6 | Endogenous agonist: | |
| 20-Hydroxyeicosatetraenoic acid | Basora et al. (2003) | |
| Exogenous agonists: | ||
| Hyperforin | Leuner et al. (2007) | |
| 2,4-Diacylphloroglucinol | Leuner et al. (2010) | |
| Exogenous antagonist: | ||
| GsMTx-4 | Spassova et al. (2006) | |
| TRPM2 | Endogenous agonists: | |
| ADP-ribose | Perraud et al. (2005) | |
| Cyclic ADP-ribose | Kolisek et al. (2005) | |
| Exogenous antagonists: | ||
| N-(p-amylcinnamoyl)anthranilic acid | Kraft et al. (2006) | |
| Clotrimazole, econazole | Hill et al. (2004a) | |
| 2-APB | Togashi et al. (2008) | |
| Flufenamic acid | Hill et al. (2004b; Naziroğlu et al. (2007) | |
| TRPM3 | Endogenous agonists: | |
| Pregnenolone sulphate | Wagner et al. (2008) | |
| D-erythro-sphingosine | Grimm et al. (2005) | |
| Endogenous antagonist: | ||
| Progesterone | Majeed et al. (2012) | |
| Exogenous antagonist: | ||
| Rosiglitazone | Majeed et al. (2011a) | |
| Mefenamic acid | Klose et al. (2011) | |
| Naringenin, hesperetin, ononetin, eriodictyol | Straub et al. (2013) | |
| TM3E3 (polyclonal antibody) | Naylor et al. (2008) | |
| TRPM4 | Exogenous agonist: | |
| BTP2 | Takezawa et al. (2006) | |
| Exogenous antagonist: | ||
| 9-Phenanthrol | Grand et al. (2008) | |
| TRPM5 | Exogenous antagonist: | |
| Triphenylphosphine oxide | Palmer et al. (2010) | |
| TRPM6 | Exogenous antagonist: | |
| 2-APB | Li et al. (2006) | |
| TRPM7 | Endogenous antagonist: | |
| Sphingosine | Qin et al. (2013) | |
| Exogenous antagonists: | ||
| 2-APB*2009 | Li et al. (2006) | |
| Carvacrol | Parnas et al. (2009) | |
| Nafamostat mesilate (dependent on extracellular divalent ions) | Chen et al. (2010) | |
| NDGA, AA861, MK886 | Chen et al. (2010) | |
| Waixenicin A | Zierler et al. (2011) | |
| FTY720 | Qin et al. (2013) | |
| Quinine, CyPPA, dequalinium, NS8593, SKA31, UCL 1684 | Chubanov et al. (2012) | |
| TRPM8 | Exogenous agonists: | |
| Menthol | Peier et al. (2002a) | |
| Linalool, geraniol, hydroxycitronellal, WS-3, WS-23, FrescolatMGA, FrescolatML, PMD38, CoolactP, Cooling Agent 10 | Behrendt et al. (2004) | |
| Cis-and trans-p-menthane3 | Bandell et al. (2004) | |
| CPS-368 | Sherkheli et al. (2010) | |
| Exogenous antagonists: | ||
| AMTB | Lashinger et al. (2008) | |
| BCTC | Behrendt et al. (2004) | |
| Benzimidazoles | Parks et al. (2011); Calvo et al. (2012) | |
| 5-Benzyloxytryptamine | DeFalco et al. (2010) | |
| Compound 9l | Matthews et al. (2012) | |
| Tetrahydroisoquinoline 87 | Tamayo et al. (2012) | |
| Arylglycine derivatives | Zhu et al. (2013) | |
| TRPA1 | Endogenous agonists: | |
| 15-Deoxy-Δ12,14-PGJ2, | Materazzi et al. (2008;) | |
| 8-Iso-PGA2, PGA2, Δ12-PGJ2 | Taylor-Clark et al. (2008a) | |
| 4-Hydroxynonenal | Trevisani et al. (2007) | |
| 4-Oxononenal | Taylor-Clark et al. (2008b) | |
| Methylglyoxal | Ohkawara et al. (2012) | |
| Endogenous antagonists: | ||
| Resolvin D1 Resolvin D2 | Bang et al. (2010b); Park et al. (2011a) | |
| Exogenous agonists: | ||
| Cinnamaldehyde, methyl salicylate, eugenol, gingerol | Bandell et al. (2004) | |
| Allicin, diallyl disulfide | Bautista et al. (2005) | |
| Δ9-tetrahydrocannabinol, Isothiocyanates | Jordt et al. (2004) | |
| Acrolein | Bautista et al. (2006) | |
| Carvacrol | Xu et al. (2006a) | |
| Formalin | McNamara et al. (2007) | |
| α,β-Unsaturated aldehydes | Andrè et al. (2008) | |
| Auranofin | Hatano et al. (2013) | |
| Capsiate | Shintaku et al. (2012) | |
| Curcumin | Leamy et al. (2011) | |
| PF-4840154 | Ryckmans et al. (2011) | |
| Apomorphine (agonist in low micromolar range and antagonist in higher concentration) | Schulze et al. (2013) | |
| Cannabichromene, cannabidiol, cannabinol*2009 | De Petrocellis et al. (2008; 2011) | |
| Exogenous antagonists: | ||
| Camphor | Xu et al. (2005) | |
| Menthol | Macpherson et al. (2006) | |
| Thymol | Lee et al. (2008) | |
| HC-030031 | McNamara et al. (2007) | |
| Chembridge-5861528 | Wei et al. (2009) | |
| AP18 | Petrus et al. (2007) | |
| A-967079 | McGaraughty et al. (2010) | |
| AZ465 | Nyman et al. (2013) | |
| GRC17536 | Kaneko and Szallasi (2013) | |
| TRPML1 | Exogenous agonists: | |
| SF-51, ML-SA1 | Shen et al. (2012) | |
| TRPML2 | Exogenous agonists: | |
| SID24801657, SID24787221 | Saldanha et al. (2010–2009) | |
| TRPML3 | Exogenous agonists: | |
| SID24801657, SID24787221 | Saldanha et al. (2010–2009) |
Figure 3.

Selected TRPV1 agonists: capsaicin (the pungent ingredient in hot chili peppers), resiniferatoxin (isolated from the cactus-like perennial E. resinifera), and the endocannabinoids anandamide and palmitoylethanolamide.
Few generalizations can be made about TRP channels. Some show a highly restricted tissue expression pattern (TRPA1 and TRPV1 are predominantly expressed in sensory neurons; Patapoutian et al., 2009) whereas others (TRPCs) are rather ubiquitously expressed (Singh et al., 2012). Because members of the TRP family of channels (Figure 1) share much less homology with one another compared with other ion channel families (Wu et al., 2010), the identification of highly subtype-selective compounds is likely to be more attainable.
Despite the striking progress in our understanding of TRP channel functions (see Owsianik et al., 2006; Wu et al., 2010; Li et al., 2011a), some inherent problems persist. According to Bernd Nilius, TRP channels have a fair and an ugly face (Nilius, 2013). Activation or inhibition of a TRP channel may be beneficial in one organ and, at the same time, may induce unacceptable adverse effects in another. Indeed, the clinical development of first-generation TRPV1 antagonists was halted because they caused hyperthermia and put patients at risk for scalding injuries by elevating the heat pain threshold (see Moran et al., 2011; Brederson et al., 2013). Drug discovery companies that find a way for exploiting the ‘fair face’ of TRP channels without revealing the ‘ugly face’ will be able to create a new generation of targeted therapies.
TRP channels: a brief overview
TRP channels were initially discovered in a blind strain of Drosophila (Montell and Rubin, 1989). When exposed to prolonged intense light, these spontaneously mutant fruit flies showed transient calcium influx into their photoreceptor cells; this is why the mutant gene was termed trp, ‘transient receptor potential’. This seminal finding paved the way to the discovery of the first mammalian TRP channels, called ‘canonical’ (TRPC) due to their homology to the Drosophila channel (Wes et al., 1995; Zhu et al., 1995).
Mammalian TRP channels comprise 28 members and are divided into six subfamilies: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPP (Polycystin), TRPML (Mucolipin) and TRPA (Ankyrin) based on their homology of amino acid sequences (Figure 1; Clapham et al., 2001; Wu et al., 2010; Nelson et al., 2011). The mucolipin and polycystin subfamilies were named after the diseases they are associated with, mucolipidosis and autosomal dominant polycystic kidney disease (ADPKD) respectively. The vanilloid subfamily was named after its founding member, the vanilloid (capsaicin) receptor TRPV1. The first melastatin channel (TRPM1) was discovered as a protein present in benign nevi and absent in malignant melanoma (Duncan et al., 1998). As of today, the ankyrin subfamily has only one member, TRPA1, which (as the name implies) is rich in ankyrin repeats at its N-terminus.
As a general rule, TRP channels have six transmembrane spanning domains (S1–S6) with a pore-forming loop between S5 and S6 (Figure 1; Wu et al., 2010). Both –NH2 and –COOH termini are located intracellularly. Many TRP channels are non-selective Ca2+-permeable channels with permeability ratios PCa/PNa < 10. TRPM4 and TRPM5, in particular, are only permeable to monovalent cations and they do not conduct Ca2+ and Mg2+, while TRPV5 and TRPV6 are highly Ca2+ selective with PCa/PNa > 100 (Owsianik et al., 2006). Most TRPs form functional channels as homotetramers, but heteromultimerization is frequently observed (Cheng et al., 2010). This creates a potential problem for drug discovery efforts as heteromultimers (that are not easily recreated in heterologous expression systems) may have distinct pharmacological properties.
TRP channels are ‘cellular sensors’ (Clapham, 2003) that respond to changes in the cellular environment, including temperature, stretch/pressure, chemicals, oxidation/reduction, osmolarity and pH, both acidic and alkaline (Moran et al., 2011; Nieto-Posadas et al., 2011a). Of note, a number of TRP channels are also activated by natural products, including herbs, spices, venoms and toxins (Vriens et al., 2008). For example, TRPV1 is a shared target for capsaicin (Caterina et al., 1997), jelly fish venoms and spider (tarantula) toxins (Cromer and McIntyre, 2008). A list of ligands for TRP channels is provided in Table 1. Representative chemical structures of TRP channel agonists (GSK101679A for TRPV4) and antagonists (GRC6211, AMG517, SB-705498, AZD1386, JTS-653 and ABT-102 for TRPV1; HC-067047 for TRPV4; Pyr3 for TRPC3; and AMTB for TRPM8) are shown in Figures 4 and 5. Despite decades of intensive search, only a few endogenous ligands of TRP channels have been identified, such as the endocannabinoids anandamide and palmitoylethanolamide, (structures shown in Figure 3) for TRPV1 (Zygmunt et al., 1999; Ambrosino et al., 2013)]. How TRP channels are modulated in vivo is still unknown.
Figure 4.

Small molecule TRPV1 antagonists: selected structures.
Figure 5.
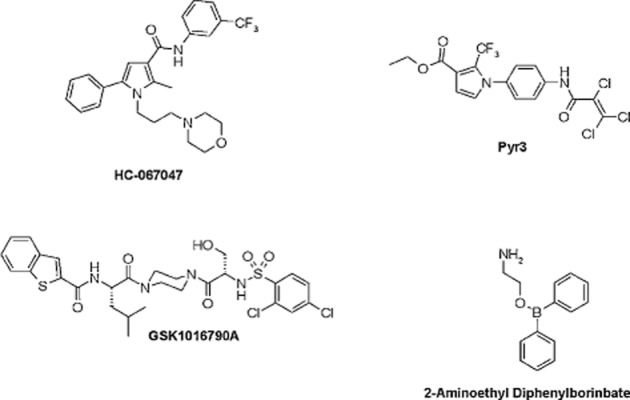
Representative examples of TRPV4 agonists (GSK1016790A), TRPV4 antagonists (HC-067047), TRPV3 agonists (2-ABT), and TRPC3 inhibitors (Pyr3).
Some TRPM channels like TRPM2 are unique in that they contain a functional nucleoside diphosphate linked to some other moiety/ADP ribose domain, as well as a kinase domain that bears some resemblance to PKA (see Eisfeld and Lückhoff, 2007). In other words, these TRPMs combine features of ion channels and enzymes and are thus referred to by some as ‘chanzymes’ (Montell, 2003). TRPM2 functions as a cellular redox (oxidative stress) sensor and has been implicated in the pathogenesis of bipolar disorder, diabetes, as well as cardiovascular and neurodegenerative disorders (Jiang et al., 2010). Indeed, a mutant TRPM2 (Pro1018Leu) has been linked to the Guamanian amyotrophic lateral sclerosis (ALS-G)/Parkinsonism-dementia complex (Hermosura et al., 2008).
Importantly, TRP channels are also stimulated by intracellular Ca2+ increase induced by the activation of GPCRs and mediate downstream signalling. Furthermore, the activity of TRP channels is modulated by various intracellular molecules including phosphatidylinositol 4,5-bisphosphate (PIP2), DAG, ATP and calmodulin (see Wu et al., 2010). Indeed, TRPCs are subdivided into two groups, TRPC1, C4 and C5 and TRPC3, C6, C7, depending on DAG-sensitivity (Wu et al., 2010). For some TRP channels, like TRPV1, phosphorylation by PKs and dephosphorylation by phosphatases provide important contribution to setting the channel activity (reviewed in Szallasi et al., 2007). Indeed, phosphorylation of TRPV1 by PKC is now thought to represent a crucial biochemical mechanism by which TRPV1 is sensitized during inflammation to cause thermal hyperalgesia (Jeske et al., 2009). Moreover, TRP channels were shown to interact with a growing number of intracellular proteins to form ‘signalplexes’ and ‘channelosomes’ (Planells-Cases and Ferrer-Montiel, 2007). Such interactions are now believed to be important for TRP channel trafficking, positioning and activity (Goswami, 2012). Recent research suggests that manipulation of the interaction between TRP channels and their regulatory proteins can be exploited for therapeutic purposes. A promising example of this approach, to prevent TRPV1 sensitization by blocking the interplay between TRPV1 and the scaffolding protein AKAP79 (Btesh et al., 2013; Fischer et al., 2013), will be discussed later under ‘pain and TRP channels’.
Activation of TRP channels allows cations pass through the membrane and depolarize cells, leading to a wide range of cellular responses. Stimulated by a broad range of stimuli and expressed probably in all the cells in the body (Nilius, 2013), TRP channels are thought to play diverse physiological roles. Besides, extensive research in the field has demonstrated that TRP channels are involved in a number of diseases affecting the peripheral and CNS (Vennekens et al., 2012; Morelli et al., 2013), the respiratory (Preti et al., 2012), genito-urinary (Skryma et al., 2011), gastrointestinal (GI; Holzer, 2011), cardiovascular (Watanabe et al., 2013) and immune systems (Schwartz et al., 2007; Smith and Nilius, 2013), as well as in metabolic disorders including obesity and diabetes (Suri and Szallasi, 2008; Zhu et al., 2011).
The direct link between TRP channels and human diseases have been revealed by human genetic studies demonstrating that mutations in TRP genes are causally associated with hereditary diseases, the so-called ‘TRP channelopathies’ (Nilius and Owsianik, 2010). Representative examples of these channelopathies include focal segmental glomerular sclerosis (FSGS), ADPKD and scapuloperoneal spinal muscular atrophy, which are linked to TRPC6 (Winn et al., 2005), TRPP2 (Igarashi and Somlo, 2002) and TRPV4 (Auer-Grumbach et al., 2010) respectively. Knockout and transgenic animal studies also revealed a pathogenic role for both the absence and hyperactivity of TRP channels. For example, TRPC3 (−/−) mice show defects in motor coordination and walking behaviour (Hartmann et al., 2008) whereas transgenic mice overexpressing TRPC6 in their heart develop massive cardiac hypertrophy (Kuwahara et al., 2006).
In summary, there is strong experimental and clinical evidence to substantiate TRP channels as appealing drug targets and a number of molecules targeting TRP channels have already advanced to clinical trials (Moran et al., 2011; Brederson et al., 2013; Table 2). Later, we provide an overview of TRP channels and diseases and discuss potential approaches for therapeutic intervention.
Table 2.
Drugs targeting TRP channels in clinical development
| Action | Drug | Company | Therapy Area | Highest development status | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| TRPV1 agonist | capsaicin | Not Assigned | Pain | Launched | |
| TRPV1 agonist | NGX-4010 | Acorda Therapeutics Inc/Astellas Pharma Inc | Postherpetic neuralgia | Launched | |
| TRPV1 agonist | zucapsaicin | Sanofi-Aventis Canada Inc | Osteoarthritis | Registered | |
| TRPV1 agonist | zucapsaicin | Winston Pharmaceuticals Inc | Cluster headache | Phase 3 | NCT00033839 |
| TRPV1 agonist | MCP-101 (resiniferatoxin) | Mt Cook Pharma | Overactive bladder | Phase 2 | N/A |
| TRPV1 antagonist | DWP-05195 | Daewoong Pharmaceutical Co Ltd | Neuropathic pain | Phase 2 | NCT01557010 |
| TRPV1 antagonist | XEN-D0501 | Provesica Ltd | Overactive bladder | Phase 2 | N/A |
| TRPV1 siRNA | SYL-1001 | Sylentis Sau | Ocular pain | Phase 2 | NCT01776658 |
| TRPV1 antagonist | Mavatrep | Johnson & Johnson Pharmaceutical Research & Development LLC | Osteoarthritis/Pain | Phase 1 | NCT00933582 |
| NCT01006304 | |||||
| TRPV1 antagonist | PHE-377 | PharmEste SRL | Neuropathic pain | Phase 1 | N/A |
| TRPV1 antagonist | MR-1817 | Mochida Pharmaceutical Co Ltd | Pain | Phase 1 | NCT00960180 |
| TRPV1 antagonist | PAC-14028 | Pacific Pharmaceuticals Co Ltd | Atopic dermatitis/IBD | Phase 1 | NCT01638117 |
| TRPV1 antagonist | SB-705498 | GlaxoSmithKline plc | Pruritus | Phase 1 | NCT01673529 |
| TRPV3 antagonist | GRC-15300 | Glenmark Pharmaceuticals Ltd/Sanofi | Neuropathic pain/Osteoarthritis | Phase 2 | NCT01463397 |
| TRPM8 agonist | Menthol | Not assigned | Carpal tunnel syndrome/Neck pain | N/A | NCT01716767 |
| NCT01542827 | |||||
| TRPM8 agonist | D-3263 | Dendreon Corp | Cancer | Phase 1 | NCT00839631 |
| TRPA1 antagonist | GRC-17536 | Glenmark Pharmaceuticals Ltd | Diabetic peripheral neuropathy/Respiratory disorders | Phase 2 | NCT01726413 |
| TRPA1 antagonist | CB-625 | Cubist Pharmaceuticals/Hydra Biosciences | Inflammatory disease/ Pain | Phase 1 | N/A |
Pain and TRP channels
A number of TRP channels (including TRPV1, V3 and V4, TRPA1, TRPM3 and M8, TRPC1, C3 and C6) are expressed in nociceptive sensory neurons. Extensive research with genetically modified animals and pharmacological agents has confirmed that these TRP channels are involved in the generation and transduction of pain and thus represent promising targets for the development of novel analgesic agents (see Patapoutian et al., 2009; Moran et al., 2011; Brederson et al., 2013).
TRPV1
A subset of nociceptive neurons with somata in sensory (dorsal root and trigeminal) ganglia is distinguished by its unique sensitivity to capsaicin (Szallasi and Blumberg, 1999). The initial excitation by capsaicin of these neurons is followed by a lasting refractory state (traditionally referred to as desensitization) in which the cells are unresponsive not only to a repeated capsaicin challenge, but also to various unrelated stimuli like noxious heat and acids (see Szallasi and Blumberg, 1999). Thus, desensitization by capsaicin has a clear therapeutic potential. The receptor for capsaicin was identified as TRPV1 (Caterina et al., 1997) and accumulating evidence suggests a crucial role for TRPV1 in pain sensation (see Szallasi et al., 2007; Gomtsyan and Faltynek, 2010). First, TRPV1 is activated by multiple painful stimuli including noxious heat, pungent chemicals (capsaicin and jelly fish venom), and protons (Szallasi et al., 2007). In addition, TRPV1 can be activated by voltage, lipids and phosphorylation (Pingle et al., 2007). Second, TRPV1-deficient mice show reduced thermal hyperalgesia in response to inflammatory mediators such as bradykinin and/or NGF (Caterina et al., 2000; Davis et al., 2000; Chuang et al., 2001). In addition, oleoylethanolamide, an endogenous TRPV1 agonist (Ahern, 2003), induces visceral pain-related behaviour in mice that is inhibited by the TRPV1 antagonist capsazepine and is absent in TRPV1-null animals (Wang et al., 2005). Third, pharmacological blockade or knockdown of TRPV1 displays analgesic activity in various preclinical pain models, including arthritic (Szabó et al., 2005; Joshi and Honore, 2010) and cancer pain (Jimenez-Andrade and Mantyh, 2010).
Somewhat unexpectedly, genotyping studies have so far failed to identify any TRPV1 polymorphism associated with neuropathic pain, although TRPV1 variants were correlated with altered somatosensory function in patients with neuropathic pain (Binder et al., 2010). Parenthetically, the TRPV1 585 Ile-Ile genotype appears to lower the risk for developing painful knee osteoarthritis (Valdes et al., 2011). But not all TRPV1 variants are harmless. For example, in a large European study, six TRPV1 gene SNPs appeared to confer higher risk for chronic cough [Smit et al., 2012; although, in a different study, the loss-of-function TRPV1 variant I585V was associated with a lower risk for childhood asthma (Cantero-Recasens et al., 2010).
Capsaicin-containing creams (e.g. Zostrix, 0.075%) have been used for decades for the treatment of chronic painful conditions such as diabetic neuropathy (Knotkova et al., 2008). Despite their popularity, controlled clinical studies found no evidence that these creams had greater analgesic potency than placebo (see Szallasi and Sheta, 2012).
To increase the exposure of cutaneous nerve endings to capsaicin, occlusive patches (NGX-4010, Qutenza) and liquid formulations (NGX-1998, 20% capsaicin) were developed by Neuroges-X (San Mateo, CA, USA; Bley, 2012). Although in 2010, Qutenza was approved to treat post-herpetic neuralgia in the USA, the sales of this $700 pain patch never matched the company's expectations. In 2012, after the US Food and Drug Administration rejected Neuroges-X's request to extend the use of Qutenza to HIV-associated peripheral neuropathy, the company ceased operations and reached a tentative deal to sell Qutenza and the investigational liquid formulation NGX-1998 to Acorda (Ardsley, NY, USA; http://www.researchviews.com/healthcare/pharma/DealReports.aspx?sector=Pharma&DealID=191932).
Resiniferatoxin is currently undergoing clinical trials at the National Cancer Institute in patients with intractable cancer pain as a ‘molecular scalpel’ to achieve permanent analgesia (NCT00804154). In preclinical models of chronic pain, intrathecal resiniferatoxin induces a lasting analgesic effect by selectively ablating TRPV1-expressing sensory neurons in the dorsal root and trigeminal ganglia (Iadarola and Gonnella, 2013). In client-owned dogs with severe osteosarcoma pain, intrathecal resiniferatoxin was well-tolerated and effective: it provided significant pain relief and restored ambulation for several months after a single administration (Brown et al., 2005; Iadarola and Gonnella, 2013). It is hoped that intrathecal resiniferatoxin will be a good alternative to narcotic analgesics in some cancer patients with localized pain, such as pain caused by bone metastasis.
After the cloning of TRPV1, there was a great deal of enthusiasm in the pharmaceutical industry to develop small-molecule TRPV1 antagonists as analgesic agents. Indeed, a number of TRPV1 antagonists including SB-705498 (GlaxoSmithKline), AMG517 (Amgen), AZD1386 (AstraZeneca), GRC-6211 (Lilly/Glenmark), MK-2295 (Merck/Neurogen), ABT-102 (Abbott) and PHE377 (PharmEste) have been advanced to Phase I and II clinical studies for indications related to pain (representative structures are shown in Figure 4; Moran et al., 2011; Brederson et al., 2013). The enthusiasm, however, was soon tempered by unforeseen adverse effects. Some TRPV1 antagonists (AMG517) caused marked hyperthermia, prompting their withdrawal from the clinical trials, whereas others (MK-2295) blunted noxious heat perception, putting patients at risk for scalding injuries (Moran et al., 2011; Brederson et al., 2013).
The magnitude of hyperthermia seems to vary depending on the chemical structure. While AZD1386 modestly increased body temperature (∼0.4 °C on average) in patients with gastroesophageal reflux disease (GERD; Krarup et al., 2011), AMG517 caused a lasting (1–4 days) and marked hyperthermia response (up to 40.2°C) in human volunteers (compare AZD1386 and AMG517 structures in Figure 4; Gavva et al., 2008). In preclinical studies (in rodents and dogs), PHE377 (structure undisclosed) was devoid of any effect on body temperature at doses at which it inhibited both thermal and mechanical hyperalgesia (http://www.pharmeste.com/repository/contenuti/paragrafi/file/PharmEste_Leaflet_2012.PDF).
The site that mediates the hyperthermic action of TRPV1 antagonists is still hotly debated. In rodents, capsaicin evokes transient hypothermia (presumably by activating cooling mechanisms after tricking the animals into believing that they are hot), followed by a loss of the animals' ability to regulate their body temperature (rats desensitized to capsaicin develop hyperthermia when placed in a hot chamber; Szallasi and Blumberg, 1999). The effects of capsaicin on thermoregulation were linked to the CNS (Hajós et al., 1985). However, TRPV1 antagonists that do or do not enter the CNS are comparable in their ability to elevate body temperature, making a CNS target extremely unlikely (Cui et al., 2006). Consequently, it was postulated that TRPV1 in the periphery has an endogenous tone that is essential for maintaining normal body temperature (Gavva, 2008). However, rodents whose TRPV1 has been eliminated by genetic recombination (TRPV1 kncok out mice) or chemical ablation (neonatal capsaicin treatment) do not develop hyperthermia.
Similar to the hyperthermic response, the magnitude of blunted heat perception also seems to depend on the TRPV1 antagonist pharmacophore. The increase in heat pain threshold was first noted after the administration of 400 mg of SB-705498 to healthy human volunteers (Chizh et al., 2007). Importantly, unlike the febrile reaction that disappeared upon repeated dosing, the impaired thermal sensitivity persisted during the whole course of the study (Chizh et al., 2007). Impaired heat perception was also observed with MK-2295 (Eid, 2011), ABT-102 (Rowbotham et al., 2011) and AZD1386 (Krarup et al., 2011). Some volunteers receiving MK-2295 perceived potentially harmful temperature as innocuous (Eid, 2011). Indeed, minor (1st and 2nd degree) burns were reported in some clinical study subjects. Interestingly, another clinical study (XEN-D0501) found no evidence of scalding injuries in the study participants (Round et al., 2011).
The heat sensor in TRPV1 is clearly distinct from the capsaicin and proton recognition sites (Szolcsányi and Sándor, 2012) and a new generation of modality-selective TRPV1 antagonists that cause neither hyperthermia nor impaired pain heat perception have been proposed (Szolcsányi and Sándor, 2012; Brederson et al., 2013).
An attractive alternative approach to circumvent the side effects of TRPV1 antagonists is to target TRPV1 in diseased, but not in healthy, tissues (Szallasi and Blumberg, 2006). Phosphorylation of the TRPV1 protein by PKA and PKC is believed to play a crucial role in inflammatory sensitization (reviewed in Szallasi et al., 2007). The interaction between both kinases and TRPV1 depends on the scaffolding protein AKAP79 (Zhang et al., 2008). Recently, specific residues in TRPV1 and AKAP79 were discovered by site-directed mutagenesis experiments where these proteins interact (Btesh et al., 2013; Fischer et al., 2013). This information allowed the design and synthesis of peptides that can block the interaction between TRPV1 and AKAP79. In mice, these blocking peptides prevented the development of inflammatory thermal hyperalgesia but it remains to be seen if a similar strategy can be successful in chronic pain patients.
Neither AMG-9810 (a TRPV1 antagonist) nor HC-030031 (a TRPA1 blocker, see later) relieved ongoing pain in a mouse model of osteoarthritis, although both ameliorated thermal hyperalgesia (Okun et al., 2012). Consisitent with these findings, in a randomized, double-blinded, prospective clinical trial with client-owned dogs suffering from severe hip osteoarthritic pain, the TRPV1 antagonist ABT-116 showed only marginal analgesic activity over placebo (Malek et al., 2012). ABT-116 did not attenuate lameness in dogs with experimentally induced urate synovitis either at doses at which it caused seriously high rectal temperatures (Cathcart et al., 2012). The striking difference in the analgesic activity of TRPV1 antagonists between rodent (where it potently reduces experimental osteoarthritic pain; Honore et al., 2005; Puttfarcken et al., 2010) and canine (without clinical benefits, see earlier) models of human osteoarthritic pain is puzzling and concerning. Indeed, osteoarthritic pain is a major clinical indication for TRPV1 antagonists and a number of TRPV1 antagonists have entered clinical trials for this indication (Table 2), but as yet, the results have not been disclosed.
TRPA1
Unlike TRPV1 (which shows distinct structure-activity relations in its ligand-binding properties, hence the original name ‘vanilloid receptor’), TRPA1 is activated by a wide range of irritant natural products, including allyl isothiocyanate (Jordt et al., 2004; Capasso et al., 2012), cinnamaldehyde (Bandell et al., 2004) and allicin (Bautista et al., 2005) found in mustard oil, cinnamon and garlic respectively. TRPA1 is also targeted by environmental irritants found in tear gas, exhaust fumes, household cleaning agents and cigarette smoke; examples include acrolein (Bautista et al., 2006), formalin (McNamara et al., 2007) and α,β-unsaturated aldehydes (Andrè et al., 2008). Of note, TRPA1 binds umbellulone, an active ingredient in the Californian ‘headache tree’ Umbellularia californica (Nassini et al., 2012a). Indeed, activation of TRPA1 expressed on meningeal afferents was implicated in the pathomechanism of migraine (Edelmayer et al., 2012). Somewhat surprisingly for an ‘irritant receptor,’ TRPA1 is also activated by the non-pungent capsaicin analogue, capsiate (Shintaku et al., 2012), as well as the non-psychotropic cannabinoid, cannabichromene (see Figure 6 for structure; De Petrocellis et al., 2008;2011). This is interesting because cannabichromene is thought to play a pivotal role in the anti-inflammatory and analgesic activity of medical marijuana and cannabichromene did ameliorate experimental murine colitis (Romano et al., 2013). TRPA1 also acts as a receptor for reactive oxygen species (ROS; Bessac et al., 2008), but it is debated if TRPA1 can be activated by noxious cold. In general, reactive chemicals activate TRPA1 by inducing covalent modification of cysteines in the N-terminus (Nilius et al., 2011).
Figure 6.
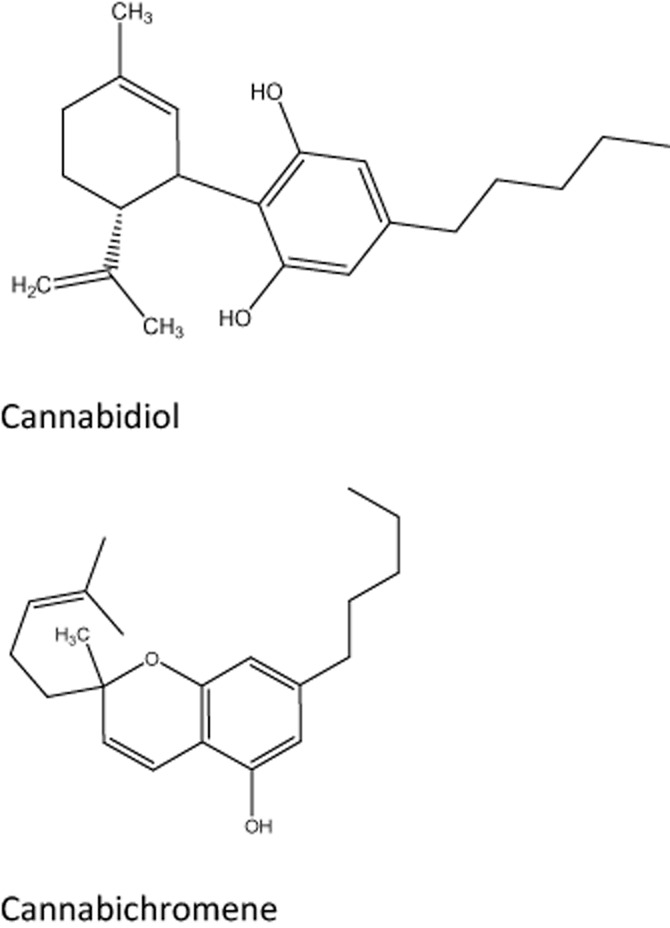
Selected plant cannabinoids that target TRP channels: cannabichromene (TRPA1 agonist) and cannabidiol (TRPM8 antagonist).
TRPA1 is well established as a pain sensor. A gain-of-function point mutation in TRPA1 (N855S) was identified as the cause of familial episodic pain syndrome, a rare human pain disorder characterized by severe upper body pain triggered by fasting and physical stress (Kremeyer et al., 2010). TRPA1 is primarily expressed in sensory neurons (where it is highly coexpressed with TRPV1), but growing evidence suggests that functional TRPA1 is also present in non-neuronal tissues such as heart, small intestine, lung and pancreas (Stokes et al., 2006). As discussed later under respiratory disorders, TRPA1 expressed by lung fibroblasts (Mukhopadhyay et al., 2011) might play a role in the pathogenesis of asthma and COPD (Nassini et al., 2012b).
TRPA1 expression in sensory neurons appears to be plastic and neuropathic injury increased neuronal expression of TRPA1 in humans (Anand et al., 2008). Cyclopentenone PGs, metabolites of PGs enhancing pain sensation, cause a robust calcium response in dorsal root ganglion neurons and induce pain behaviour in wild-type mice, but not in TRPA1-deficient mice (Materazzi et al., 2008). Interestingly, the pain phenotype of TRPA1 knockout and knock-down animals are different: inflammation-induced mechanical hyperalgesia is reduced in the knock-down mice, but not in the knockouts (see Garrison and Stucky, 2011; Nilius et al., 2011). This implies the existence of a compensatory mechanism that takes over the function of the missing TRPA1 in the knockout animals and restores mechanical hyperalgesia during inflammation.
Pharmacological inhibition of TRPA1 with HC-030031, a highly selective TRPA1 antagonist, attenuated formalin-induced pain (McNamara et al., 2007) and reversed mechanical hypersensitivity following complete Freund's adjuvant (CFA) treatment. HC-030031 also displayed analgesic activity in the spinal nerve ligation model of neuropathic pain (Eid et al., 2008). CHEM-5861528, a derivative of HC-030031, alleviated mechanical hyperalgesia in a rat model of diabetic neuropathic pain (Wei et al., 2009; Koivisto et al., 2012). Of note, methylglyoxal (an endogenous carbonyl compound that is produced in large amounts during hyperglycemic conditions) activates human TRPA1 (Ohkawara et al., 2012). Furthermore, TRPA1 has been implicated in migraine (Edelmayer et al., 2012), dental pain (Haas et al., 2011), chemotherapy-induced neuropathic pain (Nassini et al., 2013) and colicky pain of GI origin (Blackshaw et al., 2013).
The Abbott TRPA1 antagonist A-967079 attenuated both evoked and spontaneous firing recorded from wide dynamic range (WDR) spinal cord neurons during CFA-induced inflammation (McGaraughty et al., 2010) without having any effect on body temperature (Chen et al., 2011). However, in rats with osteoarthritic pain, A-967079 had no effect on spontaneous WDR firing (though it blocked evoked mechanical hyperalgesia), suggesting (somewhat disappointingly) that TRPA1 blockade may not alleviate the on-going, spontaneous ‘nagging’ pain in patients with osteoarthritis (McGaraughty et al., 2010).
There is good evidence linking TRPA1 to the cold allodynia that develops during ciguatera (Vetter et al., 2012) or following chemotherapy (Nassini et al., 2013). In some studies, TRPA1 was directly activated by noxious cold (Story et al., 2003). Paradoxically, TRPA1 expressed on polymodal C-fibres appears to be activated by hot temperatures (Hoffmann et al., 2013). It is tempting to speculate that this mechanism is responsible for the development of inflammatory thermal hyperalgesia which is absent in the TRPA1 knockout mice (P. Reeh, pers. comm.).
To date, two TRPA1 antagonists have reached clinical stage of development, GRC1753 (Glenmark) for chronic pain and CB-625 for acute surgical pain (Cubist Pharmaceuticals Inc., Lexington, MA, USA; Hydra Biosciences, Inc., Cambridge, MA, USA).
TRPV3
TRPV3 is abundantly expressed in keratinocytes where it is thought to serve various functions (Nilius and Bíró, 2013). Keratinocytes release IL-1, a pro-inflammatory cytokine, in response to eugenol, a non-selective TRPV3 agonist (Xu et al., 2006a). TRPV3 expression is significantly increased in keratinocytes in patients with breast pain (Gopinath et al., 2005); by contrast, TRPV3 is decreased in keratinocytes in patients with diabetic neuropathy (Facer et al., 2007). In addition, TRPV3 was significantly increased in brachial plexus nerves collected from patients with traumatic nerve injury (Facer et al., 2007). Interestingly, Olmsted syndrome patients (a genetic disorder caused by a gain-of-function TRPV3 mutation; Lin et al., 2012) suffer from intense itching but not pain.
TRPV3 is activated by warm temperatures in the range of 31–39°C and its activity is enhanced during repetitive heat stimulations (Xu et al., 2002; Peier et al., 2002b). TRPV3-null mice showed marked deficits in responses to innocuous and noxious heat (Moqrich et al., 2005). GRC15300, a potent, selective, orally available TRPV3 antagonist demonstrated efficacy in inflammatory and neuropathic pain models, and this compound is being investigated in clinical trials (Khairatkar-Joshi et al., 2010).
TRPM8, TRPV4, TRPM3 and TRPCs
TRPM8 is expressed in nociceptive Aδ and C fibres that are cold sensitive (McKemy et al., 2002; Kobayashi et al., 2005). TRPM8 is activated by cold temperatures in the range of 8–28°C, as well as by cooling compounds such as menthol and icilin (McKemy et al., 2002; Peier et al., 2002a). Topical menthol has been tried clinically as an analgesic in patients with carpal tunnel syndrome (NCT01716767) and neck pain (NCT01542827). Naturally occurring TRPM8 antagonists include the plant cannabinoids cannabidiol (see Figure 6 for structure), cannabinol and cannabiogerol (De Petrocellis et al., 2008;2011). Studies with TRPM8-null mice showed decreased sensitivity to cold temperature, as well as attenuated hypersensitivity to cold after nerve injury or inflammation (Bautista et al., 2007; Colburn et al., 2007). Synthetic TRPM8 antagonists were analgesic in a chronic constriction injury-induced model of neuropathic pain in rats (Parks et al., 2011; Calvo et al., 2012) but, as yet, no TRPM8 antagonist has advanced to clinical trials.
TRPV4 as a pain target is highly controversial. Intraplantar injection of the endogenous TRPV4 activator dimethylallyl pyrophosphate elicits nociceptive flinches (Bang et al., 2012). Furthermore, TRPV4-deficient mice demonstrated decreased pain behaviour in inflammatory pain models (Todaka et al., 2004; Alessandri-Haber et al., 2006), as well as models of painful peripheral neuropathy (Alessandri-Haber et al., 2008). Basal visceral nociception and TRPV4 agonist-induced visceral hypersensitivity were reduced by intervertebral injection of TRPV4-targeted siRNA (Cenac et al., 2008). That said, none of the numerous gain-of-function TRPV4 channelopathies has a painful phenotype.
TRPM3-deficient mice showed impaired behavioural response to noxious heat and failed to develop inflammatory heat hyperalgesia (Vriens et al., 2011). Naturally occurring TRPM3 blockers include the citrus fruit flavanones, naringenin and hesperetin (Straub et al., 2013). Of TRPC channels expressed by sensory neurons, TRPC5 appears to be the most interesting given its postulated role in cold-sensation (Zimmermann et al., 2011).
Respiratory disorders and TRP channels
The mammalian respiratory tract is densely innervated by sensory afferent fibres whose activation by irritant and/or inflammatory stimuli evokes a myriad of central and peripheral protective reflex responses, including cough, mucus secretion and bronchospasm (Canning, 2006). The pulmonary chemoreflex is a triad of bradycardia, bradypnea and hypotension. Of TRP channels expressed in these afferents, TRPA1 and TRPV1 have attracted the most attention as sensors of environmental irritants and reactive chemicals that threaten airway function and integrity.
TRPA1
Hypochlorite (the oxidizing mediator of chlorine) and hydrogen peroxide (a ROS) activate TRPA1 in chemosensory neurons in mice to cause respiratory depression, as well as pain behaviour, both of which were attenuated in TRPA1-deficient mice (Bessac et al., 2008). Ozone, one of the major air pollutants, stimulated a subset of nociceptive sensory neurons isolated from vagal ganglia of wild-type mice, but not those from TRPA1 knockout mice (Taylor-Clark and Undem, 2010).
TRPA1 is also targeted by endogenously generated pro-inflammatory ligands as exemplified by nitro-oleic acid, a nitrated phospholipid produced during inflammation (Taylor-Clark et al., 2009). Cigarette smoke is the major cause of COPD and is among the most prevalent triggers of asthma. Crotonaldehyde and acrolein, two main components of cigarette smoke, evoked Ca2+ influx in cultured guinea pig jugular ganglia neurons and promoted contraction of isolated guinea pig bronchi (Andrè et al., 2008). These responses were abolished by the selective TRPA1 antagonist HC-030031 (Andrè et al., 2008). Moreover, inhalation of acrolein and other irritant TRPA1 agonists causes cough both in guinea pigs and human volunteers (Andrè et al., 2009; Birrell et al., 2009). Genetic deletion and/or pharmacological blockade of TRPA1 inhibited leukocyte infiltration in the airways, reduced cytokine and mucus production, and almost completely abolished airway hyperreactivity to contractile stimuli in a murine ovalbumin model of asthma (Caceres et al., 2009).
In the lung, TRPA1 is also expressed by fibroblasts (Mukhopadhyay et al., 2011). Most recently, this non-neuronal TRPA1 has been linked to non-neurogenic airway inflammation (Nassini et al., 2012b). This is important because neurogenic inflammation, although predominant in preclinical models of asthma, plays lesser, if any, role in the human disease. Indeed, tachykinin receptor (NK1, 2 and 3) antagonists were without any clear benefit in clinical trials for asthma. Taken together, these findings suggest that selective TRPA1 antagonist may provide therapeutic benefits in respiratory diseases characterized by airway inflammation, such as asthma and COPD (Belvisi et al., 2011; Preti et al., 2012).
TRPV1
TRPV1 is another key player in the control of airway sensitivity. Indeed, inhaled capsaicin evokes multiple protective reflex responses in humans, including cough, sneezing and fluid secretion (Szallasi and Blumberg, 1999). In rodents, capsaicin also evokes the pulmonary chemoreflex, which is believed to represent the major dose-limiting factor for acute capsaicin administration (in desensitization studies, capsaicin needs to be given in increasing doses in consecutive days to avoid potentially fatal respiratory depression; Szallasi and Blumberg, 1999). The capsaicin inhalation test is broadly used to identify a subset of chronic cough patients with airway sensory hyperreactivity (Ternesten-Hasséus et al., 2008). It is believed that these patients have overactive sensory nerves responsible for the airway symptoms and may benefit from inhaled TRPV1 (and/or TRPA1) antagonists (Millqvist, 2011). It is worth mentioning here that fatal asthma attacks were reported in asthma patients following incidental capsaicin inhalation (see Szallasi and Blumberg, 1999).
Hydrogen sulfide evokes neuropeptide release from isolated guinea pig airway tissue; it also contracts the guinea pig bronchus (Trevisani et al., 2005). These effects are reduced by capsaicin desensitization or by the TRPV1 antagonist, capsazepine (Trevisani et al., 2005). In anaesthetized guinea pigs, intratracheal instillation of hydrogen sulfide increases the total lung resistance and evokes neurogenic inflammation (airway plasma protein extravasation): these effects are reduced by capsazepine (Trevisani et al., 2005). TRPV1 activators induce action potential discharge in murine vagal C-fibre terminals and this response was absent in TRPV1-deficient C-fibres (Kollarik and Undem, 2004). Pharmacological inhibition of TRPV1 significantly inhibited airway hyperresponsiveness to histamine in non-anaesthetized, ovalbumin-sensitized guinea pigs (Delescluse et al., 2012). In addition, a recent study demonstrated that PGE2 and bradykinin, two well-described endogenous inflammatory mediators, activated isolated guinea pig sensory ganglia and evoked cough in guinea pigs. Interestingly, effective blockade of this cough response required the simultaneous antagonism of both TRPV1 and TRPA1 receptors (Grace et al., 2012).
Several lines of evidence implicate TRPV1 in the pathomechanism of chronic cough (see Spina and Page, 2013). The concentration at which inhaled capsaicin evokes coughing is markedly reduced in a subpopulation of chronic cough patients. These patients show increased TRPV1 expression in their airway nerves (Groneberg et al., 2004). Furthermore, TRPV1 gene polymorphism was associated with cough sensitivity among subjects without asthma (Smit et al., 2012). Nevertheless, TRPV1 as a target for anti-tussive drugs remains controversial because inhaled SB-705498, a selective TRPV1 antagonist, failed to ameliorate spontaneous coughing in chronic cough patients (C. Page, pers. comm.).
TRPM8
TRPM8, a cold-sensing TRP channel, is expressed in a subset of autonomic afferent nerves innervating the bronchopulmonary system and activation of these nerves may increase airway resistance (Xing et al., 2008). If so, TRPM8 activation may be associated with cold-induced exacerbation of asthma and other pulmonary disorders (Xing et al., 2008). On the other hand, respiratory irritant responses evoked by vapours containing cigarette smoke constituents (acrolein, acetic acid or cyclohexanone) were alleviated by menthol, and the effect of menthol was reversed by the TRPM8 antagonist AMTB (see Figure 5 for structure; Willis et al., 2011). Indeed, menthol is added to some cigarette brands to minimize airway irritation. Furthermore, nasal application of TRPM8 agonists significantly increased the threshold of capsaicin-induced cough responses in human volunteers (Buday et al., 2012). Clearly, TRPM8 activation can be both beneficial (for example, it may reduce airway irritancy) and harmful (it may exacerbate asthma), depending on the patient.
The role of TRPM8 in airways extends beyond the sensory nerves. In bronchial epithelium of patients with COPD, TRPM8 expression is markedly increased and stimulation with cold or menthol causes MUC5AC expression. MUC5AC expression is reduced by TRPM8 shRNA in normal human bronchial epithelial cells (Li et al., 2011a). Considering that cold is one of the key triggers of COPD exacerbation and the enhanced mucus secretion contributes to morbidity of COPD by plugging airways and causing recurrent infection, TRPM8 may play an important role in the development of COPD and maybe also asthma.
TRPV4
The link between TRPV4 and human pulmonary disease was initially established by the discovery of TRPV4 gene polymorphism in COPD patients (Zhu et al., 2009). Interestingly, one of these COPD-predisposing TRPV4 variants has a gain-of-function phenotype with enhanced Ca2+ influx and MMP-1 release evoked by diesel exhaust particles, indicating that altered activity of TRPV4 could drive the pathogenesis of COPD in some patients (Li et al., 2011b). Normal TRPV4 activity may maintain ciliary movements and cilia on bronchial epithelial cells are essential for airway clearance. The Ca2+ overload secondary to this gain-of-function mutation is thought to impair ciliary function, leading to accumulation of harmful airborne particles in the lungs. Indeed, impaired ciliary movement (‘ciliopathy’) is an early sign of COPD (http://weill.cornell.edu/news/releases/wcmc_2012/07_12_12b.shtml).
TRPV4 is also implicated in the pathogenesis of pulmonary oedema caused by high pulmonary venous pressure secondary to heart failure. TRPV4 activation evoked by elevated vascular pressure induces a marked increase in pulmonary endothelial cell permeability (Jian et al., 2008). This observation is in line with the finding that systemic administration of a TRPV4 agonist elicited increased pulmonary vascular permeability, vascular haemorrhage, and circulatory collapse as a result of profound disruption of the endothelial permeability barrier (Willette et al., 2008). Conversely, TRPV4 inhibition prevented the increased vascular permeability and resultant pulmonary oedema induced by elevated pulmonary venous pressure in isolated rodent and canine lungs (Thorneloe et al., 2012). Of note, the expression of TRPV4 in the pulmonary vasculature is enhanced in lung sections obtained from heart failure patients (Thorneloe et al., 2012). Collectively, these observations imply a therapeutic benefit for TRPV4 blockade in heart failure patients with pulmonary oedema.
TRPC6
Lung ischaemia–reperfusion is another cause of pulmonary oedema. The involvement of TRPC6 in this condition is implied by the finding that TRPC6-deficient mice fail to develop oedema following lung ischaemia–reperfusion (Weissmann et al., 2012). It was suggested that TRPC6 is activated by DAG generated in a sequence of biochemical events starting from superoxide production by NADPH oxidase 2 (NOX2) and leading to elevated vascular permeability.
The role of TRPC6 in pulmonary system extends beyond oedema. For example, TRPC6 is implicated in the pathogenesis of idiopathic pulmonary hypertension. Three key studies showed that (i) TRPC6 expression was increased in pulmonary artery smooth muscle cells taken from idiopathic pulmonary hypertension patients (Yu et al., 2004); (ii) TRPC6-deficient mice failed to develop pulmonary hypertension in response to chronic hypoxia (Weissmann et al., 2006); and (iii) SNPs in the TRPC6 gene promoter region, which cause elevated expression of the channel, are associated with idiopathic pulmonary arterial hypertension (Yu et al., 2009a).
Skin disorders and TRP channels
TRP channels are present in both neuronal and non-neuronal cells in the skin where they are thought to play a key role in itch, regulation of barrier function, keratinocyte differentiation, hair growth, inflammation and wound healing (see Moran et al., 2011). TRPV1 is expressed in sensory nerves innervating the skin and genetic deletion or pharmacological inhibition of TRPV1 decreased histamine-induced scratching behaviour in mice (Shim et al., 2007; Imamachi et al., 2009). In keeping with this observation, scratching behaviour induced by LTB4 was decreased by TRPV1 blockade through a mechanism involving attenuated migration of neutrophils to the skin (Fernandes et al., 2013). Moreover, capsaicin injection into the mouse skin pretreated with CFA (but not into healthy skin) induced scratching behaviour, which was attenuated by capsazepine (Liang et al., 2011). There is anecdotal evidence that desensitization to capsaicin creams may reduce itch associated with various aetiologies (Breneman et al., 1992; Ellis et al., 1993; Lysy et al., 2003).
TRPA1 is highly co-expressed with TRPV1 in cutaneous sensory nerves. TRPA1 antagonism and/or genetic deletion, but not TRPV1 blockade, reduced scratching behaviour evoked by intradermal injection of hydrogen peroxide (Liu and Ji, 2012). Chloroquine and BAM8-22 induced itch through a TRPV1-independent mechanism by activating two other receptors, mas-related GPCR A3 (MrgprA3) and MrgprC11. Sensory neurons isolated from TRPA1-deficient mice exhibited markedly decreased responses to chloroquine and BAM8–22 and, accordingly, TRPA1-deficient mice exhibited reduced scratching in response to these pruritogens (Wilson et al., 2011). Moreover, while LTB4 induced scratching by activating both TRPV1 and TRPA1, the downstream mechanisms leading to itch differ between these channels, i.e. neutrophil migration and superoxide release respectively (Fernandes et al., 2013). These findings suggest that (i) TRPA1 antagonists may be useful for the treatment of itch; and (ii) the spectrum of itch responsive to TRPA1 blockade may be different from that of TRPV1 antagonists.
TRPV3 is abundantly expressed in keratinocytes (Peier et al., 2002b) and is thought to play a key role in barrier formation and hair morphogenesis. Activation of TRPV3 induces release of pro-inflammatory cytokines from murine keratinocytes (Xu et al., 2006a). In human keratinocytes, TRPV3 activation decreased proliferation and induced apoptosis (Borbíró et al., 2011). The same study demonstrated that TRPV3 activation resulted in hair shaft elongation, suppression of proliferation and induction of apoptosis in human organ-cultured hair follicles. Spontaneous mutant rodent strains (DS-Nh mice and WBN/Kob-Ht rats) that carry mutations for Gly573Ser and Gly573Cys in the TRPV3 gene, respectively, display a hairless or a pruritic dermatitis phenotype (Asakawa et al., 2006; Yoshioka et al., 2009). Interestingly, the same mutations together with another for Try692Gly in the TRPV3 gene were identified in patients with Olmsted syndrome, a rare disorder characterized by the combination of peri-orificial keratotic plaques, bilateral palmoplantar keratoderma, alopecia and intense itch. When expressed in heterologous systems, these TRPV3 mutants exhibited gain-of-function phenotypes (Lai-Cheong et al., 2012; Lin et al., 2012). Collectively, these observations suggest that TRPV3 plays a crucial role in skin keratinization, hair growth and itch. Therapeutic intervention by TRPV3 antagonists may reduce keratinization and itch in the skin, and potentially also alleviate alopecia. Of note, we already have a potential tool to test these predictions in GRC15300, a potent and selective TRPV3 antagonist, which is being evaluated in the clinics for pain (Khairatkar-Joshi et al., 2010).
In human keratinocytes, TRPV1 agonist treatment suppressed proliferation and induced apoptosis (Tóth et al., 2011). In human skin, TRPV1 expression was elevated in response to ultraviolet (UV) irradiation (Lee et al., 2009). In the skin of hairless mice, UV irradiation up-regulated the expression of MMPs, pro-inflammatory cytokines, COX and p53; this was reduced by TRPV1 blockade (Lee et al., 2011). Taken together, these findings suggest that TRPV1 antagonists may protect the skin from inflammation induced by UV light such as sunburn. In addition, topical TRPV1 antagonists may ameliorate thermal hyperalgesia after sunburn. TRPV1 has also been implicated in the pathogenesis of atopic dermatitis. Indeed, the TRPV1 antagonist PAC-14028 (AmorePacific Corp., Seoul, South Korea) is being investigated in patients with atopic dermatitis.
Bladder disorders and TRP channels
A number of TRP channels (including TRPV1, V2 and V4, TRPM4 and M8, TRPA1) are expressed in the bladder where they show distinct cellular distribution pattern and play different roles (see Skryma et al., 2011; Avelino et al., 2013).
TRPV1, the target for intravesical vanilloid therapy
TRPV1 is expressed in sensory neurons and urothelium. While TRPV1 in sensory C-fibre afferents is involved in the micturition reflex (Birder et al., 2002) and thought to serve as sensor of painful bladder stimuli (Charrua et al., 2007), the functional role (and the very existence) of TRPV1 in urothelium remains controversial (Everaerts et al., 2010b).
TRPV1-deficient mice fail to develop bladder hyperreflexia during cystitis (Charrua et al., 2007; Wang et al., 2008) and the TRPV1 antagonist GRC6211 attenuates bladder overactivity in a rat model of bladder inflammation induced by LPS (Charrua et al., 2009). GRC6211 also blocked the neurogenic detrusor overactivity (‘neurogenic bladder’) induced by chronic spinalization in the rat (Santos-Silva et al., 2012).
The involvement of capsaicin-sensitive nerves in the human micturition reflex is well-established (Maggi et al., 1989). When the descending neuronal control of the micturition reflex is lost (e.g. after spinal cord injury or due to multiple sclerosis, MS), the bladder becomes autonomic and the capsaicin-sensitive afferents take control of micturition (‘neurogenic bladder’). This forms the foundation for the use of intravesical capsaicin administration in patients with neurogenic bladder: in these patients, capsaicin reduces the first desire to void by increasing bladder capacity and pressure threshold for micturition (Maggi et al., 1989).
Although clinically effective in the long term, intravesical capsaicin is unacceptable for many patients because of the initial burning pain that it causes. Resiniferatoxin is a better-tolerated (much less painful) alternative for intravesical vanilloid therapy. Intravesical resiniferatoxin reduced the number of incontinent episodes (and even restored continence in some) in patients with neurogenic detrusor overactivity of spinal origin (Cruz et al., 1997; Cruz and Dinis, 2007). The beneficial effects of intravesical resiniferatoxin were long-lasting (several months) and reversible. Repeat administration replicated the therapeutic value of the initial treatment. Importantly, biopsies taken from the bladder of patients undergoing intravesical vanilloid therapy did not show any significant histopathological and/or ultrastructural (electron microscopic) alterations.
TRPV4
In the bladder, TRPV4 is abundantly expressed in urothelial cells (Yu et al., 2011). Stimulation of urothelial TRPV4 by stretch and/or hypo-osmolality induces ATP release which, in turn, activates purinergic P2X3 receptors in bladder afferents and evokes the micturition reflex (Birder et al., 2007; Mochizuki et al., 2009; Aizawa et al., 2012). TRPV4 is also expressed in the detrusor muscle. Indeed, GSK1016790A, a potent and selective TRPV4 agonist (Figure 5), induces detrusor muscle contractions even in the absence of urothelium (Thorneloe et al., 2008). Consistent with these findings, intravesical administration of GSK1016790A causes bladder overactivity in wild type, but not in TRPV4-deficient, mice (Thorneloe et al., 2008). Moreover, TRPV4-null mice displayed reduced frequency of voiding, increased urine volume per episode, and spatially altered urine spot pattern (Gevaert et al., 2007). TRPV4-null mice also showed reduced detrusor overactivity in a cyclophosphamide-induced cystitis model. HC-067047, a potent and selective TRPV4 antagonist, reduced micturition frequency in a rat model of cystitis (Everaerts et al., 2010a). Of note, a subpopulation of patients with Charcot–Marie–Tooth disease type 2C, a genetic disease caused by gain-of-function mutation in TRPV4, display bladder urgency and incontinence (Landourè et al., 2010). Collectively, these observations suggest that TRPV4 may represent a useful therapeutic target for the treatment of bladder dysfunction.
TRPM8
A subset of sensory bladder afferents expresses TRPM8 (Shibata et al., 2011) and expression of this channel is elevated after bladder outlet obstruction in rats (Hayashi et al., 2011). In patients with overactive and painful bladder syndromes, TRPM8 is similarly up-regulated in nerve fibres and its expression level significantly correlates with clinical scores (Mukerji et al., 2006a). Intravesical cold saline instillation causes uninhibited detrusor contractions in patients with either idiopathic or neurogenic detrusor overactivity, but not in healthy volunteers (Mukerji et al., 2006b). In rats, intravesical infusion of menthol evokes the micturition reflex (Nomoto et al., 2008). Volume-induced bladder contraction and nociceptive reflex responses to noxious bladder distension are reduced by AMTB, a TRPM8 antagonist (Figure 5; Lashinger et al., 2008). Additionally, in a cystometric study using rats, N-(4-t-butylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carboxamide (BCTC), another TRPM8 antagonist that also blocks TRPV1 and V4 channels, inhibited detrusor overactivity induced by menthol or cold stress (Lei et al., 2013). Taken together, these observations imply that TRPM8 is involved in bladder pain and detrusor overactivity.
TRPA1
TRPA1 is highly expressed in sensory neurons innervating bladder where it is co-expressed with TRPV1 (Streng et al., 2008). As with TRPM8, TRPA1 is up-regulated in bladder mucosa in patients with bladder outlet obstruction (Du et al., 2008). Exposure of rat bladder strips to TRPA1 agonists induced contraction (Andrade et al., 2006). In vivo, TRPA1 agonists increased the micturition frequency in rats, and desensitization of TRPV1-expressing C-fibres by capsaicin attenuated the effect of TRPA1 agonist (Du et al., 2007; Streng et al., 2008). Conversely, the TRPA1 antagonist HC-030031 attenuated bladder overactivity in models of cyclophosphamide-induced cystitis and spinal cord injury (Andrade et al., 2011; Meotti et al., 2013). These findings identify TRPA1 as a potential drug target for bladder disorders.
TRPM4
TRPM4 is functionally expressed in the detrusor muscle of the rat and guinea pig. 9-Phenanthrol, a selective TRPM4 antagonist, reduced contraction of detrusor-isolated strips induced by various stimuli including electrical field stimulation (Smith et al., 2013a,b2013b). Further studies will be needed to elucidate the pathophysiological roles of TRPM4 in human bladder.
Inflammatory bowel disease (IBD) and TRP channels
TRP channels are widely expressed in the digestive tract, with important roles in taste, visceral sensation, GI motility, as well as absorptive and secretory functions (see Boesmans et al., 2011; Holzer, 2011; Blackshaw et al., 2013). Changes in TRP channel expression have been detected in a variety of GI ailments as exemplified by increased TRPV1 expression in both GERD (Matthews et al., 2004; Bhat and Bielefeldt, 2006) and irritable bowel syndrome (Akbar et al., 2008; Keszthelyi et al., 2013). Indeed, TRPV1-null mice develop less oesophagitis after acid exposure compared with their wild-type littermates (Fujino et al., 2006).
There is good evidence to suggest an important role for TRP channels (in particular, TRPV1 and TRPA1) in the development and maintenance of IBD. Increased TRPV1-like immunoreactivity was reported in colonic biopsies taken from patients with IBD, both Crohn's disease and ulcerative colitis (Yiangou et al., 2001). In a rat model of IBD, desensitization to topical capsaicin of intestinal afferents was shown to reduce ulceration (Goso et al., 1993). Furthermore, the TRPV1 antagonist JYL1421 suppressed colorectal distension and improved colitis in rats (Miranda et al., 2007). TRPA1 expression is elevated in the inflamed mouse gut (Yang et al., 2008; Izzo et al., 2012). Experimental colitis induced by dinitrobenzene sulphonic acid (DNBS) was attenuated after both pharmacological blockade (by the TRPA1 antagonist HC-030031) and genetic inactivation of TRPA1 (Engel et al., 2011). DNBS was shown to bind to cysteine residues in the intracytoplasmic N-terminus of the TRPA1 protein, identifying TRPA1 as a direct molecular target in DNBS-induced colitis (Engel et al., 2011). The involvement of TRPA1 in IBD is, however, more complex as TRPA1 activation by cannabichromene (Figure 6) was reported to ameliorate murine colitis (Romano et al., 2013).
In the GI tract, TRPV4 is expressed both in epithelial cells and sensory afferents (Brierley et al., 2008). In IBD patients, increased TRPV4 mRNA levels were reported. In experimental animals, TRPV4 activation contributes to intestinal inflammation via chemokine release and TRPV4 blockade alleviates colitis symptoms (D'Aldebert et al., 2011; Fichna et al., 2012). Finally, TRPM8 is up-regulated both in mouse and human colon during colitis and TRPM8 activation by icilin attenuates inflammatory responses in a mouse model of IBD (Ramachandran et al., 2013).
Diabetes and TRP channels
A growing number of TRP channels (TRPM2, M4 and M5, TRPA1) have been implicated in insulin release from pancreatic beta-cells (see Colsoul et al., 2013). TRPM5 plays an important role in glucose-induced high-frequency oscillations. Indeed, membrane potential and cytosolic calcium level were reduced in islets derived from TRPM5-deficient mice, resulting in decreased glucose-induced insulin secretion (Colsoul et al., 2010). Consequently, TRPM5-deficient mice showed impaired glucose tolerance (Brixel et al., 2010; Colsoul et al., 2010). In addition, TRPM5 mediates fructose-induced insulin release (downstream of sweet taste receptors) in murine islets (Kyriazis et al., 2012). These observations indicate that TRPM5 may serve as a potential convergence point between sweet taste receptors and glucose-induced insulin secretion in pancreatic beta-cells (Kyriazis et al., 2012). In accord with this hypothesis, SNPs in TRPM5 are associated with pre-diabetic phenotypes in subjects at increased risk for type 2 diabetes in a German population (Ketterer et al., 2011). Collectively, these findings suggest that TRPM5 agonists may provide therapeutic benefits in patients with type 2 diabetes.
TRPM2 is another TRP channel implicated in insulin release from pancreatic beta-cells. Pharmacological inhibition or genetic deletion of TRPM2 reduced insulin secretion from islets induced by heat, glucose or glucagon-like peptide 1 (GLP-1) receptor agonists (Togashi et al., 2008; Uchida et al., 2011). TRPM2 is a non-selective Ca2+ permeable cation channel and TRPM2-mediated insulin secretion occurs partly by intracellular influx of Ca2+. Interestingly, insulin secretion via TRPM2 can be induced by Ca2+ influx-independent mechanisms as glucose-induced insulin secretion was lost in islets from TRPM2-deficient mice in a condition that is supposed to completely inactivate the insulin release pathway mediated by KATP channel and voltage dependent Ca2+ channel (Uchida et al., 2011). Importantly, TRPM2 knockout mice showed higher basal glucose levels and impaired glucose tolerance, indicating TRPM2 agonists may be useful as anti-diabetic agents (Uchida et al., 2011). On the other hand, other studies indicate activation of TRPM2 by ROS leads to apoptosis in beta-cell lines (Hara et al., 2002; Ishii et al., 2006; Lange et al., 2009). In addition, genotyping studies did not find any correlation between SNPs in the TRPM2 gene and type 2 diabetes (Romero et al., 2010). Clearly, further investigation is required in order to establish the validity of TRPM2 as a therapeutic target in diabetes.
The roles of TRP channels in diabetes extend beyond insulin secretion from beta-cells. For example, there is good evidence that TRPV1 in sensory afferents (presumably following the release of sensory neuropeptides) plays a role in physiological glucose control (see Suri and Szallasi, 2008). Furthermore, dysregulated TRPV1 activity was implicated in the pathomechanisms of diabetes, both type 1 (Tsui et al., 2007) and type 2 (Tsui et al., 2011). Capsaicin evokes GLP-1 release from a murine entero-endocrine cell line in vitro and when given intragastrically, capsaicin increases GLP-1 and insulin secretion in wild type, but not in TRPV1-deficient mice (Wang et al., 2012). TRPV1-deficient mice also show improved glycemic control in a diet-induced obesity model (Marshall et al., 2013). Desensitization to capsaicin was reported to increase glucose tolerance in Zucker diabetic fatty rats, a model of type-2 diabetes (Gram et al., 2007). BCTC (a non-selective TRPV1 antagonist that also blocks TRPV4 and TRPM8 channels) improved glucose tolerance in rodent models of type-2 diabetes (Tanaka et al., 2011). Finally, genetic deletion of TRPV1 protected mice from the development of autoimmune (type 1) experimental diabetes (Razavi et al., 2006). Thus, the range of the experimental evidence implies a therapeutic potential for TRPV1 blockade in diabetic patients.
Obesity is an important cause of insulin resistance and type 2 diabetes. The exact role of TRPV1 in the control of appetite and body weight remains controversial. There is anecdotal evidence that dietary capsaicin suppresses appetite and keeps both experimental animals and human volunteers lean. Genetic deletion of TRPV1 appears to protect young mice from high-fat diet-induced obesity (Motter and Ahern, 2008) but when these mice grow older, they become ‘lazy’ (hypoactive) and fat (Garami et al., 2011; Marshall et al., 2013).
The involvement of TRPV4 in body weight control is likewise controversial. While two studies demonstrated that TRPV4-deficient mice were protected from diet-induced obesity (Kusudo et al., 2012; Ye et al., 2012), another study showed the opposite effect (O'Conor et al., 2013). Even worse, in the latter study the TRPV4 (−/−) animals not only became obese but also showed severe, debilitating osteoarthritis.
The potential involvement of TRPM8 in obesity was recently suggested by the finding that chronic dietary menthol treatment prevented diet-induced obesity in wild-type, but not in TRPM8-deficient mice, through thermogenesis in brown adipose tissue, mediated by uncoupling protein 1 (UCP-1), (Ma et al., 2012b).
For the sake of completeness, it should be mentioned here that TRP channels are also potential targets for managing the complications of diabetes, such as peripheral neuropathy, nephropathy, retinopathy, as well as cardiovascular disease. As mentioned earlier, TRPA1 and TRPV1 have been investigated as analgesic targets in diabetic neuropathic pain (Koivisto et al., 2012). Of note, methylglyoxal, a metabolite of glucose, is capable of directly activating TRPA1, providing a potential link between elevated glucose levels and pain (Ohkawara et al., 2012). With regard to diabetic nephropathy, podocyte foot processes and slit diaphragms contribute to the formation of the glomerular filter in the kidney and dysfunction of podocytes leads to proteinuria (Faul et al., 2007). TRPC6 expression is elevated in podocytes by high glucose levels. Moreover, TRPC6 mediates high glucose-induced podocyte apoptosis where TRPC6 is activated by ROS generated from glucose (Liu et al., 2013; Yang et al., 2013). These observations suggest a role for TRPC6 in the development of diabetic nephropathy. Last, TRPV4 in ocular endothelial cells has been implicated in the pathogenesis of diabetic retinopathy (and other ocular neovascularization disorders).
Cancer and TRP channels
The link between TRP channels and cancer is a speculative, potentially rewarding, but highly controversial area of research. Clearly, the expression of several TRP channels (including TRPV1, V2, V6, TRPC3, C5, C6, TRPM1, M2, M3, M4, M7 and TRPM8, TRPA1) is altered in various cancers (see Santoni and Farfariello, 2011; Liberati et al., 2013). Some authors argue that TRP channels are involved in the proliferation and migration of cancer cells, as well as in their resistance to chemotherapeutic agents (see Liberati et al., 2013; Lehen'kyi and Prevarskaya, 2011). Sceptics point out that altered TRP channel expression may be simply an epiphenomenon to cancer progression and not a contributor to disease.
Of note, prostatic adenocarcinoma shows increased TRPM8 expression that appears to correlate with the aggressiveness of the disease. Recently, D-3263, a TRPM8 agonist, has entered Phase 1 clinical trials (NCT00839631) with the hope that it will kill TRM8-expressing cancer cells by calcium and sodium overload through TRPM8 activation (see Santoni and Farfariello, 2011). If this trial is successfully completed, a similar approach may be tried for other cancers that overexpress TRP channels such as TRPC6 in glioblastoma and TRPV2 in ovarian carcinoma.
Kidney disorders and TRP channels
TRPP2, TRPC6 and TRPM6 are causative genes of some genetic kidney disorders (Nilius and Owsianik, 2010). Mutations in PKD2, a gene coding TRPP2, are responsible for ADPKD, the most common inherited renal disease characterized by the growth of numerous cysts in both kidneys, and in many cases, hypertension, leading to renal failure (Peters et al., 1993; Mochizuki et al., 1996). TRPP2 forms a complex with polycystin-1 (Yu et al., 2009b), a gene product of another causative gene of ADPKD, in primary cilia of renal epithelial cells and vascular endothelial cells and this complex is proposed to transduce extracellular shear stress induced by blood flow or urine flow into intracellular Ca2+ signals (Nauli et al., 2003; AbouAlaiwi et al., 2009). Cystic renal epithelial cells have lowered intracellular Ca2+ level (Yamaguchi et al., 2006) and triptolide, a compound derived from traditional Chinese medicine, suppressed cyst formation by restoring Ca2+ signalling in a TRPP2-dependent mechanism in a murine model of ADPKD (Leuenroth et al., 2007).
Interestingly, TRPV4 is reported to form a complex with TRPP2 to serve as a mechano-and thermosensitive molecular sensor in the cilium (Köttgen et al., 2008). Chronic treatment with a low-dose TRPV4 agonist attenuated the renal cyst enlargement in an animal disease model of autosomal recessive polycystic kidney disease, which was also associated with reduced intracellular Ca2+ level (Siroky et al., 2006).
Mutations in TRPC6 are responsible for FSGS, a disease characterized by glomerular scarring, resulting in proteinuria, oedema and kidney failure. Some of the TRPC6 mutants cloned from FSGS patients show a gain-of-function phenotype (Reiser et al., 2005; Winn et al., 2005). TRPC6-deficient mice fail to develop angiotensin II (Ang-II)-induced albuminuria (Eckel et al., 2011) while podocyte-specific overexpression of TRPC6 induces kidney dysfunction analogous to human FSGS (Krall et al., 2010). In addition, expression of TRPC6 is elevated both in patients with acquired forms of proteinuric kidney disease and in a model of podocyte injury in rats (Möller et al., 2007). Although the mechanism of TRPC6-mediated podocyte dysfunction has not been clarified, NFAT-dependent gene transcription may be involved in the pathway (Wang et al., 2010). On the other hand, down-regulation of TRPC6 leads to loss of stress fibres upon Ang-II treatment in podocytes (Tian et al., 2010). TRPC6-deficient mice were originally reported to be hypertensive, although compensatory up-regulation of TRPC3 and TRPC7 may have contributed to the observed phenotype (Dietrich et al., 2005).
TRPM6 is permeable to Mg2+ and Ca2+. TRPM6 is unique among TRP channels in that it has a large carboxyl terminal PK domain, analogous to TRPM7. TRPM6 is primarily expressed in the kidney and intestine and is considered to be responsible for absorption of Mg2+. Loss-of-function mutation in TRPM6 is responsible for a rare hereditary disease characterized by profound hypomagnesaemia associated with hypocalcaemia (see Nilius and Owsianik, 2010). Accordingly, TRPM6 agonists may be useful in the management of disorders characterized by hypomagnesaemia.
CNS disorders and TRP channels
A number of TRP channels are expressed in the brain where they are believed to play key roles in the development of neurological and psychiatric disorders (see Vennekens et al., 2012). TRPC3 is a non-selective cation channel that is activated through PLC and activation of inositol trisphosphate receptors. TRPC3 is abundantly expressed in cerebellum, cortex and hippocampus where it plays a pivotal role in brain-derived neurotrophic factor (BDNF)-mediated survival and growth-cone guidance in cerebellar granule neurons (Li et al., 2005; Jia et al., 2007). In addition, TRPC3 can be activated downstream of mGlu1 receptors and induces slow excitatory postsynaptic potentials in cerebellar Purkinje cells (Hartmann et al., 2008). Interestingly, both TRPC3 knockout mice and ‘moonwalker mice’ (that possess a gain-of-function mutation in TRPC3) exhibit similarly impaired walking behaviours (Hartmann et al., 2008; Becker et al., 2009). Considering this obvious discrepancy, further investigation will be needed to assess whether or not TRPC3 provides a drug discovery opportunity for ataxia.
TRPC6 is a close homologue of TRPC3, and similarly to TRPC3, TRPC6 promotes BDNF-mediated survival and growth-cone turning in cerebellar granular cells (Li et al., 2005; Jia et al., 2007). Overexpression of TRPC6 in cultured hippocampal neurons increases the density of dendritic spine, while down-regulation of TRPC6 with siRNA reduces the spine density (Tai et al., 2008; Zhou et al., 2008). TRPC6 transgenic mice exhibited improved spatial learning and memory in the Morris water maze test, suggesting a crucial role of TRPC6 in learning and memory formation through regulation of synaptic plasticity (Zhou et al., 2008).
TRPC5 is highly expressed in hippocampus and amygdala (Riccio et al., 2009). TRPC5-deficient mice displayed anxiolytic-like phenotype in elevated plus-maze, open field and social interaction tests, but not in novelty-suppressed feeding tests, suggesting that TRPC5 is involved in innate fear (Riccio et al., 2009). In addition, synaptic responses mediated by activation of mGlu receptors and cholecystokinin CCK2 receptors, both of which are implicated in anxiety, are diminished in lateral nucleus of the amygdala derived from TRPC5-null mice (Riccio et al., 2009). Collectively, these data suggest that TRPC5 may serve as a therapeutic target for anxiety. Parenthetically, TRPV1 (−/−) mice were also reported to show reduced fear and anxiety behaviour (Marsch et al., 2007). This finding, however, has recently been questioned by another study in which only minimal brain TRPV1 expression was found using a sensitive reporter mouse model (Cavanaugh et al., 2011).
Growth cone collapse induced by semaphorin 3A is reduced in hippocampal neurons from TRPC5-deficient mice (Kaczmarek et al., 2012). If this observation is replicated by pharmacological inhibition of TRPC5, it may imply a beneficial effect for TRPC5 antagonists in neurodegenerative disorders.
TRPM2 and TRPM7 are potential therapeutic targets for stroke. TRPM2 is expressed in microglia and neurons in the brain and may serve as a redox sensor. Activation of TRPM2 by oxidative stress could lead to cell death. Indeed, TRPM2-deficient mice showed resistance against neuronal death in a focal ischaemia model of stroke (Miller and Zhang, 2011). Likewise, TRPM7-induced Ca2+ overload leads to cell death. In a murine model of brain ischaemia, down-regulation of TRPM7 by shRNA protected rats from ischaemia-induced deficits (Sun et al., 2009). Interestingly, FTY720, an immunosuppressive drug approved for the treatment of MS, inhibits TRPM7 (Qin et al., 2013). It is tempting to speculate that FTY720 suppressed axonal and neuronal loss, one of the main characteristics of MS, partly through its inhibitory effect on TRPM7.
Genetic studies found that SNPs in TRPM2 and TRPM7 genes were associated with two related neurodegenerative disorders, ALS-G and Parkinsonism dementia respectively (Plato et al., 2002). Of note, a different study in a Japanese population did not find significant correlation between SNP in TRPM7 and the ALS-Parkinsonism dementia complex (Hara et al., 2010).
TRPM4 may be involved in axonal and neuronal degeneration in MS. In experimental autoimmune encephalomyelitis, a murine model of MS, TRPM4-deficient mice were protected from axonal and neuronal injury (Schattling et al., 2012). The same study demonstrated that TRPM4-deficient neurons were resistant to excitotoxic stress and energy deficiency in vitro.
TRPML1 is a Ca2+-and Fe2+-permeable non-selective cation channel expressed predominantly in late endosomes and lysosomes (Nilius and Owsianik, 2010). Loss-of-function mutations in TRPML1 (Dong et al., 2008) are responsible for mucolipidosis type IV, a hereditary lysosomal storage disorder characterized by severe psychomotor retardation, ophthalmologic abnormalities, blood iron deficiency and achlorhydria (Sun et al., 2000; Raychowdhury et al., 2004). The pathological mechanisms arising from mutations in TRPML1 have not been clarified.
Blockade of TRPML1-mediated Ca2+ release from late endosome/lysosome vesicles may reduce fusion and trafficking of these organelles (LaPlante et al., 2004). The observed deficiency in lysosome functions could allow accumulation of lipids, followed by impaired autophagosome degradation that normally clears toxic proteins and damaged cell organelles (Vergarajauregui and Puertollano, 2008). Indeed, lysosomal lipid accumulation, defects in membrane trafficking and altered Ca2+ homeostasis are common characteristics observed in various lysosomal storage disorders including Nieman-Pick (NP) syndrome. A recent study demonstrated that increasing TRPML1 expression or using ML-SA1, a small-molecule TRPML1 agonist, restored trafficking defects and reduced lysosome storage and cholesterol accumulation in NP type C macrophages, suggesting that TRPML1 agonist may be useful for the treatment of the NP syndrome and potentially, other lysosomal storage disorders (Shen et al., 2012).
Cardiovascular disorders and TRP channels
Cardiac hypertrophy is associated with arrythmias, decompensation and sudden death. Signal transduction pathways that link pathogenic signals to cardiomyocyte hypertrophy may be exploited for therapeutic intervention. There is emerging evidence that the calcineurin/NFAT complex (probably in concert with the MAPK pathway) is one of the key mechanisms that switch on the genes that cause cardiac hypertrophy (see Eder and Molkentin, 2011). Calcineurin is a serine/threonine phosphatase controlled by intracellular Ca2+ levels and increases in intracellular Ca2+ through TRPC channels (in particular TRPC1, C3 and C6) may activate the calcineurin/NFAT pathway (see Watanabe et al., 2013). Indeed, TRPC1 knock-down by siRNA diminished the hypertrophy phenotype of cultured cardiac myocytes in response to endothelin-1 (ET-1), Ang-II and phenylephrine (Ohba et al., 2007). Consistently, siRNA targeting TRPC1 reduced NFAT activation and hypertrophic response mediated by 5-HT2A receptors in cardiomyoblasts (Vindis et al., 2010). Importantly, TRPC1-deficient mice failed to develop maladaptive cardiac hypertrophy induced by hemodynamic stress and neuro-hormonal excess (Seth et al., 2009). Taken together, these findings imply an important role for TRPC1 channels in the pathogenesis of cardiac hypertrophy.
TRPC3 is another promising target for cardiac hypertrophy. TRPC3 transgenic mice exhibit increased activation of the calcineurin/NFAT pathway, leading to cardiomyopathy and cardiac hypertrophy when challenged by neuroendocrine agonists and/or pressure overload (Nakayama et al., 2006). In keeping with this finding, Pyr3, a selective TRPC3 antagonist (Figure 5), protected mice from pressure overload-induced cardiac hypertrophy (Kiyonaka et al., 2009).
TRPC6 may be also involved in cardiac hypertrophy as knock-down of this gene by siRNAs diminished Ang–II-induced NFAT activation and hypertrophic responses in rat cardiomyocytes (Onohara et al., 2006). Conversely, cardiac-specific overexpression of TRPC6 in transgenic mice led to massive cardiac hypertrophy (Kuwahara et al., 2006).
There is good evidence that TRP channels also contribute to the pathogenesis of hypertension. Expression of TRPC3 is elevated in patients with malignant hypertension in the vascular endothelium of pre-glomerular arterioles (Thilo et al., 2009). ET-1 induces activation of the inositol trisphosphate receptor IP3R1 in arterial myocytes and causes physical coupling of the IP3R1 N-terminus to the TRPC3 channel C-terminus, leading to TRPC3 activation and vasoconstriction (Adebiyi et al., 2010). In addition, the kinase WNK4, which is a causative gene of hereditary hypertension, controls blood pressure by restricting TRPC3-mediated Ca2+ influx in the vasculature (Park et al., 2011b). Collectively, these observations suggest that TRPC3 blockade may be a novel approach to mitigate hypertension. TRPM4 may be also involved in the control of blood pressure as TRPM4-deficient mice showed a hypertensive phenotype, because of elevated catecholamine secretion from adrenal chromaffin cells (Mathar et al., 2010).
Mutations in TRPM4 are reported to be associated with multiple cardiac conduction disorders, including progressive familial heart block type I (Kruse et al., 2009), isolated cardiac conduction diseases (Liu et al., 2010) and atrioventricular block and right bundle branch block (Stallmeyer et al., 2012). The first two reports demonstrated that mutant TRPM4 underwent reduced deSUMOylation, resulting in constitutive SUMOylation and impaired endocytosis, leading to elevated levsl of TRPM4 channels on the cell surface. Such enhanced surface expression of mutant TRPM4 channel may disturb cardiac conduction by prolonging membrane depolarization and increasing inactivation of Na+ channels. On the other hand, heart rates were not altered in TRPM4-null mice while these animals showed hypertension due to enhanced release of catecholamines (Mathar et al., 2010). Lastly, TRPA1 (highly expressed in endothelial cells) has been implicated in the regulation of heart rate and blood pressure (see Earley, 2012).
Conclusions and perspectives
The TRP channel story began in 1969 with the description of a spontaneous Drosophila mutant in which, during prolonged illumination, photoreceptors showed an abnormal, transient response (Cosens and Manning, 1969). Exactly 20 years later, the mutant gene responsible for this abnormal light response was identified and termed ‘trp’ (for transient receptor potential; Montell and Rubin, 1989). 1995 witnessed the discovery of the first mammalian TRP channel, TRPC1 (Wes et al., 1995; Zhu et al., 1995). Within a few years after this seminal discovery, the family of TRP channels exploded to include such long sought-after drug targets as the vanilloid (capsaicin) receptor TRPV1 (Caterina et al., 1997), the camphor receptor TRPV3 (Peier et al., 2002b) and the menthol receptor TRPM8 (McKemy et al., 2002). The initial emphasis of drug discovery efforts was on TRP channels expressed on nociceptive neurons (Patapoutian et al., 2009). Indeed, it took less than a decade to develop the first TRPV1 antagonists to be tried in the clinics as novel analgesic drugs (Szallasi et al., 2007). Antagonists targeting TRPA1 and TRPV3 were quick to follow (Brederson et al, 2013). At the same time, exciting new discoveries have expanded the therapeutic potential of drugs targeting TRP channels into new disease areas, ranging from respiratory diseases (cough, COPD and asthma) through cardiovascular, bladder, metabolic (including obesity and diabetes) and neurological disorders to stroke and cancer.
The rapid progress in TRP channel research has brought the understanding of the roles these channels play in health and disease within reach. However, of the 28 mammalian TRP channels, only 4 (TRPV1 and V3, TRPA1, and TRPM8) have been exploited so far to reach clinical stage of drug development despite accumulating evidence to implicate other TRP channels in diseases. Clearly, several key questions remain to be answered in order to facilitate the translation of the findings in basic research to clinical applications.
First, TRP channels have polymodal gating mechanisms and most also show a broad range of tissue distribution. Even TRPV1 (traditionally considered as a ‘signature of polymodal sensory neurons’) seems to be expressed at unexpected locations such as the skin, urothelium and mast cells (see Szallasi and Blumberg, 1999; Szallasi et al., 2007). Consequently, pharmacological modulation of TRP channels may cause unacceptable, on-target, adverse effects. It was a sobering experience when many TRPV1 antagonists had to be withdrawn from the clinical trials due to either hyperthermia and/or impaired noxious heat sensation (see Moran et al., 2011; Brederson et al., 2013). Of note, TRPV1 antagonists vary significantly in the magnitude of these side effects, raising the possibility that such second-generation antagonists may be synthesized that show an improved clinical benefit to adverse effect ratio.
For other TRP channels, on-target side effects may represent an even bigger problem. For example, blockade of TRPM4 may be beneficial for the treatment of MS (Schattling et al., 2012) and anaphylaxis (Smith and Nilius, 2013), but it may cause dangerous cardiac arrhythmias and hypertension (Abriel et al., 2012). Although both gain-and loss-of-function TRPV4 mutations have been linked to human disease, this channel is another problematic drug target especially when activated by agonists (Nilius and Voets, 2013). Indeed, systemic activation of TRPV4 by GSK1016790A led to endothelial failure and cardiovascular collapse (Willette et al., 2008). To a certain degree, this issue may be circumvented by organ-specific drug delivery: for example, when applied topically to the skin, GSK1016790A promoted intercellular junction development and thus augmented barrier function with no apparent adverse effects (Kida et al., 2012). Selective modulation of TRP channels in diseased, but not in healthy, tissues (e.g. targeting TRPV1-AKAP79 interaction during inflammation) is another attractive approach to circumvent side effects.
Second, most of our understanding regarding the contribution of TRP channels to diseases is derived from studies with in vitro systems or preclinical rodent models, which do not always mirror human diseases. In this context, hereditary diseases caused by mutations in TRP channels (so-called ‘TRP channelopathies’) provide less uncertainty. Unfortunately, gain-and loss-of-function mutations often produce similar phenotypes. In terms of drug discovery, diseases caused by gain-of-function mutations in TRP channels could be more approachable as over-activation of channels could be inhibited by small molecules, while those caused by loss-of-function mutations, particularly truncation types, are difficult to target with small molecules and a less-validated approach such as gene therapy may be required to restore the normal TRP channel function. Besides the direct reversal of dysfunctional TRP channels, modulating the function of the intact TRP channels by therapeutic intervention might provide benefit when diseases related to those channelopathies are caused by mutations in other genes or environmental factors. One such example, although largely speculative, may be the use of a TRPP2 agonist for ADPKD, caused by mutations in PKD1.
Third, for many diseases we already have symptomatic therapeutic modalities and what we really need is a disease-modifying drug. For example, β-agonists improve lung functions in COPD patients, but they do not reverse (or at least halt) disease progression. Likewise, many drugs improve insulin-sensitivity in patients with type 2 diabetes, but these drugs do not fully prevent diabetic complications and neither do they prevent the exhaustion of islet cells.
The advantages and disadvantages of TRP channel agonists and antagonists over currently available therapeutic options need to be carefully weighed. For example, what would be the advantage of TRPA1 and/or TRPM8 antagonists over inhaled glucocorticoids and bronchodilators in patients with asthma? Or how about inhaled TRPV4 antagonists in patients with COPD? One may speculate, but the answers to these questions must come from clinical trials.
These obstacles are real, but probably not insurmountable, and the potential benefits are considerable. Drug discovery companies that can find creative ways to capitalize on targeting TRP channels in disease (and to spare those mediating important physiological functions) may develop novel, first-in-class drugs.
Glossary
- ADPKD
autosomal dominant polycystic kidney disease
- ALS-G
Guam variant of amyotrophic lateral sclerosis
- BDNF
brain-derived neurotrophic factor
- CFA
complete Freund's adjuvant
- COPD
chronic obstructive pulmonary disease
- DNBS
dinitrobenzene sulphonic acid
- FSGS
focal segmental glomerulosclerosis
- GERD
gastroesophageal reflux disease
- GLP-1
glucagon-like peptide-1
- IBD
inflammatory bowel disease
- WDR
wide dynamic range
Conflict of interest
Yosuke Kaneko is employed by Ono Pharmaceuticals Ltd. The company does not sell any drugs mentioned in this paper.
References
- AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, et al. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abriel H, Syam N, Sottas V, Amarouch MY, Rougier JS. TRPM4 channels in the cardiovascular system: physiology, pathophysiology, and pharmacology. Biochem Pharmacol. 2012;84:873–881. doi: 10.1016/j.bcp.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, et al. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res. 2010;106:1603–1612. doi: 10.1161/CIRCRESAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- Aizawa N, Wyndaele JJ, Homma Y, Igawa Y. Effects of TRPV4 cation channel activation on the primary bladder afferent activities of the rat. Neurourol Urodyn. 2012;31:148–155. doi: 10.1002/nau.21212. [DOI] [PubMed] [Google Scholar]
- Akbar A, Yiangou F, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory nerve fibers in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4: integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci. 2008;28:1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino P, Soldovieri MV, Russo C, Taglialatela M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br J Pharmacol. 2013;168:1430–1444. doi: 10.1111/bph.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand U, Otto WR, Facer P, Zebda N, Selmer I, Gunthorpe MJ, et al. TRPA1 receptor localization in the human peripheral nervous system and functional studies in cultured human and rat sensory neurons. Neurosci Lett. 2008;438:221–227. doi: 10.1016/j.neulet.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Andrade EL, Ferreira J, André E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol. 2006;72:104–114. doi: 10.1016/j.bcp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Andrade EL, Forner S, Bento AF, Leite DF, Dias MA, Leal PC, et al. TRPA1 receptor modulation attenuates bladder overactivity induced by spinal cord injury. Am J Physiol Renal Physiol. 2011;300:F1223–F1234. doi: 10.1152/ajprenal.00535.2010. [DOI] [PubMed] [Google Scholar]
- Andrè E, Campi B, Trevisani M, Ferreira J, Malheiros A, Yunes RA, et al. Pharmacological characterisation of the plant sesquiterpenes polygodial and drimanial as vanilloid receptor agonists. Biochem Pharmacol. 2006;71:1248–1254. doi: 10.1016/j.bcp.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrè E, Gatti R, Trevisani M, Preti D, Baraldi PG, Patacchini R, et al. Transient receptor potential ankyrin receptor 1 is a novel target for pro-tussive agents. Br J Pharmacol. 2009;158:1621–1628. doi: 10.1111/j.1476-5381.2009.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006;126:2664–2672. doi: 10.1038/sj.jid.5700468. [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M, Olschewski A, Papić L, Kremer H, McEntagart ME, Uhrig S, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42:160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avelino A, Charrua A, Frias B, Cruz C, Boudes M, de Ridder D, et al. Transient receptor potential channels in bladder function. Acta Physiol (Oxf) 2013;207:110–122. doi: 10.1111/apha.12021. [DOI] [PubMed] [Google Scholar]
- Bagal SK, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, et al. Ion channels as therapeutic targets: a drug discovery perspective. J Med Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem. 2010a;285:19362–19371. doi: 10.1074/jbc.M109.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. 2010b;161:707–720. doi: 10.1111/j.1476-5381.2010.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain. 2011;152:1156–1164. doi: 10.1016/j.pain.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Nociceptive and pro-inflammatory effects of dimethylallyl pyrophosphate via TRPV4 activation. Br J Pharmacol. 2012;166:1433–1443. doi: 10.1111/j.1476-5381.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem. 2003;278:31709–31716. doi: 10.1074/jbc.M304437200. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Becker EB, Oliver PL, Glitsch MD, Banks GT, Achilli F, Hardy A, et al. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci U S A. 2009;106:6706–6711. doi: 10.1073/pnas.0810599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvisi MG, Dubuis E, Birrell MA. Transient receptor potential A1 channels: insights into cough and airway inflammatory disease. Chest. 2011;140:1040–1047. doi: 10.1378/chest.10-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat JM, Bielefeldt K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur J Gastroenterol Hepatol. 2006;18:263–270. doi: 10.1097/00042737-200603000-00006. [DOI] [PubMed] [Google Scholar]
- Binder A, May D, Baron R, Maier C, Tölle TR, Treede RD, et al. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One. 2010;6:e17387. doi: 10.1371/journal.pone.0017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kullmann FA, Lee H, Barrick S, de Groat W, Kanai A, et al. Activation of urothelial transient receptor potential vanilloid 4 by 4alpha-phorbol 12,13-didecanoate contributes to altered bladder reflexes in the rat. J Pharmacol Exp Ther. 2007;323:227–235. doi: 10.1124/jpet.107.125435. [DOI] [PubMed] [Google Scholar]
- Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, et al. TRPA1 agonists evoke coughing in guinea pig and human volunteers. Am J Respir Crit Care Med. 2009;180:1042–1047. doi: 10.1164/rccm.200905-0665OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Brierley SM, Harrington AM, Hughes PA. TRP channels in visceral pain. Open Pain J. 2013;6:23–30. [Google Scholar]
- Bley K. Effects of topical capsaicin on cutaneous innervation: implications for pain management. Open Pain J. 2012;5:23–28. [Google Scholar]
- Boesmans W, Owsianik G, Tack J, Voets T, Vanden Berghe P. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol. 2011;162:18–37. doi: 10.1111/j.1476-5381.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141:834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbíró I, Lisztes E, Tóth BI, Czifra G, Oláh A, Szöllösi AG, et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J Invest Dermatol. 2011;131:1605–1614. doi: 10.1038/jid.2011.122. [DOI] [PubMed] [Google Scholar]
- Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol. 2013;716:61–76. doi: 10.1016/j.ejphar.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Breneman DL, Cardone JS, Blumsack RF, Lather RM, Searle EA, Pollack VE. Topical capsaicin for treatment of hemodialysis-related pruritus. J Am Acad Dermatol. 1992;26:91–94. doi: 10.1016/0190-9622(92)70013-6. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Page AJ, Hughes PA, Adam B, Liebregts T, Cooper NJ, et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterol. 2008;134:2059–2069. doi: 10.1053/j.gastro.2008.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brixel LR, Monteilh-Zoller MK, Ingenbrandt CS, Fleig A, Penner R, Enklaar T, et al. TRPM5 regulates glucose-stimulated insulin secretion. Pflugers Arch. 2010;460:69–76. doi: 10.1007/s00424-010-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DC, Iadarola MJ, Perkowski SZ, Erin H, Shofer F, Laszlo KJ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059. doi: 10.1097/00000542-200511000-00020. [DOI] [PubMed] [Google Scholar]
- Btesh J, Fischer MJ, Stott K, McNaughton PA. Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia. J Neurosci. 2013;33:9184–9193. doi: 10.1523/JNEUROSCI.4991-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, et al. Modulation of cough response by sensory inputs from the nose – role of trigeminal TRPA1 versus TRPM8 channels. Cough. 2012;8:11. doi: 10.1186/1745-9974-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo RR, Meegalla SK, Parks DJ, Parsons WH, Ballentine SK, Lubin ML, et al. Discovery of vinylcycloalkyl-substituted benzimidazole TRPM8 antagonists effective in the treatment of cold allodynia. Bioorg Med Chem Lett. 2012;22:1903–1907. doi: 10.1016/j.bmcl.2012.01.060. [DOI] [PubMed] [Google Scholar]
- Canning BJ. Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:33–47. doi: 10.1378/chest.129.1_suppl.33S. [DOI] [PubMed] [Google Scholar]
- Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, et al. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem. 2010;285:27532–27535. doi: 10.1074/jbc.C110.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Aviello G, Romano B, Borrelli F, De Petrocellis L, Di Marzo V, et al. Modulation of mouse gastrointestinal motility by allyl isothiocyanate, a constituent of cruciferous vegetables (Brassicaceae): evidence for TRPA1-independent effects. Br J Pharmacol. 2012;165:1966–1977. doi: 10.1111/j.1476-5381.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cathcart CJ, Johnson SA, Reynolds LR, Al-Nadaf S, Budsberg SC. Efficacy of ABT-116, an antagonist of the transient receptor potential vanilloid type 1, in providing analgesia for dogs with chemically induced synovitis. Am J Vet Res. 2012;73:19–26. doi: 10.2460/ajvr.73.1.19. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. doi: 10.1053/j.gastro.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537–1541. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Charrua A, Cruz CD, Narayanan S, Gharat L, Gullapalli S, Cruz F, et al. GRC-6211, a new oral specific TRPV1 antagonist, decreases bladder overactivity and noxious bladder input in cystitis animal models. J Urol. 2009;181:379–386. doi: 10.1016/j.juro.2008.08.121. [DOI] [PubMed] [Google Scholar]
- Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One. 2010;5:e11161. doi: 10.1371/journal.pone.0011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- Cheng W, Sun C, Zheng J. Heteromerization of TRP channel subunits: extending functional diversity. Protein Cell. 2010;1:802–810. doi: 10.1007/s13238-010-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, O'Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2007;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Chubanov V, Mederos y Schnitzler M, Meißner M, Schäfer S, Abstiens K, Hofmann T, et al. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol. 2012;166:1357–1376. doi: 10.1111/j.1476-5381.2012.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Clare JJ. Targeting ion channels for drug discovery. Discov Med. 2010;9:253–260. [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Colsoul B, Schraenen A, Lemaire K, Quintens R, Van Lommel L, Segal A, et al. Loss of high-frequency glucose-induced Ca2+ oscillations in pancreatic islets correlates with impaired glucose tolerance in Trpm5−/− mice. Proc Natl Acad Sci U S A. 2010;107:5208–5213. doi: 10.1073/pnas.0913107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colsoul B, Nilius B, Vennekens R. Transient receptor potential (TRP) cation channels in diabetes. Curr Top Med Chem. 2013;13:258–269. doi: 10.2174/1568026611313030004. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Cromer BA, McIntyre P. Painful toxins acting on TRPV1. Toxicon. 2008;51:163–173. doi: 10.1016/j.toxicon.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cruz F, Dinis P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn. 2007;26:920–927. doi: 10.1002/nau.20479. [DOI] [PubMed] [Google Scholar]
- Cruz F, Guimaräes M, Silva C, Reis M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet. 1997;350:640–641. doi: 10.1016/S0140-6736(05)63330-2. [DOI] [PubMed] [Google Scholar]
- Cui M, Honore P, Zhong C, Gauvin G, Mikusa J, Hernandez G, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:175–285. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Morinello A, Marini P, Magherini PC, Orlando P, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyryn type-1 and melastatin-8. Br J Pharmacol. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Morinello AS, Allara M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco J, Steiger D, Dourado M, Emerling D, Duncton MA. 5-benzyloxytryptamine as an antagonist of TRPM8. Bioorg Med Chem Lett. 2010;20:7076–7079. doi: 10.1016/j.bmcl.2010.09.099. [DOI] [PubMed] [Google Scholar]
- Delescluse I, Mace H, Adcock JJ. Inhibition of airway hyper-responsiveness by TRPV1 antagonists (SB-705498 and PF-04065463) in the unanaesthetized, ovalbumin-sensitized guinea pig. Br J Pharmacol. 2012;166:1822–1832. doi: 10.1111/j.1476-5381.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessaint J, Yu W, Krause JE, Yue L. Yohimbine inhibits firing activities of rat dorsal root ganglion neurons by blocking Na+ channels and vanilloid VR1 receptors. Eur J Pharmacol. 2004;485:11–20. doi: 10.1016/j.ejphar.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Dray A. Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br J Pharmacol. 1991;104:1045–1049. doi: 10.1111/j.1476-5381.1991.tb12547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos Y Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurtz T, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Araki I, Yoshiyama M, Nomura T, Takeda M. Transient receptor potential channel A1 involved in sensory transduction of rat urinary bladder through C-fiber pathway. Urology. 2007;70:826–831. doi: 10.1016/j.urology.2007.06.1110. [DOI] [PubMed] [Google Scholar]
- Du S, Araki I, Kobayashi H, Zakoji H, Sawada N, Takeda M. Differential expression profile of cold (TRPA1) and cool (TRPM8) receptors in human urogenital organs. Urology. 2008;72:450–455. doi: 10.1016/j.urology.2007.11.127. [DOI] [PubMed] [Google Scholar]
- Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res. 1998;58:1515–1520. [PubMed] [Google Scholar]
- Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, et al. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22:526–535. doi: 10.1681/ASN.2010050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer RM, Le LN, Yan J, Wei X, Nassini R, Materazzi S, et al. Activation of TRPA1 on dural afferents: a potential mechanism of headache pain. Pain. 2012;153:1949–1958. doi: 10.1016/j.pain.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circ Res. 2011;108:265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- Eid SR. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain. 2011;132:132–141. doi: 10.1016/j.pain.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory-and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisfeld J, Lückhoff A. TRPM2. Hand Exp Pharmacol. 2007;179:237–252. doi: 10.1007/978-3-540-34891-7_14. [DOI] [PubMed] [Google Scholar]
- Ellis CN, Berberian B, Sulica VI, Dodd WA, Jarratt MT, Katz HI, et al. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J Am Acad Dermatol. 1993;29:438–442. doi: 10.1016/0190-9622(93)70208-b. [DOI] [PubMed] [Google Scholar]
- Engel MA, Becker C, Reeh PW, Neurath MF. Role of sensory neurons in colitis: increasing evidence for a neuroimmune link in the gut. Inflamm Bowel Dis. 2011;17:1030–1033. doi: 10.1002/ibd.21422. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010a;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, et al. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010b;298:F692–F701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD, Benham CD, Chessell IP, Bountra C, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: movign away from sensory nerves. Br J Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes ES, Vong CT, Quek S, Cheong J, Awal S, Gentry C, et al. Superoxide generation and leukocyte accumulation: key elements in the mediation of leukotriene B4-induced itch by transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1. FASEB J. 2013;27:1664–1673. doi: 10.1096/fj.12-221218. [DOI] [PubMed] [Google Scholar]
- Fichna J, Mokrowieczka A, Cygankiewicz AI, Zakrzewski PA, Malecka-Panas E, Janecka A, et al. Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: a new strategy for inflammatory bowel disease treatment? Neurogastroenterol Motil. 2012;24:e557–e560. doi: 10.1111/j.1365-2982.2012.01999.x. [DOI] [PubMed] [Google Scholar]
- Fischer MJ, Betsh J, McNaughton PA. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci. 2013;33:7407–7414. doi: 10.1523/JNEUROSCI.3721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming PK, Dedman AM, Xu SZ, Li J, Zeng F, Naylor J, et al. Sensing of lysophospholipids by TRPC5 calcium channel. J Biol Chem. 2006;281:4977–4982. doi: 10.1074/jbc.M510301200. [DOI] [PubMed] [Google Scholar]
- Fujino K, de la Fuente SG, Takami Y, Takahashi G, Mantyh CR. Attenuation of acid-induced esophagitis in VR-1 deficient mice. Gut. 2006;55:34–40. doi: 10.1136/gut.2005.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. Neurosci. 2011;31:1721–1733. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison SR, Stucky CL. The dynamic TRPA1 channel: a suitable pharmacological target? Curr Pharm Biotechnol. 2011;12:1689–1697. doi: 10.2174/138920111798357302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, et al. Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans. Pain. 2008;136:202–210. doi: 10.1016/j.pain.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomtsyan A, Faltynek C, editors. Vanilloid Receptor TRPV1 in Drug Discovery: Targeting Pain and Other Pathological Disorders. Hoboken, NJ: John Wiley & Sons; 2010. [Google Scholar]
- Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, et al. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health. 2005;5:2. doi: 10.1186/1472-6874-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goso C, Evengelista S, Tramontana M, Manzini S, Blumberg PM, Szallasi A. Topical capsaicin administration protects against trinitrobenzene sulfonic acid-induced colitis in the rat. Eur J Pharmacol. 1993;249:185–190. doi: 10.1016/0014-2999(93)90431-g. [DOI] [PubMed] [Google Scholar]
- Goswami C. TRP-mediated cytoskeletal reorganization: implications for disease and drug development. In: Szallasi A, Bíró T, editors. TRP Channels in Drug Discovery. New York, Heidelberg, Dordrecht, London: Humana Press/Springer; 2012. pp. 13–39. Methods in Pharmacology and Toxicology, Springer Protocols. [Google Scholar]
- Grace M, Birrell MA, Dubuis E, Maher SA, Belvisi MG. Transient receptor potential channels mediate the tussive response to prostaglandin E2 and bradykinin. Thorax. 2012;67:891–900. doi: 10.1136/thoraxjnl-2011-201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram DX, Ahren B, Nagy I, Olsen UB, Brand CL, Sundler F, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- Grand T, Demion M, Norez C, Mettey Y, Launay P, Becq F, et al. 9-phenanthrol inhibits human TRPM4 but not TRPM5 cationic channels. Br J Pharmacol. 2008;153:1697–1705. doi: 10.1038/bjp.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Kraft R, Schultz G, Harteneck C. Activation of the melastatin-related cation channel TRPM3 by d-erythro-sphingosine [corrected. Mol Pharmacol. 2005;67:798–805. doi: 10.1124/mol.104.006734. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170:1276–1280. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: the sixth sense of the musculoskeletal system? Ann NY Acad Sci. 2010;1192:404–409. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas ET, Rowland K, Gautam M. Tooth injury increases expression of the cold sensitive TRP channel TRPA1 in trigeminal neurons. Arch Oral Biol. 2011;56:1604–1609. doi: 10.1016/j.archoralbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Hajós M, Obál F, Jr, Jancsó G, Obál F. Capsaicin impairs preoptic serotonin-sensitive structures mediating hypothermia in rats. Neurosci Lett. 1985;54:97–102. doi: 10.1016/s0304-3940(85)80124-5. [DOI] [PubMed] [Google Scholar]
- Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, et al. TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:310–313. doi: 10.1002/ajmg.b.30966. [DOI] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano N, Suzuki H, Muraki Y, Muraki K. Stimulation of human TRPA1 channels by clinical concentrations of the antirheumatic drug auranofin. Am J Physiol Cell Physiol. 2013;304:C354–C361. doi: 10.1152/ajpcell.00096.2012. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kondo T, Ishimatsu M, Takeya M, Igata S, Nakamura K, et al. Function and expression pattern of TRPM8 in bladder afferent neurons associated with bladder outlet obstruction in rats. Auton Neurosci. 2011;164:27–33. doi: 10.1016/j.autneu.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Cui AM, Go RC, Davenport B, Shetler CM, Heizer JW, et al. Altered functional properties of a TRPM2 variant in Guamanian ALS and PD. Proc Natl Acad Sci USA. 2008;105:18029–18034. doi: 10.1073/pnas.0808218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004a;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- Hill K, Benham CD, McNulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004b;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hisanaga E, Nagasawa M, Ueki K, Kulkarni RN, Mori M, Kojima I. Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells. J Biol Chem. 2009;279:35741–35748. doi: 10.2337/db08-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T, Kistner K, Miermeister F, Winkelmann R, Wittmann J, Fischer MJ, et al. TRPA1 and TRPV1 are differentially involved in heat nociception of mice. Eur J Pain. 2013;17:1472–1482. doi: 10.1002/j.1532-2149.2013.00331.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacol Ther. 2011;131:142–170. doi: 10.1016/j.pharmthera.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J Pharmacol Exp Ther. 2005;314:410–421. doi: 10.1124/jpet.105.083915. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4:159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Gonnella GL. Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J. 2013;6:95–107. doi: 10.2174/1876386301306010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Mannes AJ. The vanilloid agonist resiniferatoxin for interventional-based pain control. Curr Top Med Chem. 2011;11:2171–2179. doi: 10.2174/156802611796904942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Shimizu S, Hagiwara T, Wajima T, Miyazaki A, Mori Y, et al. Extracellular-added ADP-ribose increases intracellular free Ca2+ concentration through Ca2+ release from stores, but not through TRPM2-mediated Ca2+ entry, in rat beta-cell line RIN-5F. J Pharmacol Sci. 2006;101:174–178. doi: 10.1254/jphs.scj06001x. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Aviello G, Borrelli F, Romano B, Piscitelli F, et al. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol. 2012;166:1444–1460. doi: 10.1111/j.1476-5381.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Ruparel NB, Akopian AN, Shapiro MS, Henry MA. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain. 2009;146:301–307. doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Jian MY, King JA, Al-Mehdi AB, Liedtke W, Townsley MI. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am J Respir Cell Mol Biol. 2008;38:386–392. doi: 10.1165/rcmb.2007-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Yang W, Zou J, Beech DJ. TRPM2 channel properties, functions and therapeutic potentials. Expert Opin Ther Targets. 2010;14:973–988. doi: 10.1517/14728222.2010.510135. [DOI] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Mantyh P. TRPV1 and bone cancer pain. In: Gomtsyan A, Faltynek C, editors. Vanilloid Receptor TRPV1 in Drug Discovery. Targeting Pain and Other Pathological Disorders. Hoboken, NJ: John Wiley & Sons; 2010. pp. 191–205. [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Honore P. The role of TRPV1 receptors in osteoarthritic pain. In: Gomtsyan A, Faltynek C, editors. Vanilloid Receptor TRPV1 in Drug Discovery. Targeting Pain and Other Pathological Disorders. Hoboken, NJ: John Wiley & Sons; 2010. pp. 175–190. [Google Scholar]
- Kaczmarek JS, Riccio A, Clapham DE. Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc Natl Acad Sci USA. 2012;109:7888–7892. doi: 10.1073/pnas.1205869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Szallasi A. TRP channels as therapeutic targets. Curr Top Med Chem. 2013;13:241–243. doi: 10.2174/1568026611313030001. [DOI] [PubMed] [Google Scholar]
- Keszthelyi D, Troost FJ, Jonkers DM, Helyes Z, Hamer HM, Ludidi S, et al. Alterations in mucosal neuropeptides in patients with irritable bowel syndrome and ulcerative colitis in remission: a role in pain symptom generation? Eur J Pain. 2013;17:1299–1306. doi: 10.1002/j.1532-2149.2013.00309.x. [DOI] [PubMed] [Google Scholar]
- Ketterer C, Müssig K, Heni M, Dudziak K, Randrianarisoa E, Wagner R, et al. Genetic variation within the TRPM5 locus associates with prediabetic phenotypes in subjects at increased risk for type 2 diabetes. Metabolism. 2011;60:1325–1333. doi: 10.1016/j.metabol.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Khairatkar-Joshi N, Maharaj N, Thomas A. The TRPV3 receptor as a pain target: a therapeutic promise or just some more new biology? Open Drug Discov J. 2010;2:89–97. [Google Scholar]
- Kida N, Sokabe T, Kashio M, Haruna K, Mizuno Y, Suga Y, et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch. 2012;463:715–725. doi: 10.1007/s00424-012-1081-3. [DOI] [PubMed] [Google Scholar]
- Kitaguchi T, Swartz KJ. An inhibitor of TRPV1 channels isolated from funnel web spider venom. Biochemistry. 2005;44:15544–15549. doi: 10.1021/bi051494l. [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen TK, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, et al. Modulation of the transient receptor potential vanilloid channel TRPV4 by 4α-phorbol esters: a structure-activity study. J Med Chem. 2009;52:2933–2939. doi: 10.1021/jm9001007. [DOI] [PubMed] [Google Scholar]
- Klose C, Straub I, Riehle M, Ranta F, Krautwurst D, Ullrich S, et al. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol. 2011;162:1757–1769. doi: 10.1111/j.1476-5381.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008;24:142–154. doi: 10.1097/AJP.0b013e318158ed9e. [DOI] [PubMed] [Google Scholar]
- Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68–77. doi: 10.2174/138920111793937961. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, et al. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res. 2012;65:149–158. doi: 10.1016/j.phrs.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell. 2005;18:61–69. doi: 10.1016/j.molcel.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1−/− mice. J Physiol. 2004;555:115–123. doi: 10.1113/jphysiol.2003.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köttgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G, Montalbetti N, Simonin A, Danko T, Balazs B, Zsembery A, et al. Inhibition of the human epithelial calcium channel TRPV6 by 2-aminoethoxydiphenyl borate (2-APB) Cell Calcium. 2012;52:468–480. doi: 10.1016/j.ceca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Kraft R, Grimm C, Frenzel H, Harteneck C. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol. 2006;148:264–273. doi: 10.1038/sj.bjp.0706739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, et al. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One. 2010;5:e12859. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup AL, Ny L, Astrand M, Bajor A, Hvid-Jensen F, Hansen MB, et al. Randomised clinical trial: the efficacy of a transient receptor potential vanilloid 1 antagonist AZD1386 in human oesophageal pain. Pharmacol Ther. 2011;33:1113–1122. doi: 10.1111/j.1365-2036.2011.04629.x. [DOI] [PubMed] [Google Scholar]
- Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, et al. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. 2009;119:2737–2744. doi: 10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusudo T, Wang Z, Mizuno A, Suzuki M, Yamashita H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. Appl Physiol. 2012;112:1223–1232. doi: 10.1152/japplphysiol.01070.2011. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A. 2012;109:E524–E532. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Sethuraman G, Ramam M, Stone K, Simpson MA, McGrath JA. Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol. 2012;167:440–442. doi: 10.1111/j.1365-2133.2012.11115.x. [DOI] [PubMed] [Google Scholar]
- Landourè G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, et al. Mutations in TRPV4 cause Charcot–Marie–Tooth disease type 2C. Nature Genet. 2010;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlante JM, Ye CP, Quinn SJ, Goldin E, Brown EM, Slaugenhaupt SA, et al. Functional links between mucolipin-1 and Ca2+-dependent membrane trafficking in mucolipidosis IV. Biochem Biophys Res Commun. 2004;322:1384–1391. doi: 10.1016/j.bbrc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Lashinger ES, Steiginga MS, Hieble JP, Leon LA, Gardner SD, Nagilla R, et al. AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am J Physiol Renal Physiol. 2008;295:F803–F810. doi: 10.1152/ajprenal.90269.2008. [DOI] [PubMed] [Google Scholar]
- Leamy AW, Shukla P, McAlexander MA, Carr MJ, Ghatta S. Curcumin ( (E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci Lett. 2011;503:157–162. doi: 10.1016/j.neulet.2011.07.054. [DOI] [PubMed] [Google Scholar]
- Lee SP, Buber MT, Yang Q, Cerne R, Cortés RY, Sprous DG, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153:1739–1749. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim YK, Chung JH. Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp Dermatol. 2009;18:431–436. doi: 10.1111/j.1600-0625.2008.00806.x. [DOI] [PubMed] [Google Scholar]
- Lee YM, Kang SM, Lee SR, Kong KH, Lee JY, Kim EJ, et al. Inhibitory effects of TRPV1 blocker on UV-induced responses in the hairless mice. Arch Dermatol Res. 2011;303:727–736. doi: 10.1007/s00403-011-1153-9. [DOI] [PubMed] [Google Scholar]
- Lehen'kyi V, Prevarskaya N. Oncogenic TRP channels. Adv Exp Med Biol. 2011;704:929–945. doi: 10.1007/978-94-007-0265-3_48. [DOI] [PubMed] [Google Scholar]
- Lei Z, Ishizuka O, Imamura T, Noguchi W, Yamagishi T, Yokoyama H, et al. Functional roles of transient receptor potential melastatin 8 (TRPM8) channels in the cold stress-induced detrusor overactivity pathways in conscious rats. Neurourol Urodyn. 2013;32:500–504. doi: 10.1002/nau.22325. [DOI] [PubMed] [Google Scholar]
- Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner K, Kazanski V, Müller M, Essin K, Henke B, Gollasch M, et al. Hyperforin, a key constituent of St. John's wort, specifically activates TRPC6 channels. FASEB J. 2007;21:4101–4111. doi: 10.1096/fj.07-8110com. [DOI] [PubMed] [Google Scholar]
- Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, et al. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators-identification of a novel pharmacophore. Mol Pharmacol. 2010;77:368–377. doi: 10.1124/mol.109.057513. [DOI] [PubMed] [Google Scholar]
- Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, et al. TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect. 2011b;119:784–793. doi: 10.1289/ehp.1002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jiang J, Yue L. Functional characterization of homo-and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li Q, Yang G, Kolosov VP, Perelman JM, Zhou XD. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J Allergy Clin Immunol. 2011a;128:626–634. doi: 10.1016/j.jaci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Liang J, Xiao G, Ji W. Capsaicin induces reflex scratching in inflamed skin. Pharmacology. 2011;2011:82–87. doi: 10.1159/000330094. [DOI] [PubMed] [Google Scholar]
- Liberati S, Morelli MB, Nabissi M, Santoni M, Santoni G. Oncogenic and anti-oncogenic effects of transient receptor potential channels. Curr Top Med Chem. 2013;13:344–366. doi: 10.2174/1568026611313030011. [DOI] [PubMed] [Google Scholar]
- Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012;90:558–564. doi: 10.1016/j.ajhg.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BC, Song X, Lu XY, Li DT, Eaton DC, Shen BZ, et al. High glucose induces podocyte apoptosis by stimulating TRPC6 via elevation of reactive oxygen species. Biochim Biophys Acta. 2013;1833:1434–1442. doi: 10.1016/j.bbamcr.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, et al. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. 2010;3:374–385. doi: 10.1161/CIRCGENETICS.109.930867. [DOI] [PubMed] [Google Scholar]
- Liu L, Welch JM, Erickson RP, Reinhart PH, Simon SA. Different responses to repeated applications of zingerone in behavioral studies, recordings from intact and cultured TG neurons, and from VR1 receptors. Physiol Behav. 2000;69:177–186. doi: 10.1016/s0031-9384(00)00200-6. [DOI] [PubMed] [Google Scholar]
- Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012;28:145–154. doi: 10.1007/s12264-012-1207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysy J, Sistiery-Ittah M, Israelit Y, Shmueli A, Strauss-Liviatan N, Mindrul V, et al. Topical capsaicin-a novel and effective treatment for idiopathic intractable pruritus ani: a randomised, placebo controlled, crossover study. Gut. 2003;52:1323–1326. doi: 10.1136/gut.52.9.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. 2012b;4:88–96. doi: 10.1093/jmcb/mjs001. [DOI] [PubMed] [Google Scholar]
- Ma X, He D, Ru X, Chen Y, Cai Y, Bruce IC, et al. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br J Pharmacol. 2012a;166:349–358. doi: 10.1111/j.1476-5381.2011.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Barbanti G, Santicioli P, Beneforti P, Misuri D, Meli A, et al. Cystometric evidence that capsaicin-sensitive nerves modulate the afferent branch of micturition reflex in humans. J Urol. 1989;142:150–154. doi: 10.1016/s0022-5347(17)38701-3. [DOI] [PubMed] [Google Scholar]
- Majeed Y, Bahnasi Y, Seymour VA, Wilson LA, Milligan CJ, Agarwal AK, et al. Rapid and contrasting effects of rosiglitazone on transient receptor potential TRPM3 and TRPC5 channels. Mol Pharmacol. 2011a;79:1023–1030. doi: 10.1124/mol.110.069922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Amer MS, Agarwal AK, McKeown L, Porter KE, O'Regan DJ, et al. Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br J Pharmacol. 2011b;162:1509–1520. doi: 10.1111/j.1476-5381.2010.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed Y, Tumova S, Green BL, Seymour VA, Woods DM, Agarwal AK, et al. Pregnenolone sulphate-independent inhibition of TRPM3 channels by progesterone. Cell Calcium. 2012;51:1–11. doi: 10.1016/j.ceca.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S, Sample SJ, Schwartz Z, Nemke B, Jacobson PB, Cozzi EM, et al. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet Res. 2012;8:185–191. doi: 10.1186/1746-6148-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kössl M, Holsboer F, et al. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27:832–839. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, et al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61:246–252. doi: 10.1161/HYPERTENSIONAHA.112.201434. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Andrè E, Campi B, Amadesi S, Trevisani M, et al. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londoño JE, et al. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010;120:3267–3279. doi: 10.1172/JCI41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JM, Qin N, Colburn RW, Dax SL, et al. The design and synthesis of novel, phosphonate-containing transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett. 2012;22:2922–2926. doi: 10.1016/j.bmcl.2012.02.060. [DOI] [PubMed] [Google Scholar]
- Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibers in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897–902. doi: 10.1097/00042737-200409000-00014. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) Br J Pharmacol. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meotti FC, Forner S, Lima-Garcia JF, Viana AF, Calixto JB. Antagonism of the transient receptor potential ankyrin 1 (TRPA1) attenuates hyperalgesia and urinary bladder overactivity in cyclophosphamide-induced haemorrhagic cystitis. Chem Biol Interact. 2013;203:440–447. doi: 10.1016/j.cbi.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol. 2011;704:531–544. doi: 10.1007/978-94-007-0265-3_29. [DOI] [PubMed] [Google Scholar]
- Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, et al. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millqvist E. The airway sensory hyperreactivity syndrome. Pulm Pharmacol. 2011;24:263–266. doi: 10.1016/j.pupt.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Minke B. The history of the Drosophila TRP channel: the birth of a new channel superfamily. J Neurogenet. 2010;24:216–233. doi: 10.3109/01677063.2010.514369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Nordstrom E, Mannem A, Smith C, Banerjee B, Sengupta JN. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience. 2007;148:1021–1032. doi: 10.1016/j.neuroscience.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–21264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- Monet M, Gkika D, Lehen'kyi V, Pourtier A, Vanden Abeele F, Bidaux G, et al. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Montell C. Mg2+ homeostasis: the Mg2+nificent TRPM chanzymes. Curr Biol. 2003;13:R799–R801. doi: 10.1016/j.cub.2003.09.048. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- Morelli MB, Amantini C, Liberati S, Santoni M, Nabissi M. TRP channels: new potential therapeutic approaches in CNS neuropathies. CNS Neurol Disord Drug Targets. 2013;12:274–293. doi: 10.2174/18715273113129990056. [DOI] [PubMed] [Google Scholar]
- Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A, Rimmerman N, Bregman T, Straiker A, Felder CC, Shoham S, et al. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008;22:3024–3034. doi: 10.1096/fj.07-101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, et al. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol. 2006a;6:6. doi: 10.1186/1471-2490-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G, Waters J, Chessell IP, Bountra C, Agarwal SK, Anand P. Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol. 2006b;6:31. doi: 10.1186/1471-2490-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay I, Gomes P, Aranake S, Shetty M, Karnik P, Damle M, et al. Expression of functional TRPA1 receptor on human lung fibroblasts and epithelial cells. J Recept Signal Transduct Res. 2011;31:350–358. doi: 10.3109/10799893.2011.602413. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012a;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012b;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Benemei S, Fusi C, Trevisan dos Santos G, Materazzi S. Transient receptor potential channels in chemotherapy-induced neuropathy. Open Pain J. 2013;5:32–37. [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ. Production of a specific extracellular inhibitor of TRPM3 channels. Br J Pharmacol. 2008;155:567–573. doi: 10.1038/bjp.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroğlu M, Lückhoff A, Jüngling E. Antagonist effect of flufenamic acid on TRPM2 cation channels activated by hydrogen peroxide. Cell Biochem Funct. 2007;25:383–387. doi: 10.1002/cbf.1310. [DOI] [PubMed] [Google Scholar]
- Nelson PL, Beck A, Chen H. Transient receptor proteins illuminated: current views on TRPs and disease. Vet J. 2011;187:153–164. doi: 10.1016/j.tvjl.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Nieto-Posadas A, Jara-Oseguera A, Rosenbaum T. TRP channel gating physiology. Curr Top Med Chem. 2011a;11:2131–2150. doi: 10.2174/156802611796904870. [DOI] [PubMed] [Google Scholar]
- Nieto-Posadas A, Picazo-Juárez G, Llorente I, Jara-Oseguera A, Morales-Lázaro S, Escalante-Alcalde D, et al. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol. 2011b;8:78–85. doi: 10.1038/nchembio.712. [DOI] [PubMed] [Google Scholar]
- Nilius B. Transient receptor potential TRP channels as therapeutic drug targets: next round! Curr Top Med Chem. 2013;13:244–246. doi: 10.2174/1568026611313030002. [DOI] [PubMed] [Google Scholar]
- Nilius B, Appendino G. Spices: the savory and beneficial science of pungency. Rev Physiol Biochem Pharmacol. 2013;164:1–76. doi: 10.1007/112_2013_11. [DOI] [PubMed] [Google Scholar]
- Nilius B, Bíró T. TRPV3: a ‘more than skinny’ channel. Exp Dermatol. 2013;22:447–452. doi: 10.1111/exd.12163. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460:437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12:218–229. doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Voets T. The puzzle of TRPV4 channelopathies. EMBO Rep. 2013;14:152–163. doi: 10.1038/embor.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Owsianik G. Irritating channels: the case of TRPA1. J Physiol. 2011;589:1543–1549. doi: 10.1113/jphysiol.2010.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto Y, Yoshida A, Ikeda S, Kamikawa Y, Harada K, Ohwatashi A, et al. Effect of menthol on detrusor smooth-muscle contraction and the micturition reflex in rats. Urology. 2008;72:701–705. doi: 10.1016/j.urology.2007.11.137. [DOI] [PubMed] [Google Scholar]
- Nyman E, Franzén B, Nolting A, Klement G, Liu G, Nilsson M, et al. In vitro pharmacological characterization of a novel TRPA1 antagonist and proof of mechanism in a human dental pulp model. J Pain Res. 2013;6:59–70. doi: 10.2147/JPR.S37567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Conor CJ, Griffin TM, Liedtke W, Guilak F. Increased susceptibility of Trpv4-deficient mice to obesity and obesity-induced osteoarthritis with very high-fat diet. Ann Rheum Dis. 2013;72:300–304. doi: 10.1136/annrheumdis-2012-202272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Watanabe H, Murakami M, Takahashi Y. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Ohkawara S, Tanaka-Kagawa T, Furukawa Y, Jinno H. Methylglyoxal activates the human transient receptor potential ankyrin 1 channel. J Toxicol Sci. 2012;37:831–835. doi: 10.2131/jts.37.831. [DOI] [PubMed] [Google Scholar]
- Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, D'hoedt D, Voets T, Nilius B. Structure-function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- Palmer RK, Atwal K, Bakaj I, Carlucci-Derbyshire S, Buber MT, Cerne R, et al. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev Technol. 2010;8:703–713. doi: 10.1089/adt.2010.0334. [DOI] [PubMed] [Google Scholar]
- Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011a;31:18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Kim JY, Choi SK, Lee YH, Zeng W, Kim KH, et al. Serine-threonine kinase with-no-lysine 4 (WNK4) controls blood pressure via transient receptor potential canonical 3 (TRPC3) in the vasculature. Proc Natl Acad Sci U S A. 2011b;108:10750–11075. doi: 10.1073/pnas.1104271108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DJ, Parsons WH, Colburn RW, Meegalla SK, Ballentine SK, Illig CR, et al. Design and optimization of benzimidazole-containing transient receptor potential melastatin 8 (TRPM8) antagonists. J Med Chem. 2011;54:233–247. doi: 10.1021/jm101075v. [DOI] [PubMed] [Google Scholar]
- Parnas M, Peters M, Dadon D, Lev S, Vertkin I, Slutsky I, et al. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009;45:300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–69. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LV, Petukhov PA, Szabo T, Kedei N, Bizik F, Kozikowski AP, et al. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org Biomol Chem. 2004;2:2281–2286. doi: 10.1039/B404506H. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007;428:183–207. doi: 10.1016/S0076-6879(07)28010-3. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Perraud AL, Takanishi CL, Shen B, Kang S, Smith MK, Schmitz C, et al. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J Biol Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- Peters DJ, Spruit L, Saris JJ, Ravine D, Sandkuijl LA, Fossdal R, et al. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet. 1993;5:359–362. doi: 10.1038/ng1293-359. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- Planells-Cases R, Ferrer-Montiel A. TRP channel trafficking. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press, Taylor & Francis; 2007. pp. 319–330. [Google Scholar]
- Plato CC, Galasko D, Garruto RM, Plato M, Gamst A, Craig UK, et al. ALS and PDC of Guam: 40-year follow-up. Neurology. 2002;58:765–773. doi: 10.1212/wnl.58.5.765. [DOI] [PubMed] [Google Scholar]
- Preti D, Szallasi A, Patacchini R. TRP channels as therapeutic targets in airway disorders: a patent review. Expert Opin Ther Pat. 2012;22:663–695. doi: 10.1517/13543776.2012.696099. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Han P, Joshi SK, Neelands TR, Gauvin DM, Baker SJ, et al. A-995662 [R-8-(4-methyl-5-(4-(trifluoromethyl)phenyl)oxazol-2-ylamino)-1,2,3,4-tetrahydronaphthalen-2-ol], a novel, selective TRPV1 receptor antagonist, reduces spinal release of glutamate and CGRP in a rat knee joint pain model. Pain. 2010;150:319–326. doi: 10.1016/j.pain.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yue Z, Sun B, Yang W, Xie J, Ni E, et al. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol. 2013;168:1294–1312. doi: 10.1111/bph.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Hyun E, Zhao L, Lapointe TK, Chapman K, Hirota CL, et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci USA. 2013;110:7476–7481. doi: 10.1073/pnas.1217431110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychowdhury MK, González-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, et al. Molecular pathophysiology of mucolipidosis type IV: pH dysregulation of the mucolipin-1 cation channel. Hum Mol Genet. 2004;13:617–627. doi: 10.1093/hmg/ddh067. [DOI] [PubMed] [Google Scholar]
- Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano B, Borrelli F, Fasolino I, Capasso R, Piscitelli F, Cascio M, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169:213–229. doi: 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Germer S, Castonguay AJ, Barton NS, Martin M, Zee RY. Gene variation of the transient receptor potential cation channel, subfamily M, member 2 (TRPM2) and type 2 diabetes mellitus: a case-control study. Clin Chim Acta. 2010;411:1437–1440. doi: 10.1016/j.cca.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Round P, Priestly A, Robinson J. An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subject. Br J Clin Pharmacol. 2011;72:921–931. doi: 10.1111/j.1365-2125.2011.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Nothaft W, Duan WR, Wang Y, Faltynek C, McGaraughty S, et al. Oral and cutaneous thermosensory profile of selective TRPV1 inhibition by ABT-102 in a randomized healthy volunteer trial. Pain. 2011;152:1192–1200. doi: 10.1016/j.pain.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Ryckmans T, Aubdool AA, Bodkin JV, Cox P, Brain SD, Dupont T, et al. Design and pharmacological evaluation of PF-4840154, a non-electrophilic reference agonist of the TrpA1 channel. Bioorg Med Chem Lett. 2011;21:4857–4859. doi: 10.1016/j.bmcl.2011.06.035. [DOI] [PubMed] [Google Scholar]
- Saldanha SA, Grimm C, Mercer BA, Choi JY, Allais C, Roush WR, et al. Campaign to Identify Agonists of Transient Receptor Potential Channels 3 and 2 (TRPML3 & TRPML2) Bethesda, MD: National Center for Biotechnology Information (US); 2010. Reports from the NIH Molecular Libraries Program [Internet]2009. [PubMed] [Google Scholar]
- Santoni G, Farfariello V. TRP channels and cancer: new targets for diagnosis and chemotherapy. Endocr Metab Immune Disord Drug Targets. 2011;11:54–67. doi: 10.2174/187153011794982068. [DOI] [PubMed] [Google Scholar]
- Santos-Silva A, Charrua A, Cruz CD, Gharat L, Avelino A, Cruz F. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Auton Neurosci. 2012;166:35–38. doi: 10.1016/j.autneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Schattling B, Steinbach K, Thies E, Kruse M, Menigoz A, Ufer F, et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2012;18:1805–1811. doi: 10.1038/nm.3015. [DOI] [PubMed] [Google Scholar]
- Schleifer H, Doleschal B, Lichtenegger M, Oppenrieder R, Derler I, Frischauf I, et al. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca(2+) entry pathways. Br J Pharmacol. 2012;167:1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze A, Oehler B, Urban N, Schaefer M, Hill K. Apomorphine is a bimodal modulator of TRPA1 channels. Mol Pharmacol. 2013;83:542–551. doi: 10.1124/mol.112.081976. [DOI] [PubMed] [Google Scholar]
- Schwartz EC, Wolfs MJ, Tonner S, Wenning AS, Quintana A, Griesemer D, et al. TRP channels in lymphocytes. Hand Exp Pharmacol. 2007;179:445–456. doi: 10.1007/978-3-540-34891-7_26. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, et al. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J Pharmacol Exp Ther. 2002;303:1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, et al. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherkheli MA, Vogt-Eisele AK, Bura D, Beltrán Márques LR, Gisselmann G, Hatt H. Characterization of selective TRPM8 ligands and their structure activity response (S.A.R.) relationship. J Pharm Pharm Sci. 2010;13:242–253. doi: 10.18433/j3n88n. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Ugawa S, Imura M, Kubota Y, Ueda T, Kojima Y, et al. TRPM8-expressing dorsal root ganglion neurons project dichotomizing axons to both skin and bladder in rats. Neuroreport. 2011;22:61–67. doi: 10.1097/WNR.0b013e3283424c9c. [DOI] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, et al. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br J Pharmacol. 2012;165:1476–1486. doi: 10.1111/j.1476-5381.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- Singh BB, Pabelick CM, Prakash YS. Canonical transient receptor potential channel expression, regulation and function. In: Szallasi A, Bíró T, editors. TRP Channels in Drug Discovery. New York, Heidelberg, Dordrecht, London: Humana Press/Springer; 2012. pp. 61–88. Methods in Pharmacology and Toxicology, Springer Protocols. [Google Scholar]
- Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, et al. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol. 2006;290:F1320–F1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- Skryma R, Prevarskaya N, Gkika D, Shuba Y. From urgency to frequency: facts and controversies of TRPs in the lower urinary tract. Nat Rev Urol. 2011;8:617–630. doi: 10.1038/nrurol.2011.142. [DOI] [PubMed] [Google Scholar]
- Smit LA, Kogevinas M, Antó JM, Bouzigon E, González JR, Le Moual N, et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir Res. 2012;13:26. doi: 10.1186/1465-9921-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Parajuli SP, Hristov KL, Cheng Q, Soder RP, Afeli SA, et al. TRPM4 channel: a new player in urinary bladder smooth muscle function in rats. Am J Physiol Renal Physiol. 2013a;304:F918–F929. doi: 10.1152/ajprenal.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Hristov KL, Cheng Q, Xin W, Parajuli SP, Earley S, et al. Novel role for the transient potential receptor melastatin 4 channel in guinea pig detrusor smooth muscle physiology. Am J Physiol Cell Physiol. 2013b;304:C467–C477. doi: 10.1152/ajpcell.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Nilius B. Transient receptor potentials (TRPs) and anaphylaxis. Curr Allergy Asthma Res. 2013;13:93–100. doi: 10.1007/s11882-012-0301-4. [DOI] [PubMed] [Google Scholar]
- Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina D, Page CP. Regulating cough through modulation of sensory nerve function in the airways. Pulm Pharmacol Ther. 2013;26:486–490. doi: 10.1016/j.pupt.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hébert JL, Ferrer X, et al. Mutational spectrum in the Ca(2+)-activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat. 2012;33:109–117. doi: 10.1002/humu.21599. [DOI] [PubMed] [Google Scholar]
- Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal. 2006;18:1584–1594. doi: 10.1016/j.cellsig.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Straub I, Mohr F, Stab J, Konrad M, Philipp SE, Oberwinkler J, et al. Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM3. Br J Pharmacol. 2013;168:1835–1850. doi: 10.1111/bph.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- Sun M, Goldin E, Stahl S, Falardeau JL, Kennedy JC, Acierno JS, Jr, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9:2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Szabó A, Helyes Z, Sándor K, Bite A, Pintér E, Németh J, et al. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther. 2005;314:111–119. doi: 10.1124/jpet.104.082487. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Complex regulation of TRPV1 by vanilloids. In: Heller S, Liedtke W, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press; 2006. pp. 85–104. [Google Scholar]
- Szallasi A, Sheta M. Targeting TRPV1 for pain relief: limits, losers and laurels. Expert Opin Investig Drugs. 2012;21:1351–1369. doi: 10.1517/13543784.2012.704021. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Bloom CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J, Sándor Z. Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol Sci. 2012;33:646–655. doi: 10.1016/j.tips.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Tai Y, Feng S, Ge R, Du W, Zhang X, He Z, et al. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J Cell Sci. 2008;121:2301–2307. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, et al. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol. 2006;69:1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- Tamayo NA, Bo Y, Gore V, Ma V, Nishimura N, Tang P, et al. Fused piperidines as a novel class of potent and orally available transient receptor potential melastatin type 8 (TRPM8) antagonists. J Med Chem. 2012;55:1593–1611. doi: 10.1021/jm2013634. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shimaya A, Kiso T, Kuramochi T, Shimokawa T, Shibasaki M. Enhanced insulin secretion and sensitization in diabetic mice on chronic treatment with a transient receptor potential vanilloid 1 antagonist. Life Sci. 2011;88:559–563. doi: 10.1016/j.lfs.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008a;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, et al. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008b;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternesten-Hassèus E, Larsson C, Bende M, Millqvist E. Capsaicin provocation using two different inhalation devices. Respir Med. 2008;102:1784–1790. doi: 10.1016/j.rmed.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Thilo F, Loddenkemper C, Berg E, Zidek W, Tepel M. Increased TRPC3 expression in vascular endothelium of patients with malignant hypertension. Mod Pathol. 2009;22:426–430. doi: 10.1038/modpathol.2008.200. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, et al. N-( (1S)-1-{[4-((2S)-2-{ [ (2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4:159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J, Mizuno A, Suzuki M. Warm temperature-sensitive transient receptor potential vanilloid 4 (TRPV4) plays an essential role in thermal hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Togashi K, Inada H, Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M. The role of TRP channels in thermosensation. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton, FL: CRC Press, Taylor & Francis; 2007. pp. 271–286. [PubMed] [Google Scholar]
- Toth A, Kedei N, Szabo T, Wang Y, Blumberg PM. Thapsigargin binds to and inhibits the cloned vanilloid receptor-1. Biochem Biophys Res Commun. 2002;293:777–782. doi: 10.1016/S0006-291X(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Tóth BI, Dobrosi N, Dajnoki A, Czifra G, Oláh A, Szöllosi AG, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011;131:1095–1104. doi: 10.1038/jid.2010.421. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, et al. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H, Razavi R, Chan Y, Yantha J, Dosch HM. ‘Sensing’ autoimmunity in type 1 diabetes. Trends Mol Med. 2007;13:405–413. doi: 10.1016/j.molmed.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Tsui H, Paltser G, Chan Y, Dorfman R, Dosch HM. ‘Sensing’ the link between type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2011;27:913–918. doi: 10.1002/dmrr.1279. [DOI] [PubMed] [Google Scholar]
- Uchida K, Dezaki K, Damdindorj B, Inada H, Shiuchi T, Mori Y, et al. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 2011;60:119–126. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, De Wilde G, Doherty SA, Lories RJ, Vaughn FL, Laslett LL, et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann Rheum Dis. 2011;70:1556–1561. doi: 10.1136/ard.2010.148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, et al. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- Vay L, Gu C, McNaughton PA. The thermoTRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Menigoz A, Nilius B. TRPs in the brain. Rev Physiol Biochem Pharmacol. 2012;163:27–64. doi: 10.1007/112_2012_8. [DOI] [PubMed] [Google Scholar]
- Vergarajauregui S, Puertollano R. Mucolipidosis type IV: the importance of functional lysosomes for efficient autophagy. Autophagy. 2008;4:832–834. doi: 10.4161/auto.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter I, Touska F, Hess A, Hinsbey R, Sattler S, Lampert A, et al. Ciguatoxins activate cold pathways to elicit burning pain from cooling. EMBO J. 2012;31:3795–3808. doi: 10.1038/emboj.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, et al. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Vindis C, D'Angelo R, Mucher E, Nègre-Salvayre A, Parini A, Mialet-Perez J. Essential role of TRPC1 channels in cardiomyoblasts hypertrophy mediated by 5-HT2A serotonin receptors. Biochem Biophys Res Commun. 2010;391:979–983. doi: 10.1016/j.bbrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Nilius B, Vennekens R. Herbal compounds and toxins modulating TRP channels. Curr Neuropharmacol. 2008;6:79–96. doi: 10.2174/157015908783769644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, et al. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–2165. doi: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Miyares RL, Ahern GP. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol. 2005;564:541–547. doi: 10.1113/jphysiol.2004.081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, et al. Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2010;21:1657–1666. doi: 10.1681/ASN.2009121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain. 2008;139:158–167. doi: 10.1016/j.pain.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Iino K, Ohba T, Ito H. Possible involvement of TRP channels in cardiac hypertrophy and arrhythmia. Curr Top Med Chem. 2013;13:283–294. doi: 10.2174/1568026611313030006. [DOI] [PubMed] [Google Scholar]
- Wei H, Hämäläinen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: part 2. J Pharmacol Exp Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25:4434–4444. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H, Ling JX, Chen M, Johnson RD, Tominaga M, Wang CY, et al. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol Pain. 2008;4:22. doi: 10.1186/1744-8069-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006a;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006b;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao B, Liao C, Zhang R, Meng K, Xu J, et al. High glucose-induced apoptosis in cultured podocytes involves TRPC6-dependent calcium entry via the RhoA/ROCK pathway. Biochem Biophys Res Commun. 2013;434:394–400. doi: 10.1016/j.bbrc.2013.03.087. [DOI] [PubMed] [Google Scholar]
- Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci Lett. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell. 2012;151:96–110. doi: 10.1016/j.cell.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan NL, Knowles C, Williams NS, et al. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714–722. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- Yu W, Hill WG, Apodaca G, Zeidel ML. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am J Physiol Renal Physiol. 2011;300:F49–F59. doi: 10.1152/ajprenal.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci U S A. 2004;101:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Keller SH, Remillard CV, Safrina O, Nicholson A, Zhang SL, et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation. 2009a;119:2313–2322. doi: 10.1161/CIRCULATIONAHA.108.782458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Ulbrich MH, Li MH, Buraei Z, Chen XZ, Ong AC, et al. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci U S A. 2009b;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Zhou J, Du W, Zhou K, Tai Y, Yao H, Jia Y, et al. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci. 2008;11:741–743. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- Zhu B, Xia M, Xu X, Ludovici DW, Tennakoon M, Youngman MA, et al. Arylglycine derivatives as potent transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett. 2013;23:2234–2237. doi: 10.1016/j.bmcl.2013.01.062. [DOI] [PubMed] [Google Scholar]
- Zhu G, ICGN Investigators. Gulsvik A, Bakke P, Ghatta S, Anderson W, Lomas DA, et al. Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum Mol Genet. 2009;18:2053–2062. doi: 10.1093/hmg/ddp111. [DOI] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Luo Z, Ma S, Liu D. TRP channels and their implications in metabolic diseases. Pflugers Arch. 2011;461:211–223. doi: 10.1007/s00424-010-0902-5. [DOI] [PubMed] [Google Scholar]
- Zierler S, Yao G, Zhang Z, Kuo WC, Pörzgen P, Penner R, et al. Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem. 2011;286:39328–39335. doi: 10.1074/jbc.M111.264341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Lennerz JK, Hein A, Link AS, Kaczmarek JS, Delling M, et al. Transient receptor potential channel, subfamily C, member 5 (TRPC5) is a cold-transducer in the peripheral nervous system. Proc Natl Acad Sci USA. 2011;108:18114–18119. doi: 10.1073/pnas.1115387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


