Abstract
Migraine remains an elusive and poorly understood disease. The uncertainty is reflected by the currently unsatisfactory acute and prophylactic treatments for this disease. Genetic and pharmacological information points to the involvement of some transient receptor potential (TRP) channels in pain mechanisms. In particular, the TRP vanilloid 1 (TRPV1) and TRP ankyrin 1 (TRPA1) channels seem to play a major role in different models of pain diseases. Recent findings have underscored the possibility that TRP channels expressed in the nerve terminals of peptidergic nociceptors contribute to the migraine mechanism. Among this channel subset, TRPA1, a sensor of oxidative, nitrative and electrophilic stress, is activated by an unprecedented series of irritant and pain-provoking exogenous and endogenous agents, which release the pro-migraine peptide, calcitonin gene-related peptide, through this neuronal pathway. Some of the recently identified TRPA1 activators have long been known as migraine triggers. Furthermore, specific analgesic and antimigraine medicines have been shown to inhibit or desensitize TRPA1 channels. Thus, TRPA1 is emerging as a major contributing pathway in migraine and as a novel target for the development of drugs for pain and migraine treatment.
Linked Articles
This article is part of a themed section on the pharmacology of TRP channels. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-10
Keywords: transient receptor potential ankyrin 1 (TRPA1), migraine, calcitonin gene-related peptide (CGRP), neurogenic inflammation, thermoTRP, headache, neuropathic pain, oxidative stress, sensitization
Calcitonin gene-related peptide (CGRP) and migraine
Migraine
Migraine is a common and disabling neurovascular disorder, with heritability estimates as high as 50% and with a likely polygenic multifactorial inheritance (Pietrobon and Moskowitz, 2013). Migraine is characterized by attacks of often throbbing and frequently unilateral severe headache, which are usually associated with nausea, vomiting, and/or sensitivity to light (photophobia), sound (phonophobia), or odours (osmophobia), and aggravated by movement. If untreated, attacks typically last 4–72 h. In about 30% of patients, migraine attacks are preceded or accompanied by transient focal neurologic symptoms, which are usually visual, but that could also consist in paresthesias or language disturbances, commonly known as ‘aura’. The last update of the World Health Organization, Global Burden of Disease, states that migraine alone is responsible for almost 3% of disability attributable to a specific disease worldwide. In particular, migraine ranks first among neurological disorders, seventh among non-communicable diseases and eighth among most burdensome diseases (Murray and Lopez, 2013). The one-year prevalence of migraine registered in the United States and Western Europe is 11% overall: 6% among men and 15–18% among women (Rasmussen and Olesen, 1992; Stewart et al., 1992). Chronic migraine, which is diagnosed when patients present 15 headache attacks or more per month over at least 3 consecutive months, affects about 1–2% of the general population (Lipton, 2011). After migraine has been diagnosed, its pharmacological treatment can either be abortive or prophylactic. Poor understanding of the mechanisms underlying migraine contributes to the current unsatisfactory prophylactic pharmacological treatment of migraine and particularly of chronic migraine. There are other rarer forms of primary headaches, primarily represented by cluster headache, which shows some similarities with migraine in terms of mechanisms and treatments.
Neurogenic inflammation
A subset of nociceptors is characterized by the ability of producing and releasing from their central and peripheral terminals the tachykinins, substance P (SP) and neurokinin A (NKA) and CGRP. Current neurochemical and functional identification of the subset of peptidergic somatosensory neurons has strengthened the seminal proposals of neurogenic vasodilatation by William Bayliss (1901) and of the ‘nocifensor system’ by Sir Thomas Lewis (Lewis, 1937). Indeed, some nerve endings of the C- and Aδ type of a subpopulation of nociceptors, when activated by noxious stimuli, orchestrate, via antidromic invasion of collateral fibres by propagated action potentials, an almost instantaneous defensive response, which encompasses a rapidly developing vasodilatation and plasma protein extravasation, all phenomena mediated by SP/NKA or CGRP liberated from their peripheral (perivascular) terminals. In experimental animals, and particularly in rodents, there is overwhelming evidence that plasma extravasation is mediated by SP or NKA which, by activating neurokinin-1 (NK1) receptors in endothelial cells of postcapillary venules, promotes the opening of gaps and the leakage of plasma proteins from the lumen to the interstitial space (Geppetti and Holzer, 1996; receptor nomenclature follows Alexander et al., 2013). In contrast, arteriolar vasodilatation, which is responsible for the neurogenic hyperaemic response, is mediated exclusively by the release of CGRP (Brain and Grant, 2004).
In the past, tachykinin release from perivascular nerve endings of trigeminal nociceptors and the ensuing meningeal plasma protein extravasation had been proposed as the underlying mechanism of migraine (Moskowitz et al., 1979). However, failure of NK1 receptor antagonists to ameliorate the headache and associated symptoms of migraine (Goldstein et al., 1997) definitively excluded any contribution of tachykinins, NK1 receptors and the plasma protein extravasation component of neurogenic inflammation to the disease. These negative clinical findings have underscored the difference between rodents and humans regarding the relative contribution of specific neurogenic inflammatory responses in the different species, and now the hypothesis that neurogenic inflammation contributes to migraine headaches has apparently been discarded.
CGRP and its distribution and function
However, basic and clinical investigation on CGRP has rejuvenated the seminal idea that neurogenic responses initiated by stimulation of trigeminal sensory nerve endings in cranial vessels are a major determinant of migraine pathogenesis (Moskowitz et al., 1979). CGRP, whose existence was predicted on the basis of the alternative splicing of the calcitonin gene (Amara et al., 1985), is, on a molar basis, one of the most powerful vasodilators known (Brain and Grant, 2004). Of the two forms of the peptide identified in humans, α-CGRP is mainly expressed in primary sensory neurons, while β-CGRP is primarily found in intrinsic enteric neurons (Brain and Grant, 2004). The α-CGRP isoform is a 37-amino acid peptide markedly expressed in sensory neurons of the dorsal root ganglia (DRG), trigeminal ganglia (TG) and vagal ganglia (VG; Amara et al., 1985; Brain and Grant, 2004). The mature peptide, CGRP, is then transported to the very terminal region of central and peripheral nerve endings, where it is stored in dense core vesicles to be secreted either in the dorsal spinal cord or in a variety of peripheral tissues, particularly around blood vessels (Geppetti and Holzer, 1996). Approximately 40–50% of TG neurons are CGRP-positive (Tajti et al., 1999b; Eftekhari et al., 2010) and a dense network of CGRP-positive nociceptors is present in rodent and human meningeal vessels (Tsai et al., 1988; Edvinsson et al., 1998b). CGRP is also expressed in specific areas of the CNS, including the hypothalamus, thalamus, periaqueductal gray, superior and inferior colliculi, amygdala, trigeminocervical complex (TCC), and the cerebellum (Hokfelt et al., 1992; van Rossum et al., 1997). Some of these brain areas may be relevant in migraine pathophysiology, as indicated by the ability of CGRP to alter synaptic and neuronal activity at the TCC and transmission of nociceptive signals to the thalamus and cortical regions (Storer et al., 2004; Goadsby, 2007). More recent findings have shown that CGRP receptor antagonists inhibit cortical spreading depression in a rat model (Tozzi et al., 2012). However, the precise role of CGRP in these central structures and the contribution of such effects to the migraine mechanism remain uncertain.
CGRP receptors
The functional CGRP receptor consists of a triad of proteins, comprising a classical GPCR, the calcitonin receptor-like receptor (CLR; Aiyar et al., 1996), a single transmembrane spanning protein called receptor activity-modifying protein 1 (RAMP1; McLatchie et al., 1998), required for the binding of CGRP to CLR and the receptor component protein (Ma et al., 2003) that characterizes the G-protein associated with the receptor. Components of the CGRP receptor complex are expressed in peripheral and central structures (Eftekhari and Edvinsson, 2010), such as cell bodies in TG, periaqueductal grey, and in the trigeminal nucleus caudalis (Oliver et al., 2002; Lennerz et al., 2008). However, it has not been convincingly established whether all these components assemble in a fully functional receptor at these anatomical sites. However, there is no doubt that vascular smooth muscle cells in arteries and arterioles, including those of the cranial circulation, express the entire and functional CGRP-receptor complex, as indicated by the robust vasodilatatory effect of CGRP(mediated by activation of adenylyl cyclase) in these vessels either in vitro and in vivo (Brain and Grant, 2004).
CGRP and migraine
While the anatomical site from which migraine attack originates and the initiating mechanism is still a mystery, the key role of CGRP in the migraine pathway is supported by a series of robust findings. After the in vivo preclinical observation that TG activation results in CGRP and SP release (Goadsby et al., 1988), the first indirect evidence suggesting a role of CGRP in migraine was obtained about 20 years ago, when it was shown that, during spontaneous migraine attacks, CGRP levels were elevated in samples of cranial venous blood (Goadsby et al., 1990). Increased CGRP levels have also been found in saliva during an acute migraine attack (Cady et al., 2009) or in cranial blood during nitroglycerine-evoked cluster headache attacks (Fanciullacci et al., 1995). Interestingly, successful treatment with antimigraine compounds, such as the triptans, is accompanied by a decrease in CGRP levels during migraine or cluster headache attacks (Fanciullacci et al., 1995; Goadsby and Hargreaves, 2000). It should be noted that others have failed to detect increased CGRP levels in cranial blood of migraineurs (Tvedskov et al., 2005). Another key observation, derived from a series of highly informative provocation experiments, showed that intravenous injection of CGRP induces migraine-like attacks in migraineurs (Lassen et al., 2002).
Conclusive evidence that CGRP plays a major role in migraine originated, however, from clinical trials that used various and chemically unrelated CGRP receptor antagonists, namely olcegepant (BIBN4096BS), telcagepant (MK-0974), MK-3207 and BI 44370 TA. Intravenous administration of 2.5 mg of olcegepant produced a response 2 h after treatment in 66% of treated patients, compared with a response rate of 27% observed in the placebo group (Olesen et al., 2004), a clinical response similar to that observed for triptans (Ferrari et al., 2002). This study made two important points; firstly it showed that blockade of CGRP receptors could be a valuable alternative mechanism for migraine treatment and secondly it provided the first clinical evidence of the involvement of CGRP in the mechanism(s) for migraine. The absence of direct vasoconstrictor activity and cardiovascular effects opened a novel scenario for and gave real hope of, CGRP receptor antagonists as an effective and well-tolerated migraine treatment (Petersen et al., 2005). Telcagepant was the first orally bioavailable CGRP receptor antagonist and, up to date, the most investigated compound of this new class of drugs. It showed efficacy in pain relief of migraine headache in phase II and III clinical trials (Ho et al., 2008a,b2008b; Connor et al., 2009). Unfortunately, telcagepant increased plasma levels of liver transaminases in a few patients of a cohort of patients treated with the drug twice a day for 3 months (Han et al., 2010; Connor et al., 2011), and in patients affected by menstrual migraine enrolled in a short-term clinical study (Bigal et al., 2013). More recently, MK-3207, a new orally active CGRP receptor antagonist, demonstrated its superiority in comparison with placebo for migraine attack treatment (Hewitt et al., 2011). However, as for MK0974 (Merck & Co., 21 April 2009), MK3207 development was stopped due to the asymptomatic liver toxicity detected in some of the enrolled patients (Edvinsson and Linde, 2010). The development of both the oral CGRP antagonist, BI 44370 TA, which has been found superior to placebo end equieffective to eletriptan (Diener et al., 2011), and the orally bioavailable antagonist, BMS-927711 (Luo et al., 2012) has been stopped (Dolgin, 2013).
Studies with promising monoclonal antibodies (mAbs) against CGRP are ongoing (Bigal et al., 2013). At the time of this review, 4 mAbs are in clinical development for the treatment of migraine. Two of these mAbs, namely LY2951742 from Eli Lilly–Arteaus Therapeutics (Lilly Corporate Center, Indianapolis, IN, USA; Arteaus Therapeutics, Cambridge, MA, USA) and ALD403 from Alder Therapeutics (Alder Biopharmaceuticals, Inc., North Creek Parkway South Bothell, WA, USA), directly bind and neutralize CGRP. A third mAb, AMG 334 from Amgen Inc. (One Amgen Center Drive, Thousand Oaks, CA, USA), targets the CGRP receptor, while LBR-101 from Labrys Biologics–Pfizer (Labrys Biologics, Inc., San Mateo, CA, USA; Pfizer, New York, NY, USA) prevents the binding of CGRP to its receptor. Results of the early-phase clinical trials are not publicly available for any of these mAbs yet. According to data collected in preclinical studies, CGRP receptors are expressed on may different levels in the trigeminal vascular system of the cynomolgus monkey, such as the meningeal vasculature innervated by CGRP-positive nerve fibres, neurons and satellite cells in the trigeminal ganglion, and in the spinal trigeminal nucleus (Liu et al., 2011). Hence, in principle, mAbs targeting the CGRP system could exert their action at both the vascular and neuronal levels. Also, in the presence of a normal, un-compromised blood–brain barrier (BBB), about 0.1% of circulating IgG can enter the CNS, presumably through the circumventricular organs (Gu and Sigurdsson, 2011). Thus, even though mAbs could reach their target and prevent the interaction with the peptide and its receptors at central neuronal sites, it should be emphasized that the proportion of CGRP and /or CGRP receptors targeted by the minimal amount of mAbs that may cross the BBB is most likely negligible. Thus, blockade of the CGRP action at the perivascular level in intra- and extra-cranial vessels should currently remain the simplest and first explanation for any possible beneficial action of mAbs in migraine.
Despite the adverse reactions in the liver, which have not yet been conclusively ascribed to a class effect, the structural complexity of the CGRP receptor, which has represented the key hurdle for the development of small molecule antagonists (Moore and Salvatore, 2012), and the possible interaction of CGRP with different receptors (Walker and Hay, 2013), clinical data unequivocally demonstrate that, whatever the initiating mechanism(s) of the attacks, CGRP release and CGRP-receptor activation are major contributing mechanisms in migraine (Olesen et al., 2004; Ho et al., 2008a; Diener et al., 2011). Thus, identification of exogenous and endogenous stimuli that result in CGRP release from trigeminal neurons may be of paramount importance in decoding the molecular pathways that eventually cause or combine to worsen the headache and associated symptoms of migraine.
In the second part of this review, we first briefly present the transient receptor potential (TRP) channels and, in particular, those expressed by peptidergic primary sensory neurons, whose activation eventually results in neuropeptide release. We then summarize current knowledge regarding the role of one of these channels, the TRP ankyrin 1 (TRPA1), as a sensor of oxidative, nitrative and electrophilic stress and as a major player in various pain conditions, including migraine (see Table 1).
Table 1.
Agents which trigger or inhibit migraine and cluster headache and act on TRP channels
| Target | Agent (environmental, herbal, food, drug) | Active compound | Effect on primary headache | Action on TRP channel |
|---|---|---|---|---|
| TRPA1 | Cigarette smoke | Crotonaldehyde acrolein formaldehyde nicotine Acetaldehyde |
Migraine and cluster headache trigger (Rozen, 2010; Lima et al., 2011) | Agonist (Bang et al., 2007a; McNamara et al., 2007; Andre et al., 2008; Talavera et al., 2009) |
| Tear gas | O-chlorobenzylidene malononitrile | Headache trigger (Anderson et al., 1996) | Agonist (Brone et al., 2008) | |
| Formalin | Formalin | Migraine trigger (Wantke et al., 2000) | Agonist (McNamara et al., 2007) | |
| Umbellularia californica | Umbellulone | Migraine trigger (Immel, 2006) | Agonist (Nassini et al., 2012a) | |
| Tanacetum parthenium (feverfew) | Parthenolide | Migraine preemptive Migraine abortive (Diener et al., 2005; Cady et al., 2011) | Desensitizing agonist (Materazzi et al., 2013) | |
| Angelica sinensis | Ligustilide | Migraine preemptive | Desensitizing agonist (Zhong et al., 2011) | |
| Paracetamol | NAPQI | Migraine abortive | Desensitizing agonist (Andersson et al., 2011) | |
| Glyceryl trinitrate | NO | Migraine trigger (Iversen, 1995) | Agonist (Miyamoto et al., 2009) | |
| Ammonium chloride | Ammonium chloride | Cluster headache trigger (Irlbacher and Meyer, 2002) | Agonist (Fujita et al., 2008) | |
| TRPV1 | Alcoholic beverages | Ethanol | Migraine trigger (Kelman, 2007) | Agonist (Trevizani et al., 2002; Nicoletti et al., 2008) |
| Capsicum | Capsaicin | Migraine and cluster headache preemptive (Sicuteri et al., 1989; Fusco et al., 1994; 2003) | Desensitizing agonist (Szallasi and Blumberg, 1999; O'Neill et al., 2012) | |
| Multiple TRP | Tiger balm | Camphor | Tension type headache abortive (Schattner and Randerson, 1996) | TRPV3 agonist (Moqrich et al., 2005) TRPA1 antagonist (Sawada et al., 2008) TRPV1 desensitizing agonist (Xu et al., 2005) |
TRP channels
The TRP channel superfamily
From the original finding that vision in Drosophila melanogaster is produced by a mechanism that consists in an initial activation of a transient inward current associated with receptor stimulation (Minke, 1977; Montell, 1999), the identification of the larger family of the TRP channels has proceeded with an unprecedented pace. Currently, more than 50 members of the TRP family have been characterized (Nilius et al., 2007). Despite the wide heterogeneity of this family of ion channels, TRPs share a general role serving sensory transduction, because they contribute to vision, taste, olfaction, hearing, touch, and thermo- and osmo-sensation, making cells able to sense and respond to environmental changes.
TRP channels consist of six transmembrane domains (S1–S6) with both the amino (NH2) and carboxylic acid (COOH) termini localized to the cytosol. The COOH region is highly conserved among TRPs, whereas the NH2 region usually contains different ankyrin repeats, which consist of 33-residue motifs with a conserved backbone and variable residues that mediate specific protein–protein interactions (Sedgwick and Smerdon, 1999). The S1–S6 domains assemble as homo- or hetero-tetramers, with the pore domain formed by loops between S5 and S6, which permit a non-selective influx of cations. Although TRPs are described as non-selective Ca2+-permeable cation channels, their Ca2+/Na+ permeability ratio may vary markedly between different members of the superfamily and also among members of each subfamily (Nilius et al., 2007).
TRP channel gating may depend on direct activation of the channels by a plethora of physicochemical stimuli, including compounds of exogenous origin or endogenous signalling molecules (Nilius et al., 2007). TRP gating may also result from changes in the intracellular machinery as in the case of stimulation of the different isoforms of phospholipase C (PLC; Hardie and Minke, 1992; Niemeyer et al., 1996), following activation of GPCRs or tyrosine kinase receptors (Spehr et al., 2011). For instance, it has been hypothesized that PLC can modulate TRP channel activity through the hydrolysis of phosphatidylinositol (4,5) bisphosphate (PIP2), which leads to Ca2+ liberation from intracellular stores (Ramsey et al., 2006). Peculiar features of activation have been reported for certain TRP channels. These features may be exploited for a better understanding of how cells sense their surrounding environment, and may also represent the way to identify novel therapeutic targets.
In mammals, the TRP family consists of 28 proteins grouped into six subfamilies according to sequence identity and designated as TRP canonical (TRPC), TRP vanilloid (TRPV), TRP melastatin (TRPM), TRP polycystin (TRPP), TRP mucolipin (TRPML) and TRPA1 channels (Montell et al., 2002; Clapham, 2003). The mammalian TRPC subfamily comprises seven members (TRPC1–7), whose activation depends on the stimulation of GPCR and receptor tyrosine kinases (Montell, 1999), although TRPC1 channels seem to be directly activated by membrane stretch (Maroto et al., 2005). The TRPM comprises eight different members (TRPM1–8), which differently from TRPC and TRPV, do not contain ankyrin repeats within their NH2-terminal domain. Menthol and moderately low temperatures (<25°C) activate the TRPM8 channel. Both TRPP and TRPML families have been less extensively characterized. The TRPML family consists of three mammalian members (TRPML1–3). TRPML1 is widely expressed and appears to reside in late endosomes/lysosomes where it seems to act as a H+-sensitive channel to prevent overacidification (Soyombo et al., 2006). The heterogeneous TRPP family, consisting of three members according to structure, can be divided into PKD1-like (TRPP1-like) and PKD2-like (TRPP2-like) proteins (Hanaoka et al., 2000).
ThermoTRP channels in sensory neurons
Some TRP channels are abundantly expressed by the subsets of primary sensory neurons, which express neuropeptides. These include four of the six members of the TRPV subfamily, TRPV1, TRPV2, TRPV3 and TRPV4, characterized by their ability to sense warm–hot temperatures. TRPV channels also function as chemosensors for a number of naturally occurring (both exogenous and endogenous) and synthetic ligands. TRPV1, first identified as the receptor of the vanilloid compound, capsaicin, is responsive to high proton concentration (pH 6–5; Bevan and Geppetti, 1994; Tominaga et al., 1998), anandamide (Zygmunt et al., 1999) and a series of lipid derivatives (Ho et al., 2008c). Non-selective activators of TRPV3 and TRPV4 channels are camphor and hypotonic solutions respectively (Nilius et al., 2013). Synthetic agonists, such as the phorbol ester derivative 4α-phorbol 12,13-didecanoate (4α-PDD), low pH, citrate, endocannabinoids, arachidonic acid metabolites and NO may also activate TRPV3 channels (Watanabe et al., 2003; Vriens et al., 2005). Although the uricosuric agent probenecid has been identified as a selective stimulant of TRPV2 (Bang et al., 2007b), less information is available regarding the activators of TRPV2 channels. The TRPM8 channel is expressed in somatosensory neurons, but apparently not in those, which release tachykinins/CGRP (Bhattacharya et al., 2008). Finally, there is also evidence that TRPM3 channels, rather uniquely responsive to pregnenolone sulfate, seem to be localized to a subpopulation of primary sensory neurons (Wagner et al., 2008).
A large NH2-terminal with 17 predicted ankyrin repeat domains is the typical feature of the TRPA1 channel, the sole member of the TRPA subfamily. TRPA1 channels, first cloned from human foetal lung fibroblasts, are abundantly expressed in peptidergic nociceptors (Story et al., 2003; Bhattacharya et al., 2008), but these channels are also found in many non-neural cell types and tissues, including hair cells, pancreas, heart, brain, keratinocytes (Atoyan et al., 2009), urinary bladder (Streng et al., 2008), prostate gland (Gratzke et al., 2010), endothelium (Earley et al., 2009). and other vascular and perivascular cells (Earley, 2012), enterochromaffin cells (Nozawa et al., 2009), gastrointestinal tract (Izzo et al., 2012), odontoblasts (El Karim et al., 2010), dental pulp (El Karim et al., 2011), synovial fibroblasts (Kochukov et al., 2006), and epithelial and smooth muscle cells of the airways and lung (Nassini et al., 2012b). It has been extensively demonstrated that TRPA1 channels play a key role in the detection of pungent or irritant principles, including compounds contained in various spicy foods, such as allyl isothiocyanate (mustard oil) contained in horseradish (Jordt et al., 2004), allicin and diallyldisulfide contained in garlic (Bautista et al., 2005), cinnamaldehyde contained in cinnamon (Bandell et al., 2004), and capsiate (Shintaku et al., 2012). Additional spices or food ingredients that may activate TRPA1 channels are gingerol (in ginger), eugenol (in cloves), methyl salicylate (in wintergreen), carvacrol (in oregano), and thymol (in thyme and oregano; Bandell et al., 2004; Xu et al., 2005; Lee et al., 2008). Environmental irritants and industry pollutants, such as acetaldehyde, formaldehyde, hydrogen peroxide, hypochlorite, isocyanates, ozone, carbon dioxide, ultraviolet light and acrolein, a highly reactive α,β-unsatured aldehyde present in tear gas, cigarette smoke, smoke from burning vegetation and vehicle exhaust, and hydrogen sulfide (H2S) also activate TRPA1 channels (Bautista et al., 2006; Bang et al., 2007a; McNamara et al., 2007; Andersson et al., 2008; Bessac et al., 2008; 2009; Sawada et al., 2008; Hill and Schaefer, 2009; Taylor-Clark and Undem, 2010; Wang et al., 2010; Miyamoto et al., 2011). Recently, it has been reported the ability of cannabichromene, a non-psychotropic Cannabis-derived cannabinoid with anti-inflammatory (Romano et al., 2013) and analgesic properties (Maione et al., 2011) to activate TRPA1 channels (De Petrocellis et al., 2011). It has also been proposed that these channels function as a detector of mechanical stimuli and noxious cold (<17°C; Story et al., 2003), although these proposals remain controversial (Jordt et al., 2004; Latorre, 2009). TRPV1, TRPV2, TRPV3, TRPV4, TRPA1 and TRPM8 have been collectively labelled as thermoTRP channels because they can be activated by a large range of temperatures from noxious cold to noxious heat (Vay et al., 2012).
Members of the TRP family expressed in sensory neurons are primarily involved in the detection of noxious physical (thermal and mechanical) and chemical stimuli. Among the 6–7 TRPs expressed by nociceptors, recent pathophysiological and pharmacological findings have pointed to TRPV1 and TRPA1 channels as main contributors in models of inflammatory and neuropathic pain (Fernandes et al., 2012). It should be underlined that TRPV1 and TRPA1 channels co-localize in a subpopulation of non-myelinated or thinly myelinated C- or Aδ-fibre neurons of the DRG, TG and VG. The population of TRPV1-positive neurons seems to be larger than the TRPA1-positive subpopulation (Story et al., 2003; Bhattacharya et al., 2008). Both TRPV1 and TRPA1 channels coexist within neuropeptides (SP, NKA and CGRP) in the same nociceptive neurons. While TRPA1 expression seems to be confined to the peptidergic neuronal subpopulation, TRPV1-positive neurons appear to be also non-peptidergic (Story et al., 2003; Bhattacharya et al., 2008). However, more recent studies have identified subpopulations of DRG neurons that co-stain for IB4 (isolectin B4, a marker of non-peptidergic neurons) or the purinergic P2X3 receptor (also expressed by non-peptidergic neurons) and TRPA1 channels (Kim et al., 2010; Barabas et al., 2012).
Mutation of TRP channels has been linked to various diseases affecting different organs or systems. However, only a small number of TRP channelopathies, also known as ‘TRPpathies’, has been definitively identified so far. TRPpathies encompass neurological disorders, including scapuloperoneal hereditary motor neuropathy and Charcot–Marie–Tooth disease type-2C, due to TRPV4 mutation or Guamanian amyotrophic lateral sclerosis/parkinsonism dementia complex, due to TRPM2 or TRPM7 mutation, renal diseases, including focal segmental glomerulosclerosis, due to TRPC6 mutation or autosomal dominant polycystic kidney disease, due to TRPP2 mutation, and complex skeletal dysplasias, including brachyolmia type 3, spondylometaphyseal dysplasia, Kozlowski type and autosomal dominant metatropic dysplasia due to TRPV4 mutation, among others (Nilius and Owsianik, 2010). Of interest for the present discussion, recently, a familial episodic pain syndrome has been attributed to a gain of function mutation of TRPA1 channels (Kremeyer et al., 2010).
TRPA1 channels, oxidative stress and nitrative stress in pain and inflammation
In aerobic organisms, the balance between oxidation and reduction (redox state) is of paramount importance for physiological homeostasis. The conservation of an optimal redox state is pursued through diverse mechanisms also involving the transformation in reactive oxygen species (ROS), including O2–, OH−, H2O2 and O3, which affect cellular functions deeply, but can also lead to irreversible damage, up to cell death. Oxidative stress has been claimed as a mechanism for a number of diseases, including inflammatory pain, neuropathic pain and migraine. An unprecedented series of findings has shown that TRPA1 channels are activated by ROS, reactive nitrogen species (RNS) and other electrophiles, thus identifying the channel as a sensor of oxidative and nitrative stress generated at sites of inflammation or tissue injury. Indeed, Ca2+ influx and activation of membrane currents in sensory neurons induced by ROS, RNS or RCS (reactive carbonyl species) are absent in TRPA1−/− mice and are blocked by TRPA1 channel antagonists (Bautista et al., 2006; Trevisani et al., 2007; Bessac et al., 2008; Taylor-Clark and Undem, 2010). Activation by hyperoxia (86% O2) suggests that TRPA1 channels function as sensors, exclusively for abnormal redox states (Takahashi et al., 2011). α,β-Unsaturated aldehydes produced by membrane phospholipid peroxidation by ROS, such as acrolein (Bautista et al., 2006), 4-hydroxynonenal (Trevisani et al., 2007) or oxononenal (Taylor-Clark et al., 2008), or other by-products of oxidative stress, such as hydrogen peroxide (Bessac et al., 2008), hypochlorite (Bessac et al., 2008), or nitrative stress by-products, such as nitroleic acid or NO (Taylor-Clark et al., 2009) and other reactive molecules, all share the ability to activate TRPA1 because of their reactive properties, which result in the carbonylation, nitrosilation or oxidation of specific cysteine (C619, C639, C663, C415, C422 and C622) or lysine (K708) residues (Hinman et al., 2006; Macpherson et al., 2007).
Oxidative/nitrative stress and the ensuing TRPA1 activation result from endogenous processes driven by inflammatory or degenerative conditions, but may also derive from exogenous stimuli, which per se are already suited for TRPA1 channel stimulation. Thus, crotonaldehyde, acrolein (Andre et al., 2008), acetaldehyde (Bang et al., 2007a) and possibly other reactive molecules and nicotine (Talavera et al., 2009), all contained in cigarette smoke, gate the TRPA1 channels on airway sensory nerve terminals to release tachykinins and CGRP, which in turn mediate the early inflammatory response that follows acute exposure to cigarette smoke (Andre et al., 2008). Certain volatile anaesthetics, including the irritants isoflurane and desflurane, during induction and emergence from anaesthesia cause a strong cough reflex that can precipitate laryngospasm, a potentially life-threatening complication. Their ability to gate TRPA1 channels, thus producing sensory nerve activation and neurogenic inflammation responses, may be the underlying mechanism for such an adverse reaction (Matta et al., 2008; Eilers et al., 2010). Chemotherapeutic agents are known to produce remarkable oxidative stress, which is likely to contribute to their anticancer activity, even if this issue remains highly debated (Saeidnia and Abdollahi, 2013).
However, oxidative stress seems to be responsible for serious adverse events, including chemotherapeutic-induced peripheral neuropathy (CIPN), which characterized by paraesthesias, spontaneous pain, and typically by prolonged mechanical and cold hypersensitivity, reduces quality of life, and often causes hospitalization and therapy discontinuation (Cavaletti and Marmiroli, 2010). We recently found in a mouse model of CIPN that oxaliplatin, paclitaxel or bortezomib (Nassini et al., 2011; Materazzi et al., 2012; Trevisan et al., 2013) produce a prolonged condition of mechanical and cold hypersensitivity that lasts for 11–15 days. When established, the hypersensitivity is completely, although transiently, reverted by a ROS scavenger (α-lipoic acid) or a TRPA1 channel antagonist (HC-030031). However, in TRPA1-deleted mice, or if the ROS scavenger or the TRPA1 antagonist was given just before and for 6 h after bortezomib or oxaliplatin administration, the mice were completely and permanently protected from the development of the hypersensitivity (Trevisan et al., 2013). Because a marker of oxidative stress was transiently increased in mouse plasma within the first few hours after anticancer drug administration, the hypothesis was advanced that sensitization of TRPA1 channels by oxidative stress is required to establish and maintain the prolonged hypersensitivity by chemotherapeutic agents (Trevisan et al., 2013). These channels also contribute to mechanical hyperalgesia in models of inflammatory pain, such as those induced by carrageenan (Moilanen et al., 2012), complete Freund's adjuvant (da Costa et al., 2010), and in a caerulein-induced model of pancreatitis (Schwartz et al., 2013). However, in these cases, the association of TRPA1 channel activation/sensitization and oxidative stress has not been investigated.
TRPA1 channels in pain and migraine
Anecdotal reports and epidemiological findings indicate that a large series of endogenous or exogenous agents trigger headache in migraineurs (Courteau et al., 1994; Kelman, 2007; Lima et al., 2011). In the context of this review, it is important to emphasize that, among the highly heterogeneous series of triggering factors, certain foods or exposure to environmental agents provoke migraine headaches (Courteau et al., 1994; Kelman, 2007; Lima et al., 2011). In terms of foods, 40–50% of migraineurs are sensitive to alcohol or chocolate (Kelman, 2007). Regarding environmental agents, there is evidence suggesting that migraine and cluster headache are favoured in susceptible individuals by increased concentration of air pollutants, as indicated by a greater number of emergency room visits for headache under such circumstances (Szyszkowicz, 2008). Furthermore, exposure to tear gas induces a series of toxic effects such as cough, chest pain, dyspnoea and headache (Anderson et al., 1996). Cigarette smoke inhalation affects headache occurrence in migraineurs (Lima et al., 2011) and prolonged or repeated exposure to cigarette smoke may increase cluster headache frequency (Rozen, 2010). However, a mechanistic explanation for the ability of this apparently unrelated series of substances to trigger migraine headaches is still missing.
The α,β-unsaturated aldehyde, acrolein, contained in cigarette smoke (Bautista et al., 2006), is produced endogenously by plasma membrane peroxidation by oxidative stress. Acrolein, when acting on sensory nerve endings, causes neurogenic inflammation, by a mechanism dependent on capsaicin-sensitive peptidergic primary afferent fibres, via activation of TRPA1 channels (Bautista et al., 2006; Geppetti et al., 2008). Accordingly, compounds contained in cigarette smoke, including acrolein and crotonaldehyde, produce airway inflammatory responses through stimulation of TRPA1 channels expressed by vagal sensory nerve endings (Andre et al., 2008). Interestingly, acrolein, when applied to the nasal mucosa of rats, enhances the meningeal blood flow by a mechanism dependent on TRPA1 channel activation and the subsequent release of CGRP (Kunkler et al., 2011). Thus, it could be hypothesized that acrolein, because of its ability to activate these channels and thereby produce CGRP release, mediates neurogenic inflammation and headache induced by toxic environmental irritants, including cigarette smoke inhalation (Geppetti et al., 2008; Kunkler et al., 2011). The same mechanism could also be hypothesized for tear gases, in particular for 2-chlorobenzalmalononitrile (CS tear gas), which has shown to be able to induce headache (Anderson et al., 1996), and with similar constituents of tear gases, is one of the most potent and specific TRPA1 channel agonists characterized so far (Brone et al., 2008). Although the effect of H2S has not often been reported, intoxication with H2S has been described to trigger headache attacks (Hirsch and Zavala, 1999). Underestimation of the phenomenon may be due to the fact that the intoxication is frequently associated with loss of consciousness of the intoxicated subjects. The discovery that H2S stimulates TRPA1 channels (Miyamoto et al., 2011; Okubo et al., 2012) and that this activation results in CGRP release (Pozsgai et al., 2012; White et al., 2013) suggests a mechanistic pathway for H2S-induced headache.
Knowledge and use of herbal medicines, as often happens in biomedical investigation, may help to elucidate mechanisms of disease, to validate therapeutic targets, and eventually to direct pharmaceutical development. The California bay laurel, Umbellularia californica, is also known as the ‘headache tree’ because of the ability of its scent to cause headache attacks (Immel, 2006). Recent evidence showed that exposure to the scent of Umbellularia californica triggers cluster headache attacks (Benemei et al., 2010). Umbellulone, is a major volatile component of Umbellularia californica, with a specific chemical feature, resembling those necessary for TRPA1 agonism. Although umbellulone possesses a β,β-dialkyl substitution, which should inhibit reaction with thiol groups (LoPachin et al., 2008), it rather surprisingly reacts in a ‘click-fashion’ with the biogenic thiol cysteamine, producing a Michael adduct (Nassini et al., 2012a). As the closely related, but not irritant, terpenoid enones, (+)-verbenone and (+)-piperitone, do not produce such a chemical reaction, Michael acceptor behaviour seems to be required for the sensory noxious activity of umbellulone. Umbellulone, most likely through this chemical property, caused TRPA1-dependent CGRP release in vitro and nociceptive behaviour in vivo in rats and mice. Finally, and more importantly, when applied intranasally (likewise in the acrolein experiments), umbellulone evoked TRPA1-mediated and CGRP-dependent neurogenic meningeal vasodilation (Nassini et al., 2012a). Although a reflex pathway, originating in the nasal cavity, and resulting in CGRP-dependent meningeal vasodilatation, has been proposed to explain the vascular action of acrolein and umbellulone (Kunkler et al., 2011; Nassini et al., 2012a), the precise mechanism involved in such a response remains to be elucidated. There is evidence that TRPA1 channels are expressed by endothelial cells of rat cerebral and cerebellar pial arteries (but not in endothelium of other vascular beds), where they mediate endothelium-dependent vasodilation (Earley et al., 2009). However, a contribution of such CGRP-independent mechanism for umbellulone- and acrolein-evoked vasodilation is unlikely, as the response in meningeal arteries by the two TRPA1 channel agonists was completely abolished by CGRP receptor antagonism (Kunkler et al., 2011; Nassini et al., 2012a).
A series of substances, now identified as TRPA1 channel agonists, has been reported in the past to cause migraine or non-migraine headaches after inhalation by migraine patients or non-migraine individuals respectively (Courteau et al., 1994; Kelman, 2007; Lima et al., 2011). Ammonium chloride is an agonist for these channels (Bessac and Jordt, 2010) and its inhalation has been reported to trigger cluster-like headache attacks (Irlbacher and Meyer, 2002). Similarly, formalin has long been known as a headache-producing agent (Wantke et al., 2000), and it has recently been recognized as a TRPA1 agonist (McNamara et al., 2007).
The contribution of herbal medicines to the understanding of the role of TRPA1 channels in headache mechanism is not limited to the irritant substance, umbellulone. Very recently, it has been demonstrated that parthenolide, a bioactive compound contained in the antimigraine preparations from Tanacetum parthenium (also known as feverfew), acts as a partial agonist at TRPA1 channels (Materazzi et al., 2013). In addition, parthenolide, after an initial and moderate activation, produces a profound and persistent desensitization of these channels and a complete defunctionalization of sensory neurons (including meningeal trigeminal nerve terminals), which are rendered unable to release CGRP upon exposure to any stimulus (Materazzi et al., 2013). These findings suggest that the antimigraine action of preparations of feverfew, either used as a pre-emptive treatment (Diener et al., 2005) or as an acute medication (Cady et al., 2011), may be due to the ability of parthenolide to deactivate the trigeminovascular-CGRP system. The desensitizing action of parthenolide does not seem unique to this molecule, as ligustilide (Zhong et al., 2011), has recently been identified as a TRPA1 channel agonist, which may induce TRPA1 and sensory neuron desensitization. Ligustilide, contained in elevated concentrations in herbal remedies, is used in Chinese and North American traditional medicine to treat pain and headaches (Li et al., 2011).
Some medicines may also provoke migraine. Nitroglycerine, a NO donor with a direct vasodilator action on vascular smooth muscle, has been known for a long time as a prototypical headache-causing agent (Thomsen and Olesen, 2001) and as a valuable experimental tool to provoke migraine-like attacks (Iversen, 1995). One possible explanation for the pro-migraine action of nitroglycerine is based on the ability of NO to produce vasodilatation of cranial arteries (Shevel, 2011). This view is supported by pharmacological evidence that nitroglycerine-induced migraine-like headaches are reversed by sumatriptan (Iversen and Olesen, 1993; 1996), presumably by its direct vasoconstrictor action (Asghar et al., 2011; Amin et al., 2013), but not by a CGRP receptor antagonist (Tvedskov et al., 2010). NO may also release CGRP in vitro (Wei et al., 1992) and in vivo (Fanciullacci et al., 1995), even if this effect of NO has not always been confirmed (Eltorp et al., 2000). Although NO stimulates sensory neurons by activating TRPA1 channels (Miyamoto et al., 2009), it is not known whether NO releases CGRP from trigeminal neurons via TRPA1 stimulation. It should be, however, underlined that vasodilation in vivo (Iversen, 1995) and CGRP release in vitro (Wei et al., 1992) are very early phenomena, which occur within a few minutes after drug exposure. In contrast, clinical investigation with nitroglycerine has revealed a stereotyped and delayed time-course of the migraine-like pain that develop only 4–5 h after (Iversen, 1995) drug administration. Thus, a temporal mismatch exists between vasodilation/acute CGRP release and the occurrence of the migraine headache evoked by NO. This represents a major reason for criticism of the hypothesis that these early neurovascular actions are responsible for the pro-migraine action of the drug. Changes in neuronal sensitivity, driven by the exposure to nitroglycerine/NO, which requires 4–5 h to fully exhibit their pro-migraine potential, are more likely to be responsible for the phenomenon. However, the possible role of TRPA1 in this key process has not yet been explored.
Finally, TRPA1 channel research has provided new insights in the analgesic activity of an old medicine. The paracetamol (acetaminophen) metabolite, N-acetyl-p-benzoquinone imine (NAPQI), activated the TRPA1 channel and thereby evoked a moderate and reversible neurogenic inflammatory response, which may have contributed to airway inflammation (Nassini et al., 2010) and thus could favour asthma in susceptible individuals (Beasley et al., 2008). These initial results were followed by further data, relevant to understanding the hitherto unexplained analgesic action of paracetamol. NAPQI potentially generated by autochthone cytochrome activity within the spinal cord desensitized TRPA1 channels, which induced channel-dependent, anti-nociceptive actions (Andersson et al., 2011). However, it is not known whether this novel spinal mechanism mediated by inhibition of TRPA1 channels, possibly relevant for the general analgesic actions of paracetamol, could be of interest for the antimigraine activity of the drug.
Other TRPs in migraine and sensitization processes
TRPV1, probably because of the long-standing use of capsaicin as a pain remedy, was the first TRP channel studied in migraine. TRPV1 is a multifunctional channel involved in thermo- (heat) and chemo-sensation, functioning as a receptor for a number of seemingly unrelated noxious stimuli. The unique pharmacological property of capsaicin is based on an initial transient TRPV1 activation, associated in vivo to an irritating and painful sensation, which is quite rapidly followed by a durable refractory state in which nociceptors do not respond to subsequent challenges with capsaicin or any other irritant/painful stimulus. The marked desensitization produced by capsaicin accounts for the widespread use of capsaicin skin creams, ointments, patches and other preparations for the treatment of localized neuropathic pain (Backonja et al., 2008). A similar desensitizing property that follows site-specific injections of the capsaicin analogue and ultrapotent TRPV1 channel agonist, resiniferatoxin (Szallasi and Blumberg, 1993), has been successfully used in bladder disorders (Lazzeri et al., 2000; 2004; Peng and Kuo, 2007), and is being evaluated as a long-lasting analgesic treatment in cancer patients with refractory chronic pain (NCT00804154). Based on the assumption that this procedure, which targets the capsaicin ‘receptor’ could block meningeal afferents of the first branch of the trigeminal nerve, topical capsaicin application has been applied to the nasal mucosa to prevent cluster headache (Sicuteri et al., 1989) or migraine (Saper et al., 2002; Fusco et al., 2003) attacks. Despite the fact that results of clinical studies with intranasally delivered formulations of both capsaicin and capsaicin analogues (e.g. civamide) have often been compromised by methodological limits and small sample size (Saper et al., 2002; Fusco et al., 2003), a recent review on the treatment of cluster headache proposed a level B recommendation for intranasal civamide for prophylactic purposes (Francis et al., 2010).
TRPV1 channels under certain circumstances could also play a pathophysiological role in migraine. Alcoholic beverages are reported to induce migraine in about 40% of the patients (Kelman, 2007), but the reason for such susceptibility is unknown. Ethanol acts as a TRPV1 channel stimulant, probably because it reduces the threshold temperature for channel activation by 8°C, from 42/43 to 35°C, which is below the normal body temperature (Trevisani et al., 2002). In addition, and importantly for migraine pathophysiology, ethanol, by targeting TRPV1 channels, evokes CGRP release and the consequent vasodilation in meningeal vessels (Nicoletti et al., 2008). The ability of ethanol to sensitize TRPV1 channels is not limited to temperature, as TRPV1-mediated responses to anandamide and protons are also exaggerated by about 10- and 50-fold respectively (Trevisani et al., 2002). Thus, it may be hypothesized that the ability of alcoholic beverages to trigger migraine depends on individual susceptibility as well as the presence of co-factors, which potentiate ethanol or temperature-gating actions on TRPV1 channels.
The TRPV4 channel is activated by hypoosmolar solutions and membrane stretching and is expressed in peptidergic sensory neurons (Vergnolle et al., 2010). This feature could be of interest in migraine, the pain of which is often described as throbbing. It has also been reported that pregnenolone sulfate is a TRPM3 channel agonist in DRG neurons (Vriens et al., 2011). This finding seems of particular interest, as menstrual cycle, pregnancy and menopause are major determinants of the frequency and severity of migraine attacks. It also suggests a possible direct mechanism that may explain the strong association between female sex hormones and sensory neuron activation in migraine. However, no studies have yet addressed the role of either TRPV4 or TRPM3 channels in migraine or associated headaches.
Chronic migraine and medication overuse headache (MOH, chronic headache accompanied by overuse of symptomatic medicines) are conditions underlined by a progressively worsening hypersensitivity to a host of usually innocuous stimuli. Thus, allodynia represents a characteristic of chronic migraine and MOH (Schurks and Diener, 2008; De Felice et al., 2011). Hypersensitivity may also develop during each individual attack, where sensitization of trigeminal neurons is considered a first step, responsible for the perception of headache throbbing pain (Strassman and Levy, 2006), of a more complex process, which, involving second-order neurons, contributes to cephalic allodynia and muscle tenderness (Burstein et al., 2000; 2010). Hyperproduction of endogenous inflammatory mediators may take part in the initial phenomenon, whereas a central mechanism may activate and sensitize thalamic trigeminovascular neurons (Noseda and Burstein, 2013). A number of neurotransmitters and neural circuitries may contribute to aggravate sensitization in each individual attack, or promote the transition of migraine from an episodic to a chronic condition. There is abundant evidence that TRP channels are sensitized following exposure to proinflammatory mediators, and by intracellular pathways (Trevisani et al., 2002; Nilius et al., 2007; Nilius and Owsianik, 2010; Selescu et al., 2013; Trevisan et al., 2013). However, specific information on the role of TRP channels, and in particular TRPV1 and TRPA1, in the sensitization of trigeminal afferents and/or central nociceptive neurons, has not yet been addressed.
Conclusions
Genetic investigation has provided little help to identify the pathophysiological features that determine migraine susceptibility. Similarly, neuroimaging or biomolecular studies have not yet disclosed the underlying anatomical and neurochemical pathways that result in the migraine attack. In contrast, pharmacological studies and clinical trials have made fundamental contributions to establishing some key players in migraine headache. The ability of non-steroidal anti-inflammatory drugs (NSAIDs) to treat migraine indicates that prostanoids have a role in the disease. However, their action is far from specific, as NSAIDs are used in practically all types of pain. Triptans are of paramount importance in the acute relief of migraine attack. However, as they are receptor agonists (do not block any endogenous agent), and because of their multiple pharmacological actions (vasoconstrictors, inhibitors of CGRP release from perivascular sensory nerve terminals, inhibitors of neuronal transmission within central brain areas), they are not better suited to elucidate the underlying mechanisms of migraine. In contrast, the ability of CGRP receptor antagonists to abolish the pain and associated symptoms of migraine attack, coupled to the lack of evidence that CGRP plays any major role in other types of pain, strongly and specifically implicates CGRP in migraine. Thus, knowledge of the mechanisms that directly cause, or regulate, after a process of sensitization or desensitization, CGRP release from nerves is of paramount importance for our understanding of migraine pathophysiology. TRP channels expressed in peptidergic nociceptors are among the molecular entities that encompass all the features required for this type of investigation. TRPA1 channels, which are activated or sensitized by some known triggers of migraine, along with a wide variety of other stimuli, and are inhibited by analgesic and antimigraine medicines (see Figure 1), would represent a major target for novel drugs to treat migraine and the associated primary headaches.
Figure 1.
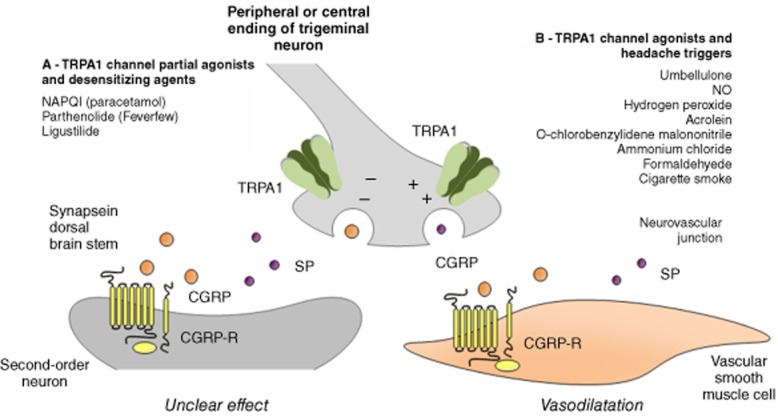
Schematic representation of the activity of several agents (drugs, herbal medicines, endogenous and exogenous compounds), which, by targeting the TRPA1 channel, may positively or negatively affect the migraine attack via the release of CGRP and SP from peripheral and central endings of trigeminal neurons. (A) Some agents may behave as partial agonists or after an initial activation may lead to a profound and enduring channel desensitization. Both mechanisms by inhibiting CGRP release may eventually ameliorate migraine and cluster headache attacks. (B) In contrast, agonists of the TRPA1, by channel stimulation and the ensuing release of neuropeptide, may trigger migraine and cluster headache attacks.
Glossary
- 4α-PDD
4α-phorbol 12,13-didecanoate
- CGRP
calcitonin gene-related peptide
- CIPN
chemotherapeutic-induced peripheral neuropathy
- DRG
dorsal root ganglia
- MOH
medication overuse headache
- NAPQI
N-acetyl-p-benzoquinone imine
- NKA
neurokinin A
- PIP2
phosphatidylinositol (4,5) bisphosphate
- SP
substance P
- TCC
trigeminocervical complex
- TG
trigeminal ganglia
- TRP
transient receptor potential
- TRPA1
TRP ankyrin 1
- TRPC
TRP canonical
- TRPM
TRP melastatin
- TRPML
TRP mucolipin
- TRPP
TRP polycystin
- TRPV
TRP vanilloid
- VG
vagal ganglia
Conflict of interest
The authors state no conflict of interest.
References
- Aiyar N, Rand K, Elshourbagy NA, Zeng Z, Adamou JE, Bergsma DJ, et al. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J Biol Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Arriza JL, Leff SE, Swanson LW, Evans RM, Rosenfeld MG. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;229:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- Amin FM, Asghar MS, Ravneberg JW, de Koning PJ, Larsson HB, Olesen J, et al. The effect of sumatriptan on cephalic arteries: a 3T MR-angiography study in healthy volunteers. Cephalalgia. 2013;33:1009–1016. doi: 10.1177/0333102413483374. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Lau GS, Taylor WR, Critchley JA. Acute effects of the potent lacrimator o-chlorobenzylidene malononitrile (CS) tear gas. Hum Exp Toxicol. 1996;15:461–465. doi: 10.1177/096032719601500601. [DOI] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol. Nat Commun. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Mass D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning P, Larsson HB, et al. Evidence for a vascular factor in migraine. Ann Neurol. 2011;69:635–645. doi: 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129:2312–2315. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P, Jr, Rauck R, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7:1106–1112. doi: 10.1016/S1474-4422(08)70228-X. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci. 2007a;26:2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007b;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS ONE. 2012;7:e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R, Clayton T, Crane J, von Mutius E, Lai CK, Montefort S, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from phase three of the ISAAC programme. Lancet. 2008;372:1039–1048. doi: 10.1016/S0140-6736(08)61445-2. [DOI] [PubMed] [Google Scholar]
- Benemei S, Appendino G, Geppetti P. Pleasant natural scent with unpleasant effects: cluster headache-like attacks triggered by Umbellularia californica. Cephalalgia. 2010;30:744–746. doi: 10.1111/j.1468-2982.2009.01988.x. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23:1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci U S A. 2008;105:20015–20020. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache. 2013;2013:12179. doi: 10.1111/head.12179. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Brone B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, et al. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol Appl Pharmacol. 2008;231:150–156. doi: 10.1016/j.taap.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123:1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RK, Vause CV, Ho TW, Bigal ME, Durham PL. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache. 2009;49:1258–1266. doi: 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- Cady RK, Goldstein J, Nett R, Mitchell R, Beach ME, Browning R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesic M) in the treatment of migraine. Headache. 2011;51:1078–1086. doi: 10.1111/j.1526-4610.2011.01910.x. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6:657–666. doi: 10.1038/nrneurol.2010.160. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Connor KM, Shapiro RE, Diener HC, Lucas S, Kost J, Fan X, et al. Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology. 2009;73:970–977. doi: 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Aurora SK, Loeys T, Ashina M, Jones C, Giezek H, et al. Long-term tolerability of telcagepant for acute treatment of migraine in a randomized trial. Headache. 2011;51:73–84. doi: 10.1111/j.1526-4610.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148:431–437. doi: 10.1016/j.pain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Courteau JP, Cushman R, Bouchard F, Quevillon M, Chartrand A, Bherer L. Survey of construction workers repeatedly exposed to chlorine over a three to six month period in a pulpmill: I. Exposure and symptomatology. Occup Environ Med. 1994;51:219–224. doi: 10.1136/oem.51.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Ossipov MH, Porreca F. Persistent medication-induced neural adaptations, descending facilitation, and medication overuse headache. Curr Opin Neurol. 2011;24:193–196. doi: 10.1097/WCO.0b013e328346af25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Pfaffenrath V, Schnitker J, Friede M, Henneicke-von Zepelin HH. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention – a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia. 2005;25:1031–1041. doi: 10.1111/j.1468-2982.2005.00950.x. [DOI] [PubMed] [Google Scholar]
- Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia. 2011;31:573–584. doi: 10.1177/0333102410388435. [DOI] [PubMed] [Google Scholar]
- Dolgin E. Antibody drugs set to revive flagging migraine target. Nat Rev Drug Discov. 2013;12:249–250. doi: 10.1038/nrd3991. [DOI] [PubMed] [Google Scholar]
- Earley S. TRPA1 channels in the vasculature. Br J Pharmacol. 2012;167:13–22. doi: 10.1111/j.1476-5381.2012.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet. 2010;376:645–655. doi: 10.1016/S0140-6736(10)60323-6. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Gulbenkian S, Barroso CP, Cunha e Sa M, Polak JM, Mortensen A, et al. Innervation of the human middle meningeal artery: immunohistochemistry, ultrastructure, and role of endothelium for vasomotility. Peptides. 1998b;19:1213–1225. doi: 10.1016/s0196-9781(98)00066-7. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Edvinsson L. Possible sites of action of the new calcitonin gene-related peptide receptor antagonists. Ther Adv Neurol Disord. 2010;3:369–378. doi: 10.1177/1756285610388343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Calamari A, Kane SA, Tajti J, Edvinsson L. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience. 2010;169:683–696. doi: 10.1016/j.neuroscience.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Eilers H, Cattaruzza F, Nassini R, Materazzi S, Andre E, Chu C, et al. Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology. 2010;112:1452–1463. doi: 10.1097/ALN.0b013e3181d94e00. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, et al. Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain. 2010;152:2211–2223. doi: 10.1016/j.pain.2010.10.016. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Curtis TM, About I, McGahon MK, Irwin CR, et al. Human dental pulp fibroblasts express the ‘cold-sensing’ transient receptor potential channels TRPA1 and TRPM8. J Endod. 2011;37:473–478. doi: 10.1016/j.joen.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Eltorp CT, Jansen-Olesen I, Hansen AJ. Release of calcitonin gene-related peptide (CGRP) from guinea pig dura mater in vitro is inhibited by sumatriptan but unaffected by nitric oxide. Cephalalgia. 2000;20:838–844. doi: 10.1046/j.1468-2982.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Fanciullacci M, Alessandri M, Figini M, Geppetti P, Michelacci S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60:119–123. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol. 2012;166:510–521. doi: 10.1111/j.1476-5381.2012.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22:633–658. doi: 10.1046/j.1468-2982.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- Francis GJ, Becker WJ, Pringsheim TM. Acute and preventive pharmacologic treatment of cluster headache. Neurology. 2010;75:463–473. doi: 10.1212/WNL.0b013e3181eb58c8. [DOI] [PubMed] [Google Scholar]
- Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, et al. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco BM, Marabini S, Maggi CA, Fiore G, Geppetti P. Preventative effect of repeated nasal applications of capsaicin in cluster headache. Pain. 1994;59:321–325. doi: 10.1016/0304-3959(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Fusco BM, Barzoi G, Agro F. Repeated intranasal capsaicin applications to treat chronic migraine. Br J Anaesth. 2003;90:812. doi: 10.1093/bja/aeg572. [DOI] [PubMed] [Google Scholar]
- Geppetti P, Holzer P. Neurogenic Inflammation. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int. 2008;101:2–6. doi: 10.1111/j.1464-410X.2008.07493.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44. doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Hargreaves RJ. Mechanisms of action of serotonin 5-HT1B/D agonists: insights into migraine pathophysiology using rizatriptan. Neurology. 2000;55:S8–14. [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wang O, Saper JR, Stoltz R, Silberstein SD, Mathew NT. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia. 1997;17:785–790. doi: 10.1046/j.1468-2982.1997.1707785.x. [DOI] [PubMed] [Google Scholar]
- Gratzke C, Weinhold P, Reich O, Seitz M, Schlenker B, Stief CG, et al. Transient receptor potential A1 and cannabinoid receptor activity in human normal and hyperplastic prostate: relation to nerves and interstitial cells. Eur Urol. 2010;57:902–910. doi: 10.1016/j.eururo.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Gu J, Sigurdsson EM. Immunotherapy for tauopathies. Mol Neurosci. 2011;45:690–695. doi: 10.1007/s12031-011-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TH, Blanchard RL, Palcza J, McCrea JB, Laethem T, Willson K, et al. Single- and multiple-dose pharmacokinetics and tolerability of telcagepant, an oral calcitonin gene-related peptide receptor antagonist, in adults. J Clin Pharmacol. 2010;50:1367–1376. doi: 10.1177/0091270010361741. [DOI] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, et al. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–722. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- Hill K, Schaefer M. Ultraviolet light and photosensitising agents activate TRPA1 via generation of oxidative stress. Cell Calcium. 2009;45:155–164. doi: 10.1016/j.ceca.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AR, Zavala G. Long-term effects on the olfactory system of exposure to hydrogen sulphide. Occup Environ Med. 1999;56:284–287. doi: 10.1136/oem.56.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008a;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008b;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008c;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Arvidsson U, Ceccatelli S, Cortes R, Cullheim S, Dagerlind A, et al. Calcitonin gene-related peptide in the brain, spinal cord, and some peripheral systems. Ann N Y Acad Sci. 1992;657:119–134. doi: 10.1111/j.1749-6632.1992.tb22762.x. [DOI] [PubMed] [Google Scholar]
- Immel DL. California Laurel. USDA Natural Resources Conservation Service; 2006. Available at: URL: http://plants.usda.gov/plantguide/pdf/cs_umca.pdf (accessed 11 December 2013) [Google Scholar]
- Irlbacher K, Meyer BU. Nasally triggered headache. Neurology. 2002;58:294. doi: 10.1212/wnl.58.2.294. [DOI] [PubMed] [Google Scholar]
- Iversen HK. Experimental headache in humans. Cephalalgia. 1995;15:281–287. doi: 10.1046/j.1468-2982.1995.1504281.x. [DOI] [PubMed] [Google Scholar]
- Iversen HK, Olesen J. The effect of sumatriptan on nitroglycerin-(NTG)-induced headache and vascular responses. Cephalalgia. 1993;13:186. [Google Scholar]
- Iversen HK, Olesen J. Headache induced by a nitric oxide donor (nitroglycerin) responds to sumatriptan. A human model for development of migraine drugs. Cephalalgia. 1996;16:412–418. doi: 10.1046/j.1468-2982.1996.1606412.x. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Aviello G, Borrelli F, Romano B, Piscitelli F, et al. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol. 2012;166:1444–1460. doi: 10.1111/j.1476-5381.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, et al. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol. 2010;518:687–698. doi: 10.1002/cne.22238. [DOI] [PubMed] [Google Scholar]
- Kochukov MY, McNearney TA, Fu Y, Westlund KN. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am J Physiol Cell Physiol. 2006;291:C424–C432. doi: 10.1152/ajpcell.00553.2005. [DOI] [PubMed] [Google Scholar]
- Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Latorre R. Perspectives on TRP channel structure and the TRPA1 puzzle. J Gen Physiol. 2009;133:227–229. doi: 10.1085/jgp.200910199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: a randomized placebo controlled study. J Urol. 2000;164:676–679. doi: 10.1097/00005392-200009010-00014. [DOI] [PubMed] [Google Scholar]
- Lazzeri M, Spinelli M, Beneforti P, Malaguti S, Giardiello G, Turini D. Intravesical infusion of resiniferatoxin by a temporary in situ drug delivery system to treat interstitial cystitis: a pilot study. Eur Urol. 2004;45:98–102. doi: 10.1016/s0302-2838(03)00418-4. [DOI] [PubMed] [Google Scholar]
- Lee SP, Buber MT, Yang Q, Cerne R, Cortes RY, Sprous DG, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153:1739–1749. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. [DOI] [PubMed] [Google Scholar]
- Lewis T. The nocifensor system of nerves and its reactions. Br Med J. 1937;1:431–435. doi: 10.1136/bmj.1.3973.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JC, Shen XF, Meng XL, Zhang Y, Lai XR. Analgesic effect and mechanism of the three TCM-herbal drug-combination Tou Feng Yu pill on treatment of migraine. Phytomedicine. 2011;18:788–794. doi: 10.1016/j.phymed.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Lima AM, Sapienza GB, Giraud Vde O, Fragoso YD. Odors as triggering and worsening factors for migraine in men. Arq Neuropsiquiatr. 2011;69:324–327. doi: 10.1590/s0004-282x2011000300011. [DOI] [PubMed] [Google Scholar]
- Lipton RB. Chronic migraine, classification, differential diagnosis, and epidemiology. Headache. 2011;51(Suppl 2):77–83. doi: 10.1111/j.1526-4610.2011.01954.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu C, Shi L, Kaufman S, Smith D, Immke D, et al. Immunohistochemical localization of the CLR/RAMP1 receptor complex in the trigeminovascular system of the cynomolgus monkey. Headache. 2011;51:6. [Google Scholar]
- LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha,beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008;104:235–249. doi: 10.1093/toxsci/kfm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Chen L, Conway CM, Denton R, Keavy D, Signor L, et al. Discovery of (5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyri din-9-yl 4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxylate (BMS-927711): an oral calcitonin gene-related peptide (CGRP) antagonist in clinical trials for treating migraine. J Med Chem. 2012;55:10644–10651. doi: 10.1021/jm3013147. [DOI] [PubMed] [Google Scholar]
- Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120:677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Maione S, Piscitelli F, Gatta L, Vita D, De Petrocellis L, Palazzo E, et al. Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. Br J Pharmacol. 2011;162:584–596. doi: 10.1111/j.1476-5381.2010.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Fusi C, Benemei S, Pedretti P, Patacchini R, Nilius B, et al. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012;463:561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Benemei S, Fusi C, Gualdani R, De Siena G, Vastani N, et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting TRPA1 channel. Pain. 2013;3959:00438–00437. doi: 10.1016/j.pain.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A. 2008;105:8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck & Co. 2009. (21 April Merck announces first-quarter 2009 financial results. Company Press Release. Last access date:16 August 2013.
- Minke B. Drosophila mutant with a transducer defect. Biophys Struct Mech. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, Otsuguro K, Ito S. Time- and concentration-dependent activation of TRPA1 by hydrogen sulfide in rat DRG neurons. Neurosci Lett. 2011;499:137–142. doi: 10.1016/j.neulet.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen LJ, Laavola M, Kukkonen M, Korhonen R, Leppanen T, Hogestatt ED, et al. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci Rep. 2012;2:380. doi: 10.1038/srep00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–268. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- Moore EL, Salvatore CA. Targeting a family B GPCR/RAMP receptor complex: CGRP receptor antagonists and migraine. Br J Pharmacol. 2012;166:66–78. doi: 10.1111/j.1476-5381.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Reinhard JF, Jr, Romero J, Melamed E, Pettibone DJ. Neurotransmitters and the fifth cranial nerve: is there a relation to the headache phase of migraine? Lancet. 1979;2:883–885. doi: 10.1016/s0140-6736(79)92692-8. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369:448–457. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- NCT00804154. Resiniferatoxin to treat severe pain associated with advanced cancer. Available at: http://clinicaltrials.gov/ct2/show/NCT00804154?term=resiniferatoxin&rank=1 (accessed 16 August 2013)
- Nassini R, Materazzi S, Andre E, Sartiani L, Aldini G, Trevisani M, et al. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. 2010;24:4904–4916. doi: 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012a;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, et al. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE. 2012b;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P, Trevisani M, Manconi M, Gatti R, De Siena G, Zagli G, et al. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia. 2008;28:9–17. doi: 10.1111/j.1468-2982.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460:437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Biro T, Owsianik G. TRPV3: time to decipher a poorly understood family member! J Physiol. 2013;2013:8. doi: 10.1113/jphysiol.2013.255968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. 2013;3959:00389–00388. doi: 10.1016/j.pain.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo K, Matsumura M, Kawaishi Y, Aoki Y, Matsunami M, Okawa Y, et al. Hydrogen sulfide-induced mechanical hyperalgesia and allodynia require activation of both Cav3.2 and TRPA1 channels in mice. Br J Pharmacol. 2012;166:1738–1743. doi: 10.1111/j.1476-5381.2012.01886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev. 2012;64:939–971. doi: 10.1124/pr.112.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Kuo HC. Multiple intravesical instillations of low-dose resiniferatoxin in the treatment of refractory interstitial cystitis. Urol Int. 2007;78:78–81. doi: 10.1159/000096940. [DOI] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Lassen LH, Kruuse C, Jonassen O, Lesko L, et al. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia. 2005;25:139–147. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- Pozsgai G, Hajna Z, Bagoly T, Boros M, Kemény Á, Materazzi S, et al. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur J Pharmacol. 2012;689:56–64. doi: 10.1016/j.ejphar.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12:221–228. doi: 10.1046/j.1468-2982.1992.1204221.x. [DOI] [PubMed] [Google Scholar]
- Romano B, Borrelli F, Fasolino I, Capasso R, Piscitelli F, Cascio M, et al. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169:213–229. doi: 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum D, Hanisch UK, Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Rozen TD. Cluster headache as the result of secondhand cigarette smoke exposure during childhood. Headache. 2010;50:130–132. doi: 10.1111/j.1526-4610.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- Saeidnia S, Abdollahi M. Antioxidants: friends or foe in prevention or treatment of cancer: the debate of the century. Toxicol Appl Pharmacol. 2013;271:49–63. doi: 10.1016/j.taap.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Saper JR, Klapper J, Mathew NT, Rapoport A, Phillips SB, Bernstein JE. Intranasal civamide for the treatment of episodic cluster headaches. Arch Neurol. 2002;59:990–994. doi: 10.1001/archneur.59.6.990. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci. 2008;27:1131–1142. doi: 10.1111/j.1460-9568.2008.06093.x. [DOI] [PubMed] [Google Scholar]
- Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216–220. [PubMed] [Google Scholar]
- Schurks M, Diener HC. Migraine, allodynia, and implications for treatment. Eur J Neurol. 2008;15:1279–1285. doi: 10.1111/j.1468-1331.2008.02343.x. [DOI] [PubMed] [Google Scholar]
- Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33:5603–5611. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- Selescu T, Ciobanu AC, Dobre C, Reid G, Babes A. Camphor activates and sensitizes transient receptor potential melastatin 8 (TRPM8) to cooling and icilin. Chem Senses. 2013;38:563–575. doi: 10.1093/chemse/bjt027. [DOI] [PubMed] [Google Scholar]
- Shevel E. The extracranial vascular theory of migraine – a great story confirmed by the facts. Headache. 2011;51:409–417. doi: 10.1111/j.1526-4610.2011.01844.x. [DOI] [PubMed] [Google Scholar]
- Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, et al. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br J Pharmacol. 2012;165:1476–1486. doi: 10.1111/j.1476-5381.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicuteri F, Fusco BM, Marabini S, Campagnolo V, Maggi CA, Geppetti P, et al. Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin J Pain. 1989;5:49–53. doi: 10.1097/00002508-198903000-00010. [DOI] [PubMed] [Google Scholar]
- Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, et al. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- Spehr J, Gelis L, Osterloh M, Oberland S, Hatt H, Spehr M, et al. G protein-coupled receptor signaling via Src kinase induces endogenous human transient receptor potential vanilloid type 6 (TRPV6) channel activation. J Biol Chem. 2011;286:13184–13192. doi: 10.1074/jbc.M110.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol. 2004;142:1171–1181. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. [3H]resiniferatoxin binding by the vanilloid receptor: species-related differences, effects of temperature and sulfhydryl reagents. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:84–91. doi: 10.1007/BF00168777. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szyszkowicz M. Ambient air pollution and daily emergency department visits for headache in Ottawa, Canada. Headache. 2008;48:1076–1081. doi: 10.1111/j.1526-4610.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- Tajti J, Uddman R, Moller S, Sundler F, Edvinsson L. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst. 1999b;76:176–183. doi: 10.1016/s0165-1838(99)00024-7. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. J Physiol. 2010;588:423–433. doi: 10.1113/jphysiol.2009.183301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, et al. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen LL, Olesen J. Nitric oxide in primary headaches. Curr Opin Neurol. 2001;14:315–321. doi: 10.1097/00019052-200106000-00009. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Costa C, Caproni S, Pisani A, et al. Critical role of calcitonin gene-related peptide receptors in cortical spreading depression. Proc Natl Acad Sci U S A. 2012;109:18985–18990. doi: 10.1073/pnas.1215435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan G, Materazzi S, Fusi C, Altomare A, Aldini G, Lodovici M, et al. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013;73:3120–3131. doi: 10.1158/0008-5472.CAN-12-4370. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Geppetti P, Davis JB, Bianchi A, Harrison S, Randall AD, et al. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SH, Tew JM, McLean JH, Shipley MT. Cerebral arterial innervation by nerve fibers containing calcitonin gene-related peptide (CGRP): I. Distribution and origin of CGRP perivascular innervation in the rat. J Comp Neurol. 1988;271:435–444. doi: 10.1002/cne.902710310. [DOI] [PubMed] [Google Scholar]
- Tvedskov JF, Lipka K, Ashina M, Iversen HK, Schifter S, Olesen J. No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann Neurol. 2005;58:561–568. doi: 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- Tvedskov JF, Tfelt-Hansen P, Petersen KA, Jensen LT, Olesen J. CGRP receptor antagonist olcegepant (BIBN4096BS) does not prevent glyceryl trinitrate-induced migraine. Cephalalgia. 2010;30:1346–1353. doi: 10.1177/0333102410363491. [DOI] [PubMed] [Google Scholar]
- Vay L, Gu C, McNaughton PA. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol. 2012;165:787–801. doi: 10.1111/j.1476-5381.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Cenac N, Altier C, Cellars L, Chapman K, Zamponi GW, et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br J Pharmacol. 2010;159:1161–1173. doi: 10.1111/j.1476-5381.2009.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Hofmann T, Philipp SE, Stab J, Chen X, et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70:482–494. doi: 10.1016/j.neuron.2011.02.051. [DOI] [PubMed] [Google Scholar]
- Wagner TF, Loch S, Lambert S, Straub I, Mannebach S, Mathar I, et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nat Cell Biol. 2008;10:1421–1430. doi: 10.1038/ncb1801. [DOI] [PubMed] [Google Scholar]
- Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170:1293–1307. doi: 10.1111/bph.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Liman ER. TRPA1 is a component of the nociceptive response to CO2. J Neurosci. 2010;30:12958–12963. doi: 10.1523/JNEUROSCI.2715-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wantke F, Focke M, Hemmer W, Bracun R, Wolf-Abdolvahab S, Gotz M, et al. Exposure to formaldehyde and phenol during an anatomy dissecting course: sensitizing potency of formaldehyde in medical students. Allergy. 2000;55:84–87. doi: 10.1034/j.1398-9995.2000.00307.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Wei EP, Moskowitz MA, Boccalini P, Kontos HA. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ Res. 1992;70:1313–1319. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- White BJ, Smith PA, Dunn WR. Hydrogen sulphide-mediated vasodilatation involves the release of neurotransmitters from sensory nerves in pressurized mesenteric small arteries isolated from rats. Br J Pharmacol. 2013;168:785–793. doi: 10.1111/j.1476-5381.2012.02187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2005;9:628–635. doi: 10.1038/nn1692. 2006. [DOI] [PubMed] [Google Scholar]
- Zhong J, Minassi A, Prenen J, Taglialatela-Scafati O, Appendino G, Nilius B. Umbellulone modulates TRP channels. Pflugers Arch. 2011;462:861–870. doi: 10.1007/s00424-011-1043-1. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


