Abstract
Background and Purpose
Evodiamine, a racemic quinazolinocarboline alkaloid isolated from the traditional Chinese medicine Evodiae fructus, has been reported to act as an agonist of the transient receptor potential vanilloid type-1 (TRPV1) cation channel both in vitro and in vivo. Evodiamine is structurally different from all known TRPV1 activators, and has significant clinical potential as a thermogenic agent. Nevertheless, the molecular bases for its actions are still poorly understood.
Experimental Approach
To investigate the structure-activity relationships of evodiamine, the natural racemate was resolved, and a series of 23 synthetic analogues was prepared, using as the end point the intracellular Ca2+ elevation in HEK-293 cells stably overexpressing either the human or the rat recombinant TRPV1.
Key Results
S-(+) evodiamine was more efficacious and potent than R-(−) evodiamine, and a new potent lead (Evo30) was identified, more potent than the reference TRPV1 agonist, capsaicin. In general, potency and efficacy correlated with the lipophilicity of the analogues. Like other TRPV1 agonists, several synthetic analogues could efficiently desensitize TRPV1 to activation by capsaicin.
Conclusions and Implications
Evodiamine qualifies as structurally unique lead structure to develop new potent TRPV1 agonists/desensitizers.
Linked Articles
This article is part of a themed section on the pharmacology of TRP channels. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-10
Keywords: vanilloids, TRP channels, evodiamine, TRPV1, palvanil, capsaicin
Introduction
Transient receptor potential (TRP) channels are a large family of non-selective cation channels variously regulated (De Petrocellis and Di Marzo, 2005). They respond to physical and chemical stimuli such as temperature, pH, light, osmolarity, touch, pheromones, oxidative stress and lipids (Venkatachalam and Montell, 2007). Mutations in many of the genes that encode TRP channels cause pathological conditions known as ‘TRP channelopathies’ (Nilius and Owsianik, 2010). Members of the TRP vanilloid-(TRPV1-V4), melastatin-(TRPM2, M3, M5 and M8) and ankiryn-type (TRPA1) subfamilies are gated by temperature changes (<15 to >53°C), and are known as ‘thermoTRPs’. ThermoTRPs are expressed in sensory nerve terminals and initiate sensory nerve impulses following the detection of chemical and thermal stimuli (Patapoutian et al., 2009).
TRPV1 (Caterina et al., 1997) is a unique pain-sensing transducer playing a pivotal role in the maintenance of inflammatory conditions secondary to tissue injury by trauma, infection, surgery, burns or diseases with an inflammatory component (Jara-Oseguera et al., 2008; Basbaum et al., 2009). It is also known as the ‘capsaicin receptor’, and was initially described in dorsal root, trigeminal and nodose ganglia neurons (Caterina et al., 1997). TRPV1 displays wide tissue and cellular expression in both peripheral and central nervous systems (Cristino et al., 2006; Moran et al., 2011), and in both neuronal and non-neuronal cells, its highest expression being in sensory neurons (Sanchez et al., 2001). It is activated by pungent compounds such as capsaicin and resiniferatoxin, spider and tarantula toxins, noxious temperatures (>42°C) and low pH (<5.9) (Caterina et al., 1997; Siemens et al., 2006; Bohlen et al., 2010). Endogenous mediators, like the endocannabinoid anandamide (Zygmunt et al., 1999) and some eicosanoids (Hwang et al., 2000; De Petrocellis et al., 2012; Wen et al., 2012), also activate TRPV1 channels. Molecular-modelling techniques demonstrated that the preferential conformations of these compounds in solution substantially overlap with those of capsaicin (Movahed et al., 2005), although none of these endogenous compounds is as potent as this natural product. TRPV1 undergoes desensitization in the continuous presence of an activating stimulus, explaining why TRPV1 agonists can produce paradoxical analgesic and anti-inflammatory actions. Receptor desensitization is Ca2+-dependent and implies dephosphorylation of the receptor (whereas phosphorylation usually causes its sensitization) and its endocytosis and degradation in lysosomes (Sanz-Salvador et al., 2012). The validation of TRPV1 as a therapeutic target for pain prompted the development of several TRPV1 antagonists that have entered clinical trials for the treatment of acute, chronic and neuropathic pain (Basbaum et al., 2009; Ferrer-Montiel et al., 2012). Additionally, in the nose, stimulation of TRPV1 in nasal fibres elicits release of proinflammatory mediators, which contribute to rhinitic symptoms, so that blockade of the channel with SB-705498 counteracts such symptoms (Changani et al., 2013). The TRPV1 antagonists SB-705498 and PF-04065463 also inhibited evoked-airway hyper-responsiveness to histamine. Indeed, TRPV1 is present on peripheral terminals of airway sensory nerves and modulation of its activity represents a potential target for the pharmacological treatment of airway hyper-responsiveness (Delescluse et al., 2012). Functional TRPV1 channels are also expressed in rat peripheral arteries where they appear to exhibit different pharmacological properties from those located in sensory neurons (Czikora et al., 2012). Thus, vascular TRPV1 in activation may represent an unwanted effect of TRPV1 antagonists when used as analgesics in vivo. On the other hand, vascular TRPV1 may be a new therapeutic target for the regulation of tissue blood distribution (Czikora et al., 2012). Finally, TRPV1 and the neuropeptide substance P located on sensory neurones and non-neuronal cells were recently suggested to be important targets in sepsis and in this context TRPV1 seems to play a protective role (Bodkin and Fernandes, 2013).
The pharmacophore of TRPV1 antagonists fits within the pore region of a TRPV1 receptor homology model, with critical hydrogen bond interactions proposed between the antagonist and Tyr 667 on helix six. The molecular determinants that are required for activation or inhibition of TRPV1 by chemical ligands have been largely identified (Jordt and Julius, 2002; Gavva et al., 2004; Phillips et al., 2004), with the pore helix playing an important role in channel functionality and activation (Myers et al., 2008).
TRPV1 can be activated by evodiamine (Figure 1), a racemic quinazolinocarboline alkaloid isolated from the fruits of the traditional Chinese medicine Evodiae fructus (Pearce et al., 2004). This is a remedy known as Wu-Chu-Yu and used for the management of a diverse series of conditions (angina pectoris, hypertension, impotence, postpartum haemorrhage, pain, vomiting, pyresis, gastrointestinal disorders and headache), some of which are apparently unrelated to TRPV1 activity (Kobayashi, 2003). Evodiamine activates guinea pig, rat and mouse TRPV1 (Kobayashi et al., 2000; 2001a,2001b; Kobayashi, 2003; Pearce et al., 2004; Rang et al., 2004) and shows anti-anxiety (Lu et al., 2012), anti-obesity (Kobayashi et al., 2001b; Wang et al., 2008; Bak et al., 2010), antinociceptive (Kobayashi, 2003), anti-inflammatory (Ko et al., 2007), anti-allergic (Rang et al., 2003) and anticancer (Kan et al., 2004; 2007; Liao et al., 2005; Yang et al., 2008; 2009; 2013; Jiang and Hu, 2009; Chen et al., 2010; Wang et al., 2010; Dong et al., 2012) properties, which are often attenuated by TRPV1 antagonists. Evodiamine inhibits the proliferation of a wide variety of tumour cells by inducing their apoptosis (Lee et al., 2006; Zhang et al., 2010; Tu et al., 2013) and shows anti-angiogenesis effects (Shyu et al., 2006). In endothelial cells, evodiamine and capsaicin induce nitric oxide production and endothelial nitric oxide synthase phosphorylation, by interacting with intracellular proteins important for the regulation of vascular tone, like calmodulin and PI3K/Akt (Chiou et al., 1996; Domenicali et al., 2005; Wang and Wang, 2005; Yao and Garland, 2005; Ching et al., 2011). Administered chronically, evodiamine also produces TRPV1-dependent protection against atherosclerosis in mice (Wei et al., 2013). The thermogenic potential of evodiamine has made it popular in the health food market as a non-pungent slimming agent, even though this activity has never been conclusively demonstrated in the clinic, and only relies on animal and cellular experiments (Wang et al., 2008).
Figure 1.
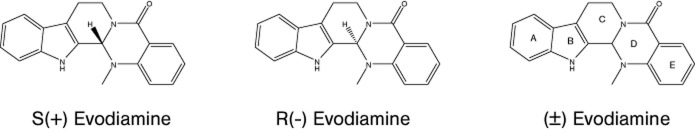
Structure of S(+) Evodiamine; R(−) Evodiamine and (±) Evodiamine.
The interaction between evodiamine and TRPV1 is not fully understood. Previous mutation studies have suggested that the affinity of agonists with rat TRPV1 is affected by Tyr511, Ser512, Leu515, Phe543, Met547 and Lys571 (Lee et al., 2011). With the aim of understanding the molecular basis for the recognition of evodiamine by TRPV1, a homology model has been constructed and the specific interaction between the two molecules has been studied using computational approaches. According to this model, ring A of evodiamine (Figure 1) establishes a hydrophobic interaction with Tyr511, while ring E forms π-π interactions with Tyr555 of human TRPV1. In addition, evodiamine makes two H-bonds between the carbonyl oxygen and the side chains of Lys571 and between the indole nitrogen and the side chains of Ile569 (Wang et al., 2012). Capsaicin and evodiamine share certain pharmacophoric elements, but their lipophilic moiety is different, encompassing a saturated isononenyl group in capsaicin, and two phenyl rings in evodiamine. Animal species differences in the capability of activating TRPV1 have been reported for capsaicin but not evodiamine.
Apart from any structural difference between capsaicin and evodiamine, a critical, and surprisingly so far overlooked, issue is that capsaicin is achiral, while evodiamine is chiral. The recognition model for evodiamine is chiral, and a large difference in bioactivity is therefore to be expected between its enantiomers. In this context, the identification of the active enantiomeric form should provide the basis for any future structure-activity study on this interesting lead compound. To address these issues, we have compared the activity of the two enantiomers of evodiamine, and a series of synthetic enantiomerically pure analogues at both human and rat TRPV1 stably overexpressed in HEK-293 cells.
Methods
Synthesis, purification, resolution and characterization of evodiamine analogues
The synthesis of racemic evodiamine was carried out according to the related literature procedure (Danieli and Palmisano, 1978). Racemic evodiamine was condensed with (1S)-10-camphorsulfonyl chloride to provide diastereomer derivatives, the hydrolysis of which by NaOH (10%) provided the pure enantiomers (S)-evodiamine and (R)-evodiamine in almost quantitative yields (90%). The synthesis of evodiamine derivatives was accomplished in a synthetic sequence starting from L-tryptophan methyl ester hydrochloride, which in a two-step sequence provided Evo 05. Then, two cyclizations furnished its epimer at position 3, Evo 06, in a diastereomeric ratio of 8:2. Hydrolysis of the ester with LiOH and treatment of the corresponding acid or acyl chloride with various amines or hydroxyl derivatives afforded the desired compounds. The enantiomeric compounds were obtained starting from d-tryptophan methyl ester hydrochloride (D. Passarella, A. Sacchetti, M. Christodoulou, in preparation).
Assays of TRPV1-mediated elevation of intracellular [Ca2+]
HEK-293 cells stably overexpressing recombinant human TRPV1 were selected by G-418 (Geneticin, Invitrogen Life Technologies, Grand Island, NY, USA; 600 μg·mL−1), grown on 100-mm diameter Petri dishes as monolayers in minimum essential medium supplemented with non-essential amino acids, 10% FBS, and 2 mM glutamine, and maintained under 5% CO2 at 37°C. On the day of the experiment, the cells were loaded for 1 h at 25°C with the cytoplasmic calcium indicator Fluo-4AM (Invitrogen Life Technologies) 4 μM in DMSO containing 0.02% Pluronic F-127 (Invitrogen). After loading, cells were washed twice in Tyrode's buffer (145 mM NaCl, 2.5 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 10 mM d-glucose and 10 mM HEPES, pH 7.4), resuspended in the same buffer, and transferred to a quartz cuvette of the spectrofluorimeter (Perkin-Elmer LS50B; PerkinElmer Life and Analytical Sciences, Waltham, MA, USA) under continuous stirring (about 100 000 cells for assay). [Ca2+]i was determined before and after the addition of various concentrations of test compounds by measuring cell fluorescence (excitation λ = 488 nm; emission λ = 516 nm). The potency (EC50 values) was determined as the concentration of test compound required to produce half-maximal increases in [Ca2+]i. The efficacy of the agonists was determined at 10 μM by comparing their effect with the analogous effect observed with 4 μM ionomycin (LKT Laboratories, Inc. St. Paul, MN, USA). Dose-response curves were fitted by a sigmoidal regression with variable slope. Curve fitting and parameter estimation were performed with GraphPad Prism® (GraphPad Software Inc., San Diego, CA, USA). All determinations were conducted at least in triplicate, and the compounds were tested also on wild-type HEK293 cells (i.e. not transfected with any TRP construct): when significant, the values of the effect on [Ca2+]i in wild-type HEK293 cells were taken as baselines and subtracted from the values obtained from transfected cells. Statistical analysis of the data was performed by analysis of variance at each point using anova followed by the Bonferroni's test. LogP was calculated according to Tetko et al. (2005).
All molecular target nomenclature conformed with the BJP's Concise Guide to PHARMACOLOGY (Alexander et al., 2013).
Results
In agreement with previous literature, natural and racemic evodiamine produced a dose-dependent increase in intracellular calcium in HEK-293 cells stably transfected with the human recombinant TRPV1, with an EC50 = 155.20 ± 8.2 nM. When tested on the rat recombinant TRPV1, the EC50 was about fourfold higher (652.2 ± 26.7). Resolution of the racemate afforded the two enantiomers, and S-(+) evodiamine resulted more efficacious and almost more than fourfold more potent than R-(−) evodiamine at both human (EC50 = 113.4 ± 8.9 and 546.0 ± 24.2 nM, respectively) and rat (391.5 ± 3.3 and 1491 ± 46 nM, respectively) TRPV1 (Table 1, Figure 2). The specificity of the receptor response was verified by pretreating the cells for 5 min with the selective TRPV1 antagonist 5-iodo-resiniferatoxin (Wahl et al., 2001) at a concentration of 10 nM before the addition of the compounds. The effects of 1 μM (±)-, (+)-and (−)-evodiamine were all inhibited by 100% (Figure 2 and data not shown). Likewise, (±)-, (+)-and (−)-evodiamine were all inactive in untransfected HEK-293 cells (Figure 2 and data not shown).
Table 1.
Effect of racemic and resolved evodiamine enantiomers on elevation of intracellular [Ca2+] in HEK-293 cells overexpressing either the human or rat (r) TRPV1 channel
| Compound | Efficacy (at 10 μM ± SE) | Potency EC50 ± SE, nM | Desensitization of 0.1 μM capsaicin response IC50 ± SE, nM | LogP ± SD |
|---|---|---|---|---|
| S(+) Evodiamine | 82.9 ± 1.5 | 113.4 ± 8.9 | 176.9 ± 3.7 | 2.95 ± 0.37 |
| r 66.3 ± 0.1 | r 391.5 ± 3.3 | r 260.9 ± 17.6 | ||
| R(−) Evodiamine | 76.0 ± 0.8 | 546.0 ± 24.2 | 674.9 ± 99.1 | 2.95 ± 0.37 |
| r 55.4 ± 0.1 | r 1491 ± 46 | r 1580 ± 40 | ||
| (+/−) Evodiamine | 79.4 ± 0.9 | 155.2 ± 8.2 | 215.8 ± 7.3 | 2.95 ± 0.37 |
| r 71.9 ± 0.4 | r 652.2 ± 26.7 | r 523.4 ± 17.0 |
All tests were carried out six times, and the compounds were tested also on HEK-293 cells not transfected with the TRPV1 receptor: none produced a significant elevation of intracellular [Ca2+] (Figure 2). The specificity of the receptor response was also verified by pretreating the cells transfected with human or rat TRPV1 for 5 min with the specific antagonist 5-iodo-resiniferatoxin (Wahl et al., 2001) at a concentration of 10 nM before the addition of the compound at 1 μM: this resulted in a complete inhibition of evodiamine activity at TRPV1 (Figure 2).
Figure 2.
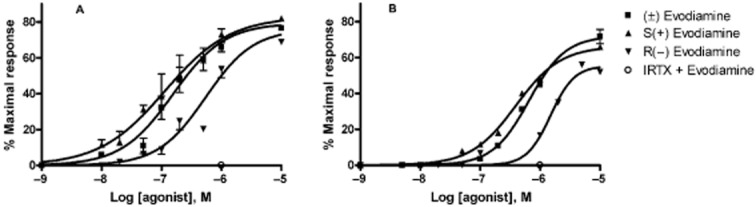
Dose-dependent effects of (±) evodiamine; S(+) evodiamine and R(−) evodiamine on TRPV1-mediated elevation of intracellular calcium in HEK-293 cells stably overexpressing the human (A) or the rat (B) recombinant TRPV1 channel. Data are means of n = 6 separate experiments. The specificity of the receptor response was also verified by pretreating the cells transfected with TRPV1 for 5 min with the specific antagonist 5-iodo-resiniferatoxin (IRTX) (Wahl et al., 2001) at a concentration of 10 nM before the addition of the compound at the concentration of 1 μM. This resulted in a complete inhibition of the activity of all three evodiamines at TRPV1 (empty circle).
In the light of these observations, a series of synthetic analogues of evodiamine were prepared, resolved and independently assayed. In all cases, the analogues of S(+)-evodiamine were more potent than those of R(−)-evodiamine (Table 2), with the following rank of potency:
Table 2.
Effect of synthetic evodiamine analogues and their enantiomers on elevation of intracellular calcium in HEK-293 cells overexpressing either the human or rat (r) TRPV1 channel
| Compound | Structure | Efficacy (at 10 μM ± SE) | Potency EC50 ± SE | Desensitization of 0.1 μM capsaicin response IC50 ± SE | LogP ± SD |
|---|---|---|---|---|---|
| Evo 05 | 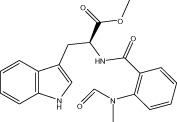 |
<10 | NA | NA | 1.48 ± 0.46 |
| 0 | NA | NA | |||
| Evo 15 | 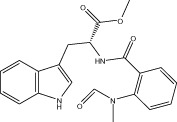 |
0 | NA | NA | 1.48 ± 0.46 |
| r < 10 (8.5 ± 0.1) | NA | NA | |||
| Evo 06 | 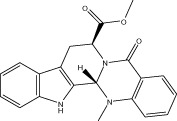 |
70.0 ± 0.9 | 1.18 ± 0.08 μM | 1.13 ± 0.04 μM | 2.77 ± 0.50 |
| r 69.0 ± 3.2 | r 2.21 ± 0.40 μM | r 1.68 ± 0.17 μM | |||
| Evo 21 | 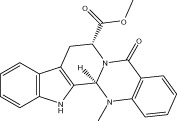 |
73.2 ± 1.7 | 3.06 ± 0.37 μM | 4.42 ± 0.09 μM | 2.77 ± 0.50 |
| r 62.2 ± 1.0 | r 5.42 ± 0.33 μM | r 5.26 ± 0.19 μM | |||
| Evo 09 | 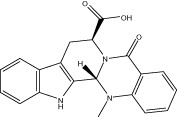 |
10.6 ± 0.6 | 4.56 ± 1.16 μM | NA | 2.45 ± 0.51 |
| r < 10 (4.9 ± 0.1) | NA | NA | |||
| Evo 22 | 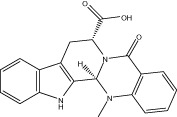 |
<10 (4.4 ± 0.6) | NA | NA | 2.45 ± 0.51 |
| r < 10 (6.5 ± 0.1) | NA | NA | |||
| Evo 23 | 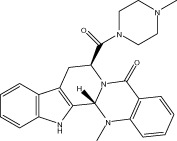 |
41.7 ± 1.0 | 4.25 ± 0.50 μM | 7.31 ± 0.60 μM | 2.10 ± 0.72 |
| r 25.1 ± 0.6 | r 3.12 ± 0.37 μM | r 12.32 ± 0.74 μM | |||
| Evo 29 | 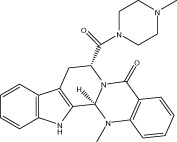 |
12.9 ± 1.0 | 9.76 ± 2.17 μM | NA | 2.10 ± 0.72 |
| r < 10 (9.5 ± 0.1) | NA | NA | |||
| Evo 28 |  |
17.8 ± 0.7 | 4.00 ± 0.83 μM | 34.06 ± 0.06 μM | 1.54 ± 0.85 |
| r 16.1 ± 0.5 | r 5.74 ± 0.1 μM | r 27.24 ± 7.10 μM | |||
| VR002 | 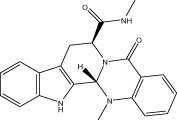 |
<10 (3.9 ± 0.1) | NA | NA | 1.91 ± 0.80 |
| r < 10 (7.4 ± 0.1) | NA | NA | |||
| VR001 | 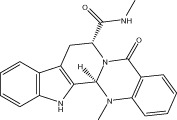 |
<10 (1.0 ± 0.1) | NA | NA | 1.91 ± 0.80 |
| r < 10 (1.2 ± 0.1) | NA | NA | |||
| Evo 30 | 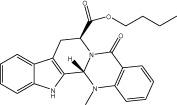 |
67.1 ± 0.9 | 2.01 ± 0.11 nM | 1.65 ± 0.15 nM | 3.96 ± 0.57 |
| r 72.1 ± 1.0 | r 2.45 ± 0.20 nM | r 1.61 ± 0.04 nM | |||
| Evo 31 | 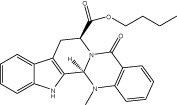 |
51.2 ± 1.2 | 89.6 ± 14.5 nM | 0.18 ± 0.01 μM | 3.96 ± 0.57 |
| r 38.3 ± 0.8 | r 0.20 ± 0.02 μM | r 0.28 ± 0.01 μM | |||
| Evo 34 | 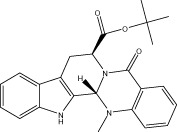 |
69.4 ± 0.8 | 25.9 ± 1.2 nM | 25.95 ± 0.44 nM | 3.79 ± 0.49 |
| r 69.5 ± 2.4 | r 89.1 ± 17.7 nM | r 31.5 ± 1.4 nM | |||
| Evo 35 | 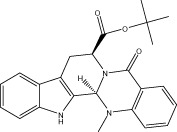 |
41.8 ± 1.0 | 0.33 ± 0.04 μM | 0.36 ± 0.02 μM | 3.79 ± 0.49 |
| r 46.2 ± 1.9 | r 0.76 ± 0.16 μM | r 1.05 ± 0.13 μM | |||
| Evo 38 | 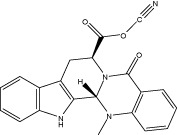 |
68.4 ± 0.6 | 59.2 ± 2.4 nM | 45.1 ± 2.0 nM | 2.59 ± 1.02 |
| r 50.2 ± 0.2 | r 76.1 ± 1.5 nM | r 80.1 ± 2.4 nM | |||
| Evo 39 | 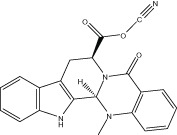 |
58.3 ± 0.8 | 96.5 ± 6.1 nM | 0.12 ± 0.01 μM | 2.59 ± 1.02 |
| r 48.4 ± 1.1 | r 0.12 ± 0.02 μM | r 0.22 ± 0.03 μM | |||
| Evo 42 | 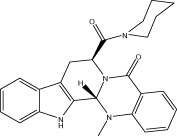 |
58.4 ± 0.6 | 0.21 ± 0.01 μM | 0.38 ± 0.01 μM | 3.26 ± 0.48 |
| r 48.9 ± 0.2 | r 0.87 ± 0.02 μM | r 0.64 ± 0.03 μM | |||
| VR007 | 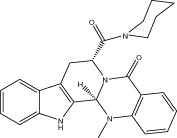 |
60.4 ± 1.1 | 3.08 ± 0.19 μM | 2.67 ± 0.10 μM | 3.26 ± 0.48 |
| r 54.3 ± 2.1 | r 6.69 ± 0.94 μM | r 6.94 ± 0.58 μM | |||
| Evo 44 | 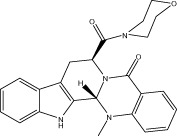 |
29.1 ± 1.9 | 5.43 ± 1.23 μM | 5.35 ± 0.20 μM | 2.01 ± 0.63 |
| r 34.3 ± 0.6 | r 5.43 ± 0.85 μM | r 6.24 ± 0.40 μM | |||
| VR003 | 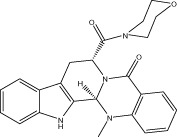 |
<10 (4.3 ± 0.1) | 10 μM | 57.76 ± 6.63 μM | 2.01 ± 0.63 |
| r < 10 (5.6 ± 0.1) | NA | NA | |||
| Evo 46 | 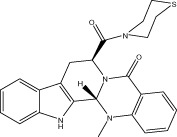 |
55.5 ± 0.8 | 0.27 ± 0.01 μM | 0.47 ± 0.01 μM | 2.83 ± 0.64 |
| r 44.5 ± 0.6 | r 0.74 ± 0.03 μM | r 0.98 ± 0.04 μM | |||
| VR005 | 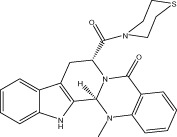 |
66.7 ± 0.5 | 6.99 ± 0.14 μM | 5.91 ± 0.09 μM | 2.83 ± 0.64 |
| r 51.7 ± 1.0 | r 6.85 ± 0.42 μM | r 10.33 ± 0.63 μM |
All tests were carried out at least in triplicate, and the compounds were tested also on HEK-293 cells not transfected with the TRPV1 receptor: none produced a significant elevation of intracellular [Ca2+] (not shown). The specificity of the receptor response for the substances that showed a substantial effect on intracellular [Ca2+], was verified also by pretreating the human or rat TRPV1 transfected cells for 5 min with the specific antagonist 5-iodo-resiniferatoxin at the concentration of 10 nM before the addition of the compound (1 μM), and full antagonism was observed in each case (not shown). NA = not active.
VR002 < Evo 44 < Evo 09 < Evo 23 < Evo 28 < Evo 06 < Evo 46 < Evo 42 < Evo 38 < Evo 34 < Evo 30. The corresponding EC50 values ranked between ∼5 μM and ∼2 nM for both human and rat TRPV1, with VR002 being inactive.
The rank of potency of the synthetic R(−)-enantiomers was considerably different (VR001 = VR003 = Evo 22 < Evo 29 < VR005 < Evo 21 ∼ VR007 < Evo 35 < Evo 31 ∼ Evo 39), and the corresponding EC50 values ranked between ∼10 and 0.1 μM, with VR001, VR003 and Evo 22 being inactive. The lipophilicity of these compounds is reported in Table 2, with LogP values ranking between ∼1.9 and ∼4. The rank, excluding compound Evo 28, which had a very low logP value (logP = 1.5), was as follows: VR002 = VR001 < Evo 44 = VR003 < Evo 23 = Evo 29 < Evo 09 = Evo 22 < Evo 38 = Evo 39 < Evo 46 = VR005 = Evo 06 = Evo 21 < Evo 42 = VR007 < Evo 34 = Evo 35 < Evo 30 = Evo 31. The Spearman's rank order correlation index between the EC50 at human and rat TRPV1 and the logP values of the synthetic S(+)-evodiamine enantiomers compounds was calculated and was r = −0.85 (P < 0.001) and −0.82 (P < 0.001), respectively; for the synthetic R(−)-evodiamine enantiomers it was r = −0.80 (P = 0.003) and −0.74 (P = 0.01) respectively. Therefore, a strong positive correlation exists between lipophilicity and potency of evodiamine analogues at both human and rat TRPV1.
Finally, the compounds, given to cells with a 5 min pre-incubation, were also found to desensitize the human recombinant TRPV1 to the intracellular Ca2+-elevating effects of 100 nM capsaicin (Tables 1 and 2).
Discussion
The natural indoloquinazolone alkaloid evodiamine has a pentacyclic U-shaped structure, with a peripheral lipophilic core, the basic amino group in the concavity of the molecular structure, and the polar amide group on the central part of the outer rim. The evodiamine chemotype is unique within TRPV1 agonists, and, in general, very few heterocyclic chemotypes of natural activators are known, the best examples being the acyl amides of salsolinol (N-arachidonoylsalsolinol), an endogenous tetrahydroisoquinoline formed by a Pictet–Spengler condensation of dopamine with acetaldehyde (O'Dell et al., 2007), and a number of tetrahydro-β-carbolines (Ortar et al., 2013). Among TRPV1 agonists, little chiral discrimination had been observed so far within vanillamides of ricinoleic acid (Appendino et al., 2006) and analogues of anandamide (Appendino et al., 2009). Instead, within TRPV1 antagonists, enantiomeric pairs of indazole derivatives showed chiral discrimination, with the (R)-enantiomers being up to 30-fold more potent than their (S)-counterparts (Gomtsyan et al., 2007). Within antagonists of another TRP channel, TRPA1, enantiomeric discrimination was demonstrated in dihydropyrimidones (Gijsen et al., 2012) and for a N-1-Alkyl-2-oxo-2-chlorophenyl amide (Vallin et al., 2012) ligand. Within antagonists of TRPM8, arylglycine derivatives were very recently identified, the two diastereomers of which were separated and the absolute configurations determined by Vibrational Circular Dichroism: the (S,S)-isomer [(S)-1-((S)-2-(2-fluorophenyl)pyrrolidin-1-yl)-2-(2-fluorophenylamino)-2-(4-(trifluoromethyl)phenyl)ethanone] and the corresponding (R,S)-isomer. The former diastereomer was more potent at inhibiting icilin-induced canine TRPM8 functional activity in vitro (IC50 = 0.006 μM) than the latter (IC50 = 0.045 μM) (Zhu et al., 2013).
Chirality is often an important feature of drug efficacy; remarkably, the chiral discrimination observed with evodiamine could also be confirmed in a series of synthetic analogues, with compounds from the S-series being more potent than those from the R-series (Table 2). A similar level of enantiodifferentiation was also observed when a selection of enantiomeric pairs was assayed here on rat TRPV1.
Regarding the structure-activity relationships of evodiamine analogues, most chemical modifications were detrimental for activity. Thus, the opening of the five-membered ring (Evo 05 vs. Evo 15), the introduction of an acetamide-(Evo 28), an N-methyl-acetamide-(VR001), a methoxycarbonyl-(Evo 06 and Evo 21), a carboxyl-(Evo 09 and Evo 22) or a piperazylamido group-(Evo 23) on ring C, all caused a decrease of potency. The introduction of an oxycyano carbonyl group in the same position resulted in compound Evo 38 with a potency similar to S(+)-evodiamine, and compound Evo 39 with a potency higher than R(−)-evodiamine. Particularly interesting appeared to be the butoxycarbonyl derivatives Evo 30 and Evo 34, which showed activity in the low nanomolar range. Recent investigations have been devoted to the identification of TRPV1 regions involved in the recognition by some ligands and in the prediction of their binding modes (Kym et al., 2009; Lee et al., 2011; Wang et al., 2012). The presence of a tert-butylphenyl group is frequently found in TRPV1 ligands, presumably because of the potentiation of π-π stacking and hydrophobic interactions (Vriens et al., 2009). Finally, amidation with morpholine (Evo 44) (EC50 = 5.43 μM), piperidine (Evo 42) (EC50 = 210 nM) and thiomorpholine (Evo 46) (EC50 = 270 nM) produced compounds less potent than S(+)-evodiamine (EC50 = 113 nM).
With the exception of Evo28, exhibiting a very low LogP value (1.5), the rank of potency of the synthetic (S) enantiomers correlated nicely with lipophilicity. Recently, a strong correlation was reported, within TRPV1 agonists, between the kinetics of induction of calcium influx, pungency and lipophilicity, although the correlation was not linear for the extreme values of LogP (Ursu et al., 2010). Other recent studies have shown that the lipophilicity of capsaicinoids can strongly influence both their efficacy at, and kinetics of activation of, TRPV1. We previously described the activity at the human TRPV1 of several capsaicin analogues, and found that N-arachidonyl-vanillamide (arvanil) (De Petrocellis et al., 2000) and N-retinoyl-vanillamide (retvanil) (Appendino et al., 2005), are among the most potent ‘capsaicinoid’ TRPV1 agonists. It was shown that the ligand (capsaicin, resiniferatoxin and anandamide) binding site of TRPV1 lies on the inner face of the plasma membrane and that much of TRPV1 itself is localized to internal membranes (Jung et al., 1999; 2002; Zygmunt et al., 1999; De Petrocellis et al., 2001). Thus, lipophilicity of the agonist plays an important role in its ability to penetrate the cell membrane and interact with TRPV1 binding site, and influences both the kinetics and potency of vanilloids at the channel (Lazar et al., 2006). N-palmitoyl-vanillamide (palvanil) (Melck et al., 1999) exhibited a kinetics of activation of human recombinant TRPV1-mediated intracellular calcium elevation significantly slower than that of capsaicin (logP = 7.15 ± 0.79 and 3.74 ± 0.52, respectively) and exhibited no pungency in the eye-wiping assay in mice, as well as stronger desensitizing effects on TRPV1 and anti-hyperalgesic activity (De Petrocellis et al., 2011). Two other capsaicin analogues with decreased pungency, arvanil and N-oleoyl-vanillamide (olvanil), are highly lipophilic due to the increased length of the fatty acid chain (logP = 7.20 ± 1.14 and 7.55 ± 0.91, respectively) and exhibit an EC50 of about 0.5 nM in a calcium assay, much lower than that of capsaicin (De Petrocellis et al., 2000).
In conclusion, we present evidence that chiral discrimination exists in the vanilloid activity of evodiamine, and that lipophilicity is critical for this activity. These results indicate that evodiamine may represent a useful starting point for the development of new potent TRPV1 agonists/desensitizers, and confirm the pharmacological potential of the combination of natural building blocks to provide new bioactive compounds.
Glossary
- DMSO
dimethyl sulfoxide
- IRTX
5-iodo-resiniferatoxin
- PI3K
phosphoinositite 3-kinase
Conflict of interest
GF is an employee of Indena S.p.a.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Overview. Br J Pharmacol. 2013;170:1449–1867. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, et al. Development of the first ultra-potent ‘capsaicinoid’ agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther. 2005;312:61–70. doi: 10.1124/jpet.104.074864. [DOI] [PubMed] [Google Scholar]
- Appendino G, Cascio MG, Bacchiega S, Moriello AS, Minassi A, Thomas A, et al. First ‘hybrid’ ligands of vanilloid TRPV1 and cannabinoid CB2 receptors and non-polyunsaturated fatty acid-derived CB2-selective ligands. FEBS Lett. 2006;580:568–574. doi: 10.1016/j.febslet.2005.12.069. [DOI] [PubMed] [Google Scholar]
- Appendino G, Ligresti A, Minassi A, Cascio MG, Allarà M, Taglialatela-Scafati O, et al. Conformationally constrained fatty acid ethanolamides as cannabinoid and vanilloid receptor probes. J Med Chem. 2009;52:3001–3009. doi: 10.1021/jm900130m. [DOI] [PubMed] [Google Scholar]
- Bak EJ, Park HG, Kim JM, Kim JM, Yoo YJ, Cha JH. Inhibitory effect of evodiamine alone and in combination with rosiglitazone on in vitro adipocyte differentiation and in vivo obesity related to diabetes. Int J Obes. 2010;34:250–260. doi: 10.1038/ijo.2009.223. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JV, Fernandes ES. TRPV1 and SP: key elements for sepsis outcome? Br J Pharmacol. 2013;170:1279–1292. doi: 10.1111/bph.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141:834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Changani K, Hotee S, Campbell S, Pindoria K, Dinnewell L, Saklatvala P, et al. Effect of the TRPV1 antagonist SB-705498 on the nasal parasympathetic reflex response in the ovalbumin sensitized guinea pig. Br J Pharmacol. 2013;169:580–589. doi: 10.1111/bph.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Yu CH, Wang SW, Pu HF, Kan SF, Lin LC, et al. Anti-proliferative effects of evodiamine on human thyroid cancer cell line ARO. J Cell Biochem. 2010;110:1495–1503. doi: 10.1002/jcb.22716. [DOI] [PubMed] [Google Scholar]
- Ching L-C, Kou YR, Shyue S-K, Su K-H, Wei J, Cheng L-C, et al. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc Res. 2011;91:492–501. doi: 10.1093/cvr/cvr104. [DOI] [PubMed] [Google Scholar]
- Chiou WF, Liao JF, Chen CF. Comparative study on the vasodilatory effects of three quinazoline alkaloids isolated from Evodia rutaecarpa. J Nat Prod. 1996;59:374–378. doi: 10.1021/np960161+. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Czikora Á, Lizanecz E, Bakó P, Rutkai I, Ruzsnavszky F, Magyar J, et al. Structure-activity relationships of vanilloid receptor agonists for arteriolar TRPV1. Br J Pharmacol. 2012;165:1801–1812. doi: 10.1111/j.1476-5381.2011.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli B, Palmisano G. A new approach to quinazolinocarboline alkaloids: synthesis of (±) evodiamine, rutaecarpine and dehydroevodiamine. Heterocycles. 1978;9:803–806. [Google Scholar]
- De Petrocellis L, Di Marzo V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci. 2005;77:1651–1666. doi: 10.1016/j.lfs.2005.05.021. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agrò A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Guida F, Moriello AS, De Chiaro M, Piscitelli F, de Novellis V, et al. N-palmitoyl-vanillamide (palvanil) is a non-pungent analogue of capsaicin with stronger desensitizing capability against the TRPV1 receptor and anti-hyperalgesic activity. Pharmacol Res. 2011;63:294–299. doi: 10.1016/j.phrs.2010.12.019. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Schiano Moriello A, Imperatore R, Cristino L, Starowicz K, Di Marzo V. A re-evaluation of 9-HODE activity at TRPV1 channels in comparison to anandamide: enantioselectivity and effects at other TRP channels and in sensory neurons. Br J Pharmacol. 2012;167:1643–1651. doi: 10.1111/j.1476-5381.2012.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delescluse I, Mace H, Adcock JJ. Inhibition of airway hyper-responsiveness by TRPV1 antagonists (SB-705498 and PF-04065463) in the unanaesthetized, ovalbumin-sensitized guinea pig. Br J Pharmacol. 2012;166:1822–1832. doi: 10.1111/j.1476-5381.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenicali M, Ros J, Fernandez-Varo G, Cejudo-Martin P, Crespo M, Morales-Ruiz M, et al. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Wang S, Miao Z, Yao J, Zhang Y, Guo Z, et al. New tricks for an old natural product: discovery of highly potent evodiamine derivatives as novel antitumor agents by systemic structure-activity relationship analysis and biological evaluations. J Med Chem. 2012;55:7593–7613. doi: 10.1021/jm300605m. [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel A, Fernández-Carvajal A, Planells-Cases R, Fernández-Ballester G, González-Ros JM, Messeguer A, et al. Advances in modulating thermosensory TRP channels. Expert Opin Ther Pat. 2012;22:999–1017. doi: 10.1517/13543776.2012.711320. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, et al. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem. 2004;279:20283–20295. doi: 10.1074/jbc.M312577200. [DOI] [PubMed] [Google Scholar]
- Gijsen HJ, Berthelot D, De Cleyn MA, Geuens I, Brône B, Mercken M. Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg Med Chem Lett. 2012;22:797–800. doi: 10.1016/j.bmcl.2011.12.068. [DOI] [PubMed] [Google Scholar]
- Gomtsyan A, Bayburt EK, Keddy R, Turner SC, Jinkerson TK, Didomenico S, et al. Alpha-methylation at benzylic fragment of N-aryl-N′-benzyl ureas provides TRPV1 antagonists with better pharmacokinetic properties and higher efficacy in inflammatory pain model. Bioorg Med Chem Lett. 2007;17:3894–3899. doi: 10.1016/j.bmcl.2007.04.105. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jara-Oseguera A, Simon SA, Rosenbaum T. TRPV1: on the road to pain relief. Curr Mol Pharmacol. 2008;1:255–269. doi: 10.2174/1874467210801030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852–1859. doi: 10.3390/molecules14051852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to ‘hot’ chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Lee SY, Hwang SW, Cho H, Shin J, Kang YS, et al. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J Biol Chem. 2002;277:44448–44454. doi: 10.1074/jbc.M207103200. [DOI] [PubMed] [Google Scholar]
- Kan SF, Huang WJ, Lin LC, Wang PS. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int J Cancer. 2004;110:641–651. doi: 10.1002/ijc.20138. [DOI] [PubMed] [Google Scholar]
- Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ, Wang PS. Anti-proliferative effects of evodiamine on human prostate cancer cell lines DU145 and PC3. J Cell Biochem. 2007;101:44–56. doi: 10.1002/jcb.21036. [DOI] [PubMed] [Google Scholar]
- Ko HC, Wang YH, Liou KT, Chen CM, Chen CH, Wang WY, et al. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur J Pharmacol. 2007;555:211–217. doi: 10.1016/j.ejphar.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. The nociceptive and anti-nociceptive effects of evodiamine from fruits of Evodia rutaecarpa in mice. Planta Med. 2003;69:425–428. doi: 10.1055/s-2003-39701. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Nakano Y, Hoshikuma K, Yokoo Y, Kamiya T. The bronchoconstrictive action of evodiamine, an indoloquinazoline alkaloid isolated from the fruits of Evodia rutaecarpa, on guinea-pig isolated bronchus: possible involvement on vanilloid receptors. Planta Med. 2000;66:526–530. doi: 10.1055/s-2000-8615. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Hoshikuma K, Nakano Y, Yokoo Y, Kamiya T. The positive inotropic and chronotropic effects of evodiamine and rutaecarpine, indoloquinazoline alkaloids isolated from the fruits of Evodia rutaecarpa, on the guinea-pig isolated right atria: possible involvement of vanilloid receptors. Planta Med. 2001a;67:244–248. doi: 10.1055/s-2001-12008. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 2001b;67:628–633. doi: 10.1055/s-2001-17353. [DOI] [PubMed] [Google Scholar]
- Kym PR, Kort ME, Hutchins CW. Analgesic potential of TRPV1 antagonists. Biochem Pharmacol. 2009;78:211–216. doi: 10.1016/j.bcp.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Lazar J, Braun DC, Tóth A, Wang Y, Pearce LV, Pavlyukovets VA, et al. Kinetics of penetration influence the apparent potency of vanilloids on TRPV1. Mol Pharmacol. 2006;69:1166–1173. doi: 10.1124/mol.105.019158. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee Y, Ryu H, Kang DW, Lee J, Lazar J, et al. Structural insights into transient receptor potential vanilloid type 1 (TRPV1) from homology modeling, flexible docking, and mutational studies. J Comput Aided Mol Des. 2011;25:317–327. doi: 10.1007/s10822-011-9421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TJ, Kim EJ, Kim S, Jung EM, Park JW, Jeong SH, et al. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol Cancer Ther. 2006;5:2398–2407. doi: 10.1158/1535-7163.MCT-06-0167. [DOI] [PubMed] [Google Scholar]
- Liao C-H, Pan S-L, Guh J-H, Chang Y-L, Pai H-C, Lin C-H, et al. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis. 2005;26:968–975. doi: 10.1093/carcin/bgi041. [DOI] [PubMed] [Google Scholar]
- Lu J-J, Bao J-L, Chen X-P, Huang M, Wang Y-T. Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med. 2012;485042:1–12. doi: 10.1155/2012/485042. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, et al. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- Moran MM, McAlexander MA, Biro T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10:601–620. doi: 10.1038/nrd3456. [DOI] [PubMed] [Google Scholar]
- Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, Ermund A, et al. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Myers BR, Bohlen CJ, Julius D. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron. 2008;58:362–373. doi: 10.1016/j.neuron.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460:437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- O'Dell DK, Rimmerman N, Pickens SR, Walker JM. Fatty acyl amides of endogenous tetrahydroisoquinolines are active at the recombinant human TRPV1 receptor. Bioorg Med Chem. 2007;15:6164–6169. doi: 10.1016/j.bmc.2007.06.032. [DOI] [PubMed] [Google Scholar]
- Ortar G, De Petrocellis L, Schiano Moriello A, Allarà M, Morera E, Nalli M, et al. Tetrahydro-β-carboline derivatives targeting fatty acid amide hydrolase (FAAH) and transient receptor potential (TRP) channels. Bioorg Med Chem Lett. 2013;23:138–142. doi: 10.1016/j.bmcl.2012.10.137. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LV, Petukhov PA, Szabo T, Kedei N, Bizik F, Kozikowski AP, et al. Evodiamine functions as an agonist for the vanilloid receptor TRPV1. Org Biomol Chem. 2004;2:2281–2286. doi: 10.1039/B404506H. [DOI] [PubMed] [Google Scholar]
- Phillips E, Reeve A, Bevan S, McIntyre P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J Biol Chem. 2004;279:17165–17172. doi: 10.1074/jbc.M313328200. [DOI] [PubMed] [Google Scholar]
- Rang WQ, Du YH, Hu CP, Ye F, Tan GS, Deng HW, et al. Protective effects of calcitonin gene-related peptide-mediated evodiamine on guinea-pig cardiac anaphylaxis. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:306–311. doi: 10.1007/s00210-002-0677-0. [DOI] [PubMed] [Google Scholar]
- Rang WQ, Du YH, Hu CP, Ye F, Xu KP, Peng J, et al. Protective effects of evodiamine on myocardial ischemia–reperfusion injury in rats. Planta Med. 2004;70:1140–1143. doi: 10.1055/s-2004-835841. [DOI] [PubMed] [Google Scholar]
- Sanchez JF, Krause JE, Cortright DN. The distribution and regulation of vanilloid receptor VR1 and VR1 5′ splice variant RNA expression in rat. Neuroscience. 2001;107:373–381. doi: 10.1016/s0306-4522(01)00373-6. [DOI] [PubMed] [Google Scholar]
- Sanz-Salvador L, Andres-Borderia A, Ferrer-Montiel A, Planells-Cases R. Agonist-and Ca2+-dependent desensitization of TRPV1 targets the receptor to lysosomes for degradation. J Biol Chem. 2012;287:19462–19471. doi: 10.1074/jbc.M111.289751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu KG, Lin S, Lee CC, Chen E, Lin LC, Wang BW, et al. Evodiamine inhibits in vitro angiogenesis: implication for antitumorgenicity. Life Sci. 2006;78:2234–2243. doi: 10.1016/j.lfs.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Siemens J, Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, et al. Virtual computational chemistry laboratory – design and description. J Comput Aid Mol Des. 2005;19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- Tu YJ, Fan X, Yang X, Zhang C, Liang HP. Evodiamine activates autophagy as a cytoprotective response in murine Lewis lung carcinoma cells. Oncol Rep. 2013;29:481–490. doi: 10.3892/or.2012.2125. [DOI] [PubMed] [Google Scholar]
- Ursu D, Knopp K, Beattie RE, Liu B, Sher E. Pungency of TRPV1 agonists is directly correlated with kinetics of receptor activation and lipophilicity. Eur J Pharmacol. 2010;641:114–122. doi: 10.1016/j.ejphar.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Vallin KS, Sterky KJ, Nyman E, Bernström J, From R, Linde C, et al. N-1-Alkyl-2-oxo-2-aryl amides as novel antagonists of the TRPA1 receptor. Bioorg Med Chem Lett. 2012;22:5485–5492. doi: 10.1016/j.bmcl.2012.07.032. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Wang C, Li S, Wang MW. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro. 2010;24:898–904. doi: 10.1016/j.tiv.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang DH. TRPV1 gene knockout impairs post-ischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang Y, Kontani Y, Kobayashi Y, Sato Y, Mori N, et al. Evodiamine improves diet-induced obesity in a uncoupling protein-1-independent manner: involvement of antiadipogenic mechanism and extracellularly regulated kinase/mitogen-activated protein kinase signaling. Endocrinology. 2008;149:358–366. doi: 10.1210/en.2007-0467. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sun L, Yu H, Zhang Y, Gong W, Jin H, et al. Binding mode prediction of evodiamine within vanilloid receptor TRPV1. Int J Mol Sci. 2012;13:8958–8969. doi: 10.3390/ijms13078958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Ching L-C, Zhao JF, Shyue SK, Lee HF, Kou YR, et al. Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol. 2013;207:299–307. doi: 10.1111/apha.12005. [DOI] [PubMed] [Google Scholar]
- Wen H, Östman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, et al. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem. 2012;287:13868–13876. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Wu LJ, Tashino S, Onodera S, Ikejima T. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic Res. 2008;42:492–504. doi: 10.1080/10715760802112791. [DOI] [PubMed] [Google Scholar]
- Yang J, Cai X, Lu W, Hu C, Xu X, Yu Q, et al. Evodiamine inhibits STAT3 signaling by inducing phosphatase shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett. 2013;328:243–251. doi: 10.1016/j.canlet.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Yang ZG, Chen AQ, Liu B. Antiproliferation and apoptosis induced by evodiamine in human colorectal carcinoma cells (COLO-205) Chem Biodivers. 2009;6:924–933. doi: 10.1002/cbdv.200800256. [DOI] [PubMed] [Google Scholar]
- Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- Zhang C, Fan X, Xu X, Yang X, Wang X, Liang HP. Evodiamine induces caspase-dependent apoptosis and S phase arrest in human colon lovo cells. Anticancer Drugs. 2010;21:766–776. doi: 10.1097/CAD.0b013e32833d26a9. [DOI] [PubMed] [Google Scholar]
- Zhu B, Xia M, Xu X, Ludovici DW, Tennakoon M, Youngman MA, et al. Arylglycine derivatives as potent transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett. 2013;23:2234–2237. doi: 10.1016/j.bmcl.2013.01.062. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


