Abstract
Background and Purpose
Osteoporosis is a condition characterized by a decrease in bone density, which decreases its strength and results in fragile bones. The endocannabinoid/endovanilloid system has been shown to be involved in the regulation of skeletal remodelling. The aim of this study was to investigate the possible modulation of bone mass mediated by the transient receptor potential vanilloid type 1 channel (TRPV1) in vivo and in vitro.
Experimental Approach
A multidisciplinary approach, including biomolecular, biochemical and morphological analysis, was used to investigate the involvement of TRPV1 in changes in bone density in vivo and osteoclast activity in vitro, in wild-type and Trpv1−/− mice, that had undergone ovariectomy or had a sham operation.
Key Results
Genetic deletion of Trpv1 as well as pharmacological inhibition/desensitization of TRPV1 signalling dramatically reduced the osteoclast activity in vitro and prevented the ovariectomy-induced bone loss in vivo, whereas the expression of cannabinoid type 2 (CB2) receptors was increased.
Conclusions and Implications
These findings highlight the pivotal role TRPV1 channels play in bone resorption and suggest a possible cross-talk between TRPV1 and CB2 receptors. Based on these results, hybrid compounds acting on both TRPV1 and CB2 receptors in an opposite manner could provide a future pharmacological tool for the treatment of diseases associated with disturbances in the bone remodelling process.
Linked Articles
This article is part of a themed section on the pharmacology of TRP channels. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2014.171.issue-10
Keywords: vanilloids/cannabinoids, TRPV1, CB2 receptor, ovariectomy, osteoclasts, osteoporosis, menopause, transgenic mouse, TRPV1 KO, bone remodelling
Introduction
Osteoporosis (OP) is a condition characterized by a decrease in bone density, which decreases its strength and results in fragile bones. The enhanced occurrence of fractures and the subsequent morbidity make OP a huge public health concern (Rachner et al., 2011; Palacios et al., 2014). Postmenopausal OP, the most frequent type of primary OP, is considered to be consequence of several factors, such as the lifestyle, dietary habits and, most importantly, the age-related decrease in sex steroidal hormones (Pacifici, 1996; Corina et al., 2012). Indeed, the loss of oestrogen after ovariectomy (OVX) induces a dramatic reduction in bone mineralization that decreases bone mass with a marked increase in bone resorption, which is responsible for weakening of the skeleton and increased fracture risk (Kim et al., 2013; Zhao et al., 2013). The postmenopausal OP occurring after interruption of gonad function benefits from hormonal replacement treatment. Oestrogen acts on the skeleton via the classical genomic oestrogen receptor (ER)α and β (Sample et al., 2012) and ERα knockout (KO) mice have a decreased adaptive response to bone loading (Lee et al., 2004). Recently, the endocannabinoid/endovanilloid (EC/EV) system has been shown to be involved in the regulation of skeletal remodelling (Ofek et al., 2006; Bab and Zimmer, 2008; Bab et al., 2008; 2009; Rossi et al., 2009; 2011).
We have shown that in osteoclasts (OCs) from osteoporotic patients, the transient receptor potential vanilloid type 1 (TRPV1) channel (Alexander et al., 2013) is up-regulated and clustered and less prone to drive calcium entry (desensitization), while levels of the cannabinoid receptor type 2 (CB2 receptor) are much reduced. This finding suggests that a functional cross-talk between CB2 and TRPV1 receptors may exist in OCs; therefore, by inducing TRPV1 desensitization/inhibition (as well as enhancing its cell internalization), it is possible to evoke the overexpression of CB2 receptors, which then reduce the activation of OCs (Rossi et al., 2011). We have shown that stimulation of CB2 receptors dramatically reduces the number of active multinucleated human OCs in vitro, and 17-β-oestradiol is able to up-regulate the expression of CB2 receptors in these cells (Rossi et al., 2013a). Accordingly, mice deficient in TRPV1 channels display a significant reduction in pain (Caterina et al., 2000) and the TRPV1 antagonist capsazepine inhibits OC and osteoblast differentiation in vitro and ovariectomy-induced bone loss in vivo (Idris et al., 2010). These findings indicate that pharmacological blockade of this channel could be of therapeutic value in patients with bone diseases. It has also been suggested that TRPV1 channel activation may partly account for the stimulating effect of the endocannabinoid anandamide (AEA) on OC formation and bone turnover (Idris et al., 2005; 2008; 2009), as AEA is known to activate TRPV1 (Smart et al., 2000; Ahluwalia et al., 2003). On this subject, molecules designed to stimulate (or up-regulate the expression of) CB2 receptors and, hence, inhibit tartrate-resistant acid phosphatase (TRAP) overactivity, may prove useful in bone diseases where the balance between osteoblast and OC activity is disturbed. Consistent with the data pointing to the EC/EV system as a novel therapeutic target for treating bone diseases, such as OP (Idris et al., 2005; 2008; 2009; 2010; Abed et al., 2009), and in view of the interest in the role that TRPV1 blockers might play in the treatment of bone resorption, in this study we examined whether the absence of TRPV1 could affect the cellular activity of OCs in mice with OVX-induced osteoporosis. Specifically, we investigated the changes in expression of the cannabinoid receptors as well as the specific synthetic or catabolic EC/EV enzymatic machinery in femur homogenates and in OCs in vitro, differentiated from peripheral mononucleated blood cells from healthy wild-type (WT) and Trpv1 KO mice that had undergone a sham surgery or OVX. Accordingly, four different groups of OCs were obtained. In these different mice cell cultures, we performed: (i) biomolecular analysis to reveal a possible alteration in the co-expression of CB1 and CB2 receptors; or in the expression of the enzymes N-acyl-phosphatidyl-ethanolamine phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH), but also of diacylglycerol lipase (DAGL) alpha or monoacylglycerol lipase (MAGL), which are involved in the biosynthesis and catabolism, respectively, of the endocannabinoids; (ii) enzymatic assay for evaluating the activity of TRAP, a specific biomarker of OC activation; (iii) biochemical analysis for evaluating the expression of TRPV1 and TRAP protein; (iv) immunohistochemical analysis for evaluating bone mass morphology in the absence of TRPV1 signalling induced either by genetic ablation or by pharmacological antagonism/desensitization.
Methods
Animals
Mice with a targeted deletion of the Trpv1 or TRPV1 encoding for gene (Trpv1 KO mice; GeneID 193034) were bought from Jackson Laboratory (The Jackson Laboratory, JAX® Mice and Services, Bar Harbor, ME, USA). A C57BL-6J strain was used as WT controls and female C57BL-6J for ovariectomia experiments. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Second University of Naples and conducted according to the National and European guidelines. Mice were housed three per cage under controlled illumination (12:12 h light:dark cycle; light on 0600 h) and environmental conditions (room temperature 20–22°C, humidity 55–60%) for at least 1 week before the commencement of experiments. Mouse chow and tap water were available ad libitum. All manipulations were performed under sterile conditions and animals were subjected to regular health checks. Animal care was in compliance with the IASP and European Community (E.C. L358/1 18/ 12/86) guidelines on the use and protection of animals in experimental research. All efforts were made to minimize animal suffering and the number of animals used.
Female C57BL/6J and Trpv1 KO (8 months old) were anaesthetized with avertin (250 mg·kg−1 i.p.) and, after assessing the absence of any movement such as eyelid flicking, tail reflex and paw retraction, induced through a point tip instrument, subjected to bilateral ovariectomy. Briefly, two incisions were made and the dorsal s.c. adipose tissue was removed from the skin. The muscle layer below was opened and the ovary isolated and removed. Muscle and skin were closed with a 4.0 silk suture. Sham-operated mice underwent the same surgical procedure, but without ovariectomy. WT and KO mice were killed 21 days post-ovariectomy and the femurs were isolated in order to perform biomolecular and morphological analyses. WT mice were treated with palvanil 1 mg·kg−1 twice a day. Moreover, five infertile WT and five infertile Trpv1−/− mice (18–24 months), were killed without undergoing OVX. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
mRNA analyses
Total RNA was prepared from primary cultures of peripheral blood mononuclear cells from WT C57BL-6J and Trpv1 KO C57BL-6J mice, either sham-operated or OVX, grown in ‘osteoclastogenic’ medium containing M-CSF and TNSF11 (RANKL) as previously described (Rossi et al., 2009). In addition, mRNA was also obtained directly from homogenized bone tissue. Total mRNA was extracted using Tri-Reagent (Molecular Research Center Inc., Cincinnati, OH, USA) and retrotranscripted using the RT High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Real time PCR analysis was carried out using the Fast SYBR Green Master mix kit (Applied Biosystems) on a 7900 HT Sequence Detection System (Applied Biosystems). Primers that were picked with Primer3 software and thermal conditions are available on request. Data were normalized with respect to the housekeeping genes Hprt (GeneID 15452).
Western blot
Total lysates of OCs from OVX and sham-operated WT and transgenic animals and from WT mice after chronic pharmacological challenge with the vanilloid agonist palvanil, were obtained through RIPA buffer lysis (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Fifty micrograms of denatured protein were loaded onto 10% polyacrylamide gels and transferred on a PVDF membrane (Millipore, Milan, Italy). Membrane strips were alternatively incubated overnight at 4°C with the following HRP-conjugated antibodies: rabbit polyclonal anti-TRAP (1:200; Abcam, Cambridge, UK), TRPV1 (1:500) and then with the relative secondary antibody for 1 h; reactive bands were visualized on a X-ray film (Fuji Corporation, Tokyo, Japan). Whereas the same membrane strip was used for revealing the expression of more than one protein of interest, a mild stripping at 60°C for 10 min was performed. Monoclonal anti-β-tubulin antibody (1:1000 or 1:2000; Sigma, Milan, Italy) was used to check for identical protein loading and as housekeeping protein.
Scanning electron microscopy (sEM)
The sEM gives information on morphology and composition of sample surfaces, with a resolution of a few nm. The basic principle of operation of sEM is the formation of an electron beam that interacts with a surface producing secondary electrons, which are collected by a sensor that processes signals and reconstructs the sample's morphology. In recent years, an instrument called ESEM (environmental scanning electron microscopy) has been developed. The construction of ESEM is similar to sEM, but the sample is not subjected to a high vacuum in order to maintain a degree of hydration while in the column, where the electron gun is situated, but is kept at a pressure of 1333 Pa, which is essential for the formation of an electron beam. The use of ESEM is preferable for the study of morphology in biological samples, as it has a lower resolution power than sEM (Muscariello et al., 2005) and in recent years it has been increasingly used in the diagnostic field (Cafiero et al., 2011; Papale et al., 2014).
Samples were sectioned with a scalpel and fixed in formaldehyde, and subsequently washed with distilled water and observed with ESEM. The samples were kept at a temperature of 5°C and pressure of 480 Pa in order to maintain a relative humidity of 60%.
Drugs and treatments
Palvanil and I-RTX (Tocris, Avonmouth, UK) were dissolved in PBS containing DMSO. DMSO final concentration in vivo was 10%. DMSO final concentration in cultures was 0.01%. OC cultures were treated with palvanil or I-RTX, alone or in combination, for 48 h. For a single treatment, cultured OCs were treated with 5 μM, 500, 50 and 10 nM of palvanil. Untreated cultured OCs were maintained in incubation media during the relative treatment time with or without vehicle (DMSO 0.01%). In vivo mice were treated twice a day with i.p. palvanil 1 mg·kg−1.
Statistics
Molecular and biochemical data are shown as means ± SEM, or as mean ± SD. All the experiments were performed at least in triplicate. A t-test was performed for evaluating differences between means of two groups. A P-value less than 0.05 was considered statistically significant. All the statistical analyses were conducted using Statgraphics CENTURION XV.II (Adalta, Arezzo, Italy; Statpoint Technologies Inc., Warrenton, VA, USA).
Results
Trpv1−/− mice
Young Trpv1−/− mice were healthy, fertile and indistinguishable from their age-matched WT controls in terms of size and weight, whereas older Trpv1−/− mice were the same size but weighed more (27.3 ± 1.83 g vs. 32.8 ± 1.7 g, respectively, P = 0.02). As expected, neither TRPV1 mRNA or TRPV1 protein was detectable in the bone from Trpv1 KO mice (Figures 1A and 3A).
Figure 1.
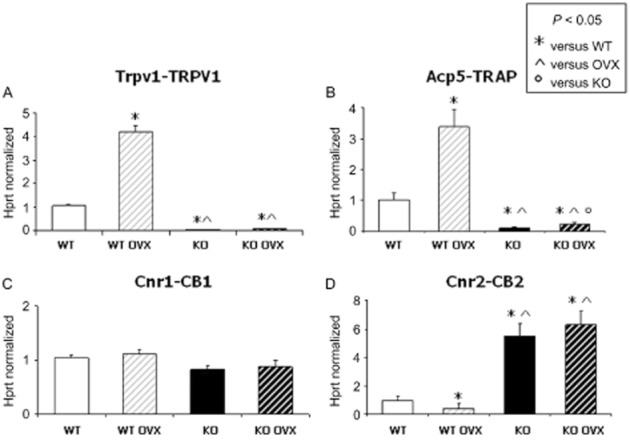
Expression analysis of osteoclatic marker TRAP and EC/EV receptors in homogenized murine femur: (A) TRPV1 variant 1 expression increased in WT OVX. As expected, bone derived from Trpv1 KO mice did not show a measurable amount of TRPV1 mRNA. (B) Increased TRAP expression was detectable in bone derived from WT OVX as compared with those from WT and significantly decreased in bone from KO mice. TRAP levels significantly increased also in KO bone after OVX with respect to bone from sham-operated KO mice. (C) CB1receptor levels did not show any significant change. (D) CB2 receptor mRNA levels decreased in bone derived from WT OVX mice and showed a significant enhancement in bone from transgenic Trpv1 KO mice either OVX or sham-operated as compared to bone from WT mice. Data shown were from homogenized murine femur obtained by real time PCR starting from 100 ng of total mRNA for the RT reaction and were normalized to the housekeeping gene Hprt. Data are presented as a mean ± SD. Differences in the mean values were evaluated by Student's unpaired t-test. P < 0.05 was considered statistically significant.
Figure 3.

Western blot analysis for TRAP and TRPV1 receptor in homogenized murine femur: (A) Western blot analysis and relative quantification for TRPV1 receptor expression in homogenized murine femur normalized with respect to β-tubulin revealed a significant increase in WT-OVX, but not in KO and KO-OVX with respect to WT. (B) Western blot analysis and relative quantification for the specific marker of osteoclast activity TRAP in homogenized murine femur normalized with respect to β-tubulin: a significant increase in TRAP in WT-OVX and a significant decrease in TRAP in Trpv1 KO were observed with respect to WT bone lysates. The genetic ablation of Trpv1 significantly inhibited the OVX-induced overexpression of TRAP. Data are presented as a mean ± SD. Differences in the mean values were evaluated by Student's unpaired t-test. P < 0.05 was considered statistically significant.
Expression of EC/EV system in WT and Trpv1−/− mice bone tissue
To investigate the role of TRPV1 channels in the EC/EV-mediated bone resorption, we analysed the expression levels of the osteoclastic marker TRAP, the cannabinoid receptors and the enzymatic machinery of the EC/EV system in the femur of ovariectomized and sham-operated WT and Trpv1 KO mice.
Real time PCR revealed an increased expression (about fourfold) of the osteoclastic marker TRAP in OVX-WT mice with respect to WT without ovariectomy (Figure 1B) together with a significant decrease in CB2 receptor mRNA levels (60% reduction; Figure 1C). Moreover, TRAP levels were strongly reduced in Trpv1 KO-derived bone and the lack of Trpv1 inhibited the OVX-induced TRAP overexpression as compared with those of OVX-WT bone (Figure 1B). Interestingly, CB2 receptor mRNA levels were significantly higher in Trpv1 KO mice and were also increased in the bone from OVX-Trpv1 KO mice (Figure 1C). Levels of CB1 receptors did not significantly change in all the groups investigated (Figure 1D).
Real time PCR was performed to investigate the expression of AEA metabolic enzymes encoding for genes, NAPE-PLD and FAAH, and 2-arachidonoyl-glycerol (2-AG) metabolic enzymes encoding for genes, DAGL and MAGL. We observed an increase in NAPE-PLD mRNA levels and a decrease in FAAH mRNA levels in the bone of OVX WT mice, whereas we observed the opposite in the bone from Trpv1−/− mice, both OVX and sham-operated groups (Figure 2A, B).
Figure 2.
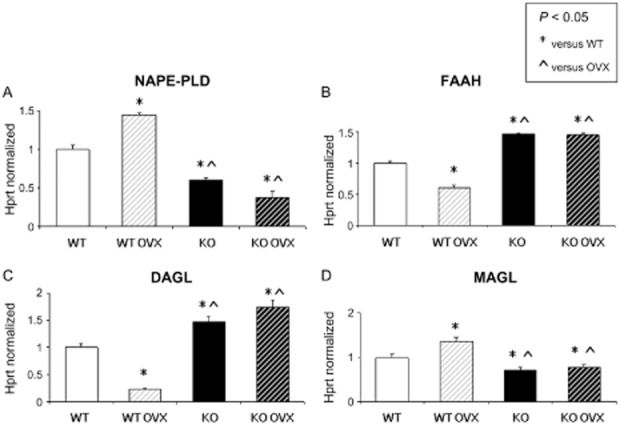
Expression of receptors and enzymes of the endocannabinoid/endovanilloid system in homogenized murine femur: (A) N-acyl-phosphatidyl-ethanolamine phospholipase D (NAPE-PLD) increased in bone derived from WT OVX mice and decreased in bone from both KO and KO OVX mice with respect to bone from WT animals. (B) Fatty acid amide hydrolase (FAAH) decreased in bone from WT OVX mice with respect to WT-derived bone, whereas FAAH increased in both KO and KO OVX-derived bones. (C) Diacylglycerol lipase α (DAGLα) decreased in WT OVX bone with respect to WT bone and increased both in KO and KO OVX-derived bones. (D) Monoglyceride lipase (MAGL) increased in bone derived from WT OVX mice and significantly decreased in both KO and KO OVX-derived bone with respect to WT bone. (A–D) OVX induced significant changes in enzymes levels in bone from KO mice. Data were obtained from homogenized murine femur by real time PCR starting from 100 ng of total mRNA for the RT reaction and have been normalized for the housekeeping gene Hprt. Data are presented as a mean ± SD. Differences in the mean values were evaluated by Student's unpaired t-test. P < 0.05 was considered statistically significant.
In contrast, molecular levels of DAGL decreased and those of MAGL were enhanced in the OVX mice and the opposite effect was observed in the absence of TRPV1 (Figure 2C, D).
TRAP levels increase after OVX and in Trpv1 KO mice
Similar to the mRNA, the TRPV1 protein levels also increased significantly in bone derived from OVX mice. As expected, Trpv1 KO mice did not show a signal for TRPV1 protein (Figure 3A).
TRAP protein levels were dramatically enhanced in OVX WT as compared with the WT sham mice. Whereas, Trpv1−/− mice showed a marked reduction in basal levels of TRAP. Moreover, Trpv1 gene deletion did not completely inhibit the OVX-induced up-regulation in TRAP protein, but significantly reduced it (Figure 3B).
Palvanil, a potent TRPV1 agonist, modulates the number and activity of murine bone-derived OCs in vitro
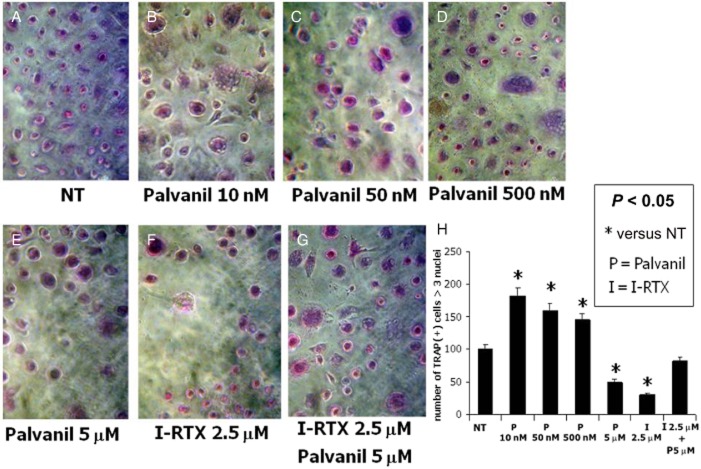
The TRAP assay revealed that the vanilloid agonist palvanil induced a dose-dependent effect on the number and activity of WT-derived OCs in vitro (Figure 4). The lowest concentration (10nM) significantly activated OCs (Figure 4B), while the highest (5 μM) significantly reduced the number of OCs (Figure 4E), acting in a similar manner to that of the vanilloid antagonist I-RTX (2.5 μM; Figure 4F).
Figure 4.
TRAP assay after pharmacological modulation of the TRPV1 receptor: (A–E) TRAP(+) multinucleated (n ≥ 3) OCs increased in a concentration-dependent manner after 48 h treatment with the vanilloid agonist palvanil, until channel desensitization: the lowest concentration (10 nM) significantly activated OCs, while the highest concentration (5 μM) significantly reduced OC numbers with respect to control. (F) OC numbers decreased after exposure to the vanilloid antagonist I-RTX (2.5 μM). (G) Fifteen minutes pretreatment with I-RTX (2.5 μM) before 24 h exposure to palvanil (5 μM) inhibited the desensitization of the TRPV1 channel. (H) Graphic representation of the multinucleated TRAP positive cell count. All the assays were performed at least in triplicate. Data are presented as a mean ± SEM. Differences between the mean values were evaluated by Student's unpaired t-test. P < 0.05 was considered statistically significant.
Palvanil exerts opposite effects in sham and OVX mice in vivo
As revealed from Western blot data, bone derived from OVX mice expressed higher levels of TRAP as compared with sham animals (Figure 5). Chronic treatment with palvanil (1 mg·kg−1, i.p.) increased the TRAP level in bone dissected from sham mice, while reducing it in bone from OVX mice (Figure 5).
Figure 5.
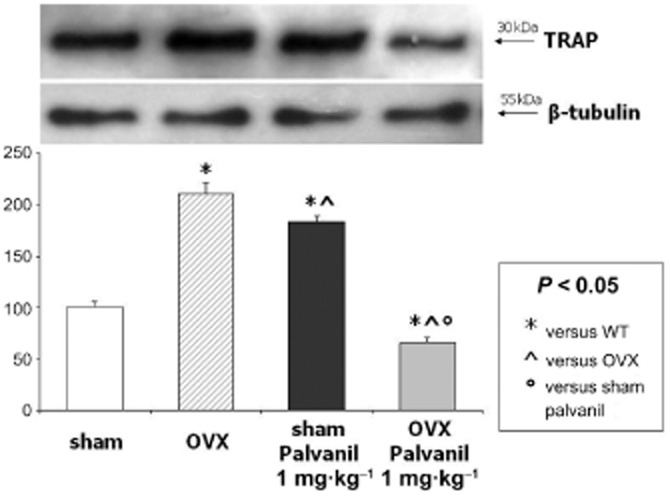
Western blot analysis for TRAP after chronic treatment with palvanil in sham and OVX mice in vivo: Western blot analysis and relative quantification of TRAP expression in homogenized murine femur normalized with respect to β-tubulin. A significant increase in TRAP levels in bone from OVX mice with respect to sham animals was found. Chronic treatment with palvanil 1 mg·kg−1 increased TRAP levels in bone dissected from sham mice, while reduced them in bone from OVX mice. Data are presented as a mean ± SD. Differences in the mean values were evaluated by Student's unpaired t-test. P < 0.05 was considered statistically significant.
Qualitative bone mass morphology
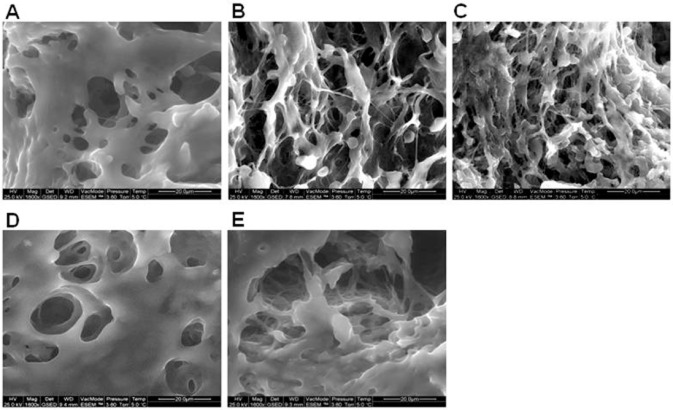
Spongy bone mass was affected by the genetic ablation of the vanilloid receptor and/or its pharmacological manipulation. Bone from 21 days ovariectomized mice showed a very reduced trabeculae thickness as compared with the sham animals (Figure 6A, B). Chronic treatment with a selective TRPV1 agonist with high desensitization power (palvanil) and the genetic ablation of Trpv1 reduced and prevented the OVX-induced bone loss respectively (Figure 6C, D, E).
Figure 6.
Bone morphology: ESEM analysis at 1600 × magnification shows reduced bone mass in bone derived from 21 days ovariectomized mice as compared with that from naïve mice (A, B). Chronic treatment with palvanil reduced the spongy bone loss (C). Bone from Trpv1−/− mice-showed a high bone density and the bone loss after ovariectomy was not observed (D, E).
TRAP levels decrease in bone tissue from old mice
We measured the TRAP levels in old (18–24 months old) mice to evaluate a possible functional, age-dependent TRPV1 switch, as suggested by recent report showing a TRPV1 shift from anti-to pro-inflammatory phenotype associated with aging (Wanner et al., 2012).
We found decreased levels of both TRAP mRNA and protein in old WT mice as compared with young WT mice (7–9 months old). In contrast, TRAP levels did not significantly change in old Trpv1−/− mice with respect to the old WT (Figure 7A, B).
Figure 7.
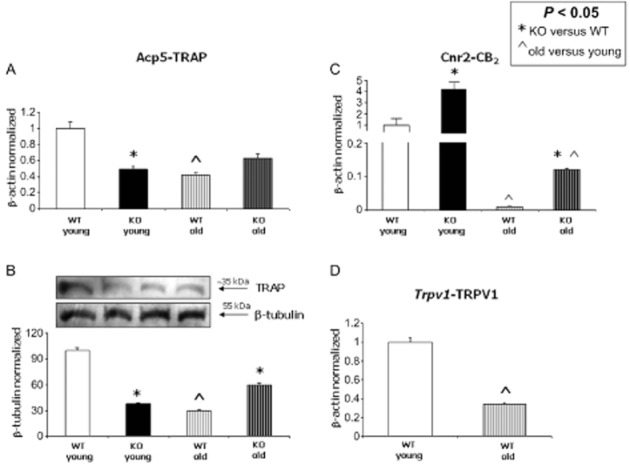
TRAP levels decrease in bone tissue from old mice. (A) TRAP decreases in bone tissue from Trpv1−/− mice as compared with that from WT mice. Ageing significantly reduced TRAP levels. The genetic ablation of Trpv1 did not exert any significant change in bone tissue from old mice. (B) Western blot analysis and relative quantification for TRAP expression in homogenized murine femur normalized with respect to β-tubulin. A significant decrease in TRAP levels was observed in bone from Trpv1−/− young animals, as well as in old bone tissue. (C) CB2 receptor levels were increased in bone tissue from Trpv1−/− mice as compared with that from WT, both in young and old animals. Ageing dramatically reduced CB2 receptor levels. (D) TRPV1 mRNA levels were significantly reduced in bone tissue from old mice.
Moreover, we found that the genetic ablation of Trpv1 significantly increased the CB2 receptor levels both in young and old mice. However, the expression of CB2 receptors in old mice was significantly lower with respect to young mice (Figure 7C). Finally, we found decreased levels of TRPV1 in old mice compared to young ones (Figure 7D).
Discussion and conclusions
The critical importance of a balanced bone remodelling is demonstrated by human diseases, that is OP, in which a massive increase in bone resorption is responsible for skeleton weakening and increased fracture risk. Bone loss occurs after interruption of gonad function, leading to the onset of the so-called postmenopausal OP, that not surprisingly benefits from hormonal replacement treatment (Horst-Sikorska and Wawrzyniak, 2011). Notably, oestrogens modulate cannabinoid receptor expression and EC/EV levels in rats and humans (Guida et al., 2010; Rossi et al., 2013b). Accordingly, we found in this study that TRAP overactivity in OCs derived from OVX WT-mice paralleled a decreased expression of CB2 receptors. The CB2 receptor exerts an inhibitory effect on the activity of various cells and, by inhibiting OCs, may represent a counteracting receptor system for bone mineralization and remodelling (Ofek et al., 2006; Rossi et al., 2009; 2011). Moreover, consistent with our previous findings in human OCs (Rossi et al., 2009), in the present study we found that the enzymes responsible for the synthesis or catabolism of the endogenous vanilloid/cannabinoid AEA or of the pure endocannabinoid 2-AG, were conversely up-regulated or down-regulated similar to the expected increase in AEA and decrease in 2-AG (Di Marzo and Deutsch, 1998). The increased expression of the NAPE-PLD and the decreased expression of FAAH enzymes involved in the AEA turnover might be responsible of its increased level and, therefore, for the overstimulation of the TRPV1 channel, which was overexpressed in the OVX mice. Conversely, the decreased expression of DAGL and the increased expression of MAGL, enzymes involved in the turnover of 2-AG, might lower the level of 2-AG and, hence, decrease the basal or tonic stimulation of the CB2 receptor, which was in fact down-regulated in the OVX mice. Intriguingly, in the absence of TRPV1, we found the opposite occurred in terms of the endocannabinoid metabolic machinery and of CB2 receptor levels. Indeed, we found that genetic ablation of Trpv1 induced an increased level of the 2-AG synthetic enzyme together with an enhanced expression of CB2 receptors, whereas AEA synthetic enzyme was reduced. This trend was even more evident in bone derived from Trpv1 KO-OVX mice. These data further indicate that oestrogen withdrawal is associated with a reduction in CB2 receptor expression and signalling in OCs from OVX mice and that pharmacological inhibition or genetic deletion of the TRPV1 channel, respectively, is sufficient to promote an up-regulation of CB2 receptors and decrease TRAP. In accord with these findings, we previously demonstrated that: (i) an oestrogen receptor antagonist down-regulates the expression of CB2 receptors in human OCs (Rossi et al., 2013a) (ii) TRPV1 overactivation/desensitization up-regulates the expression of CB2 receptors (Rossi et al., 2011) and (iii) cell activation is enhanced in the presence of a selective CB2 receptor antagonist/inverse agonist (Rossi et al., 2009). In this study, we further confirmed that TRPV1 mediates tonic regulation of the CB2 receptor, which was in fact increased (more than fivefold) in Trpv1 KO mice.
In the present study, we showed for the first time that a lack of TRPV1 signalling is beneficial to the restoration of quiescent OC activity in ovariectomized mice. A more prominent pathogenic-like response, that is recognized by an increased number of giant multinucleated cells with a higher positive TRAP activity, also indicated by greater cell differentiation, was observed in OCs from OVX-WT mice as compared with those derived from of Trpv1 KO mice. These different responses were also reproduced by pharmacological manipulation (i.e. agonism/antagonism/desensitization) of the TRPV1 channel in OVX-WT mice.
To further confirm that the TRPV1 channel plays a role in activation of OCs, we pharmacologically inhibited the TRPV1 signalling in WT-derived OCs, either by using I-RTX, a selective antagonist, or N-palmitoyl-vanillamide (palvanil), a non-pungent capsaicin analogue capable of quickly desensitizing the TRPV1 channel (De Petrocellis et al., 2011; Luongo et al., 2012). In our previous studies, we demonstrated that palvanil has slower kinetics of TRPV1 activation and is significantly more potent at desensitizing TRPV1 than capsaicin and, unlike capsaicin, is not pungent and does not induce significant hypo/hyperthermia and bronchoconstriction (De Petrocellis et al., 2011; Luongo et al., 2012). We observed that both treatments, I-RTX or palvanil, at desensitizing concentrations, exerted a beneficial effect on the restoration of quiescent OCs. Moreover, systemic, chronic palvanil treatment in OVX mice reduced the TRAP levels, which were dramatically increased in bone derived from OVX mice. In contrast, in bone from sham-operated animals, chronic treatment with palvanil increased the TRAP levels, and it is assumed that this effect only occurs when the TRPV1 channel is overactivated; further stimulation at the same dose could induce channel desensitization and, hence, by behaving as an antagonist, reduce the TRAP levels. Finally, ultrastuctural analysis showed that systemic palvanil significantly reduced the bone resorption in a similar fashion to that induced by Trpv1 deletion.
Moreover, recent evidence suggests that ageing reverses the role of the TRPV1 from anti-inflammatory to pro-inflammatory (Wanner et al., 2012). Based on these recent data, we thought it would be interesting to further investigate, through a pilot experiment, the possible effect of functional changes TRPV1 on bone metabolism in ageing. Therefore, we investigated the expression of TRAP at both the molecular and protein levels, in old infertile WT and Trpv1−/− mice. Surprisingly, old WT animals showed a significant decrease in TRAP levels with respect to young WT mice, possibly due to (i) an overall reduced bone metabolism occurring during ageing, (ii) a down-regulation in terms of expression and/or an effective functional switch of the TRPV1 channel (Wanner et al., 2011; 2012), (iii) a counteracting mechanism towards the overall chronic inflammatory state, which accords with the dramatic down-regulation in the anti-inflammatory CB2 receptors. However, this effect was not reversed by TRPV1 ablation, assuming that other compensatory mechanisms also start to be employed in bone metabolism.
In conclusion, our results show that disrupting TRPV1 signalling activity, by either genetic modification or pharmacological inhibition/desensitization inhibits, the activity of OCs in vitro and in vivo. We found that Trpv1 KO mice were strongly protected against OCs activation and the development of osteoporosis induced by OVX. In accord with these results, the systemically administered vanilloid agonist, palvanil, had a strong and TRPV1-mediated anti-osteoporotic effect in OVX mice. Thus, palvanil, which is found in low levels in capsicum (Kobata et al., 2010), after appropriate toxicological studies, might warrant further investigation as a possible treatment for chronic progressive and degenerative diseases, including osteoporosis. Moreover, as oestrogen has opposite effects on the expression of TRPV1 channels and CB2 receptors, future studies are needed to investigate how hybrid drugs acting on both TRPV1 and CB2 receptors can be used to restore balanced bone mineralization and resorption.
Glossary
- AEA
anandamide
- CB1 receptor
cannabinoid receptor type 1
- CB2 receptor
cannabinoid receptor type 2
- DAGL
diacylglycerol lipase alpha
- DMSO
dimethyl-sulfoxide
- EC/EV
endocannabinoid/endovanilloid
- ER
oestrogen receptor
- ESEM
environmental scanning electron microscopy
- FAAH
fatty acid amide hydrolase
- I-RTX
iodoresiniferatoxin
- KO
knock-out
- MAGL
monoacylglycerol lipase
- M-CSF
macrophage colony-stimulating factor
- NAPE-PLD
N-acyl-phosphatidylethanolamine phospholipase D
- NT
not treated
- OC
osteoclast
- OP
osteoporosis
- OVX
ovariectomy
- SD
standard deviation
- SEM
standard error of the mean
- TNSF11 (RANKL)
receptor activator of nuclear factor kappa-B ligand
- TRAP
tartrate-resistant acid phosphatase
- TRPV1
transient receptor potential vanilloid type 1
- WT
wild-type
Conflict of interests
None.
References
- Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol. 2009;26:146–158. doi: 10.1080/09687680802612721. [DOI] [PubMed] [Google Scholar]
- Alexander SPH,, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- Bab I, Zimmer A. Cannabinoid receptors and the regulation of bone mass. Br J Pharmacol. 2008;153:182–188. doi: 10.1038/sj.bjp.0707593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bab I, Ofek O, Tam J, Rehnelt J, Zimmer A. Endocannabinoids and the regulation of bone metabolism. J Neuroendocrinol. 2008;20:69–74. doi: 10.1111/j.1365-2826.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- Bab I, Zimmer A, Melamed E. Cannabinoids and the skeleton: from marijuana to reversal of bone loss. Ann Med. 2009;41:560–567. doi: 10.1080/07853890903121025. [DOI] [PubMed] [Google Scholar]
- Cafiero G, Papale F, Grimaldi A, Rosso F, Barbarisi M, Tortora C, et al. Immunogold labelling in environmental scanning electron microscopy: applicative features for complementary cytological interpretation. J Microsc. 2011;241:83–93. doi: 10.1111/j.1365-2818.2010.03405.x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Corina M, Vulpoi C, Bra˘nişteanu D. Relationship between bone mineral density, weight, and estrogen levels in pre and postmenopausal women. Rev Med Chir Soc Med Nat Iasi. 2012;116:946–950. [PubMed] [Google Scholar]
- De Petrocellis L, Guida F, Moriello AS, De Chiaro M, Piscitelli F, de Novellis V, et al. N-palmitoyl-vanillamide (palvanil) is a non-pungent analogue of capsaicin with stronger desensitizing capability against the TRPV1 receptor and anti-hyperalgesic activity. Pharmacol Res. 2011;63:294–299. doi: 10.1016/j.phrs.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Deutsch DG. Biochemistry of the endogenous ligands of cannabinoid receptors. Neurobiol Dis. 1998;5:386–404. doi: 10.1006/nbdi.1998.0214. [DOI] [PubMed] [Google Scholar]
- Guida M, Ligresti A, De Filippis D, D'Amico A, Petrosino S, Cipriano M, et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology. 2010;151:921–928. doi: 10.1210/en.2009-0883. [DOI] [PubMed] [Google Scholar]
- Horst-Sikorska W, Wawrzyniak A. The role of hormonal therapy in osteoporosis. Endocrynol Pol. 2011;62:61–64. [PubMed] [Google Scholar]
- Idris AI, van ‘t Hof RJ, Greig IR, Ridge SA, Baker D, Ross RA, et al. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat Med. 2005;11:774–779. doi: 10.1038/nm1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AI, Sophocleous A, Landao-Bassonga E, van ‘t Hof RJ, Ralston SH. Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149:5619–5626. doi: 10.1210/en.2008-0150. [DOI] [PubMed] [Google Scholar]
- Idris AI, Sophocleous A, Landao-Bassonga E, Canals M, Milligan G, Baker D, et al. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009;10:139–147. doi: 10.1016/j.cmet.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Idris AI, Landao-Bassonga E, Ralston SH. The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo. Bone. 2010;46:1089–1099. doi: 10.1016/j.bone.2010.01.368. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JL, Kim YH, Kang MK, Gong JH, Han SJ, Kang YH. Antiosteoclastic activity of milk thistle extract after ovariectomy to suppress estrogen deficiency-induced osteoporosis. Biomed Res Int. 2013;2013:919374. doi: 10.1155/2013/919374. doi: 10.1155/2013/919374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata K, Saito K, Tate H, Nashimoto A, Okuda H, Takemura I, et al. Long-chain N-vanillyl-acylamides from Capsicum oleoresin. J Agric Food Chem. 2010;58:3627–3631. doi: 10.1021/jf904280z. [DOI] [PubMed] [Google Scholar]
- Lee KC, Jessop H, Suswillo R, Zaman G, Lanyon LE. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-alpha and-beta. J Endocrinol. 2004;182:193–201. doi: 10.1677/joe.0.1820193. [DOI] [PubMed] [Google Scholar]
- Luongo L, Costa B, D'Agostino B, Guida F, Comelli F, Gatta L, et al. Palvanil, a non-pungent capsaicin analogue, inhibits inflammatory and neuropathic pain with little effects on bronchopulmonary function and body temperature. Pharmacol Res. 2012;66:243–250. doi: 10.1016/j.phrs.2012.05.005. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscariello L, Rosso F, Marino G, Giordano A, Barbarisi M, Cafiero G, et al. A critical overview of ESEM applications in the biological field. J Cell Physiol. 2005;205:328–334. doi: 10.1002/jcp.20444. [DOI] [PubMed] [Google Scholar]
- Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc Natl Acad Sci USA. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- Palacios S, Neyro JL, de Cabo SF, Chaves J, Rejas J. Impact of osteoporosis and bone fracture on health-related quality of life in postmenopausal women. Climacteric. 2014;17:60–70. doi: 10.3109/13697137.2013.808182. [DOI] [PubMed] [Google Scholar]
- Papale F, Cafiero G, Grimaldi A, Marino G, Rosso F, Mian C, et al. Galectin-3 expression in thyroid fine needle cytology (t-FNAC) uncertain cases: validation of molecular markers and technology innovation. J Cell Physiol. 2013;228:968–974. doi: 10.1002/jcp.24242. [DOI] [PubMed] [Google Scholar]
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Siniscalco D, Luongo L, De Petrocellis L, Bellini G, Petrosino S, et al. The endovanilloid/endocannabinoid system in human osteoclasts: possible involvement in bone formation and resorption. Bone. 2009;44:476–484. doi: 10.1016/j.bone.2008.10.056. [DOI] [PubMed] [Google Scholar]
- Rossi F, Bellini G, Luongo L, Torella M, Mancusi S, De Petrocellis L, et al. The endovanilloid/endocannabinoid system: a new potential target for osteoporosis therapy. Bone. 2011;48:997–1007. doi: 10.1016/j.bone.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Rossi F, Bellini G, Luongo L, Mancusi S, Torella M, Tortora C, et al. The 17-β-oestradiol inhibits osteoclast activity by increasing the cannabinoid CB2 receptor expression. Pharmacol Res. 2013a;68:7–15. doi: 10.1016/j.phrs.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Rossi F, Papale F, Barbarisi A. Environmental scanning electron microscopi gold immunolabeling in cell biology. Methods Mol Biol. 2013b;931:517–523. doi: 10.1007/978-1-62703-056-4_27. [DOI] [PubMed] [Google Scholar]
- Sample SJ, Racette MA, Hao Z, Thomas CF, Behan M, Muir P. Functional adaptation in female rats: the role of estrogen signaling. PLoS ONE. 2012;7:e43215. doi: 10.1371/journal.pone.0043215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, et al. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner SP, Garami A, Romanovsky AA. Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging. 2011;3:450–454. doi: 10.18632/aging.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner SP, Garami A, Pakai E, Oliveira DL, Gavva NR, Coimbra CC, et al. Aging reverses the role of the transient receptor potential vanilloid-1 channel in systemic inflammation from anti-inflammatory to proinflammatory. Cell Cycle. 2012;11:343–349. doi: 10.4161/cc.11.2.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Liu X, Zhang L, Shen X, Qi J, Wang J, et al. Bone selective protective effect of a novel bone-seeking estrogen on trabecular bone in ovariectomized rats. Calcif Tissue Int. 2013;93:172–183. doi: 10.1007/s00223-013-9739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]