Abstract
Studies of adults with attention-deficit/hyperactivity disorder (ADHD) have suggested that they have deficient response inhibition, but findings concerning the neural correlates of inhibition in this patient population are inconsistent. We used the Stop-Signal task and functional magnetic resonance imaging (fMRI) to compare neural activation associated with response inhibition between adults with ADHD (N = 35) and healthy comparison subjects (N = 62), and in follow-up tests to examine the effect of current medication use and symptom severity. There were no differences in Stop-Signal task performance or neural activation between ADHD and control participants. Among the ADHD participants, however, significant differences were associated with current medication, with individuals taking psychostimulants (N = 25) showing less stopping-related activation than those not currently receiving psychostimulant medication (N = 10). Follow-up analyses suggested that this difference in activation was independent of symptom severity. These results provide evidence that deficits in inhibition-related neural activation persist in a subset of adult ADHD individuals, namely those individuals currently taking psychostimulants. These findings help to explain some of the disparities in the literature, and advance our understanding of why deficits in response inhibition are more variable in adult, as compared with child and adolescent, ADHD patients.
Keywords: Inhibitory control, Hyperactivity, Psychostimulants, Functional magnetic resonance imaging (fMRI), Adults, Stop-Signal task
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD), which is characterized by age-inappropriate symptoms of inattention, impulsivity and hyperactivity, is the most prevalent psychiatric disorder of childhood. ADHD may continue into adulthood, with reports of symptom persistence in as many as 65% of cases (Mannuzza et al., 2003). Compared with controls, children with ADHD exhibit hypoactivation in frontoparietal and attention networks involved in executive function, but hyperactivation across large-scale networks, including the default-mode network, and somatomotor and visual networks (Cortese et al., 2012). Investigations in adults with ADHD are needed to clarify the basis of deficits that persist through the course of the disorder.
Deficient response inhibition, or the ability to suppress a prepotent or habitual response, has been proposed as a central feature of ADHD (Barkley, 2005). Findings obtained with functional magnetic resonance imaging (fMRI) suggest that deficient inhibition in ADHD samples reflects corresponding abnormality in fronto-striatal activation. During response inhibition, healthy individuals show recruitment of a network of brain regions that includes the bilateral ventrolateral prefrontal cortex (VLPFC) (encompassing the inferior frontal cortex (IFC) and insula), the pre-supplementary motor area (SMA)/SMA, medial superior frontal gyrus (SFG) and cingulate cortex, as well as subcortical regions including the striatum and thalamus (Aron and Poldrack, 2006; Aron et al., 2007; Swick et al., 2011). Subjects with ADHD show less activation in these regions compared with controls (Dickstein et al., 2006; Epstein et al., 2007). In fact, fMRI studies have consistently shown fronto-striatal hypoactivation in ADHD children and adolescents relative to controls during tasks requiring not only response inhibition but also those requiring interference inhibition, attention, and temporal processing, which together have provided considerable support for a fronto-striatal deficit hypothesis of ADHD (for review, see Cubillo et al. (2012)).
Only a few fMRI investigations of response inhibition, however, have involved adults with ADHD, and these studies have provided mixed results. In some cases, adult ADHD patients showed less activation than controls during response inhibition, including effects in VLPFC, cingulate, and striatal stopping-related regions (as reviewed by Cubillo et al. (2012) and as demonstrated in a meta-analysis by Hart et al. (2013)). For example, Mulligan et al. (2011) reported that a sample of 12 controls recruited a more extensive network of brain regions during inhibition on a Go/No-Go task as compared with 12 adult ADHD patients, and that ADHD subjects showed less activation in regions key for response inhibition, including the right PFC and preSMA. Similarly, Sebastian et al. (2012) reported less activation in an adult ADHD sample as compared with healthy controls during performance of the Stop-Signal, Go/No-Go, and Simon interference tasks, with significant effects in the right pallidum and left IFC in 20 ADHD adults as compared with 24 controls during inhibition of an already-initiated response (Stop-Signal task). Other reports, however, indicated that adults with ADHD showed no differences in (Carmona et al., 2012) or greater (Dillo et al., 2010; Karch et al., 2010) fronto-striatal activation during response inhibition as compared to controls. For example, Dibbets et al. (2009) reported no statistically significant differences in activation in fronto-striatal regions between 16 adult ADHD males and 13 healthy controls performing a modified Go/No-Go task. Similarly, while Dillo et al. (2010) found no difference in fronto-cingulo-striatal activity between 15 adult ADHD and 15 healthy control individuals performing a Go/No-Go task, they did find increased activation in parietal regions. The greater activation in parietal (Dillo et al., 2010) and cerebellar (Cubillo et al., 2012) regions during response inhibition by ADHD patients has been interpreted as reflecting the engagement of compensatory attentional processes. A number of factors may account for these discrepancies, including differences in task parameters (specifically differences between Go/NoGo and Stop-signal tasks), medication status, and symptom severity, as well as small sample size.
In an attempt to address these limitations in the literature, we examined differences in task performance and associated patterns of neural activation, as measured using fMRI, in a relatively large sample of adult participants with ADHD, as compared to controls, using a tracking version of the Stop-signal task. We hypothesized that adults with ADHD would exhibit less activation in stopping-related regions than would controls, and we conducted exploratory follow-up analyses to examine potential effects of medication status and symptom severity.
2. Methods
2.1. Participants
All participants were recruited from the Los Angeles area as part of the Consortium for Neuropsychiatric Phenomics at UCLA (www.phenomics.ucla.edu), in which they completed extensive neuropsychological testing (additional details provided in Supplementary Materials). All candidates were screened by telephone and then in person. Participants were men or women ages 21-50 years; NIH ethnic category either White, not Hispanic or Latino, or Hispanic or Latino, of any racial group; primary language (as determined by a verbal fluency test) either English or Spanish; completed at least 8 years of formal education; had no significant medical illness; adequately cooperative to complete assessments; and had visual acuity 20/60 or better. Urinalysis was used to screen for drugs of abuse (cannabis, amphetamine, opioids, cocaine, benzodiazepines), and participants were excluded if results were positive. Additional exclusion criteria for participants in the imaging portion of the study were left-handedness, pregnancy, history of head injury with loss of consciousness or cognitive sequelae, or other contraindications to scanning (e.g., claustrophobia, metal in body).
After receiving a verbal explanation of the study, participants gave written informed consent following procedures approved by the Institutional Review Boards at UCLA and the LACDMH. All subjects underwent a semi-structured assessment with the Structured Clinical Interview for the Diagnostic and Statistics Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID-I; (First MB, 2004)), supplemented for ADHD diagnoses with the Adult ADHD Interview (a structured interview form derived from the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime Version (KSADS-PL) (Kaufman et al., 1997)), in order to enable a more detailed characterization of lifetime history of ADHD in adults. For the purpose of this investigation, participants were excluded for lifetime diagnoses of schizophrenia or other psychotic disorders, bipolar I or II disorder; or current major depressive disorder, suicidality, anxiety disorder (obsessive-compulsive disorder, panic disorder, generalized anxiety disorder, post-traumatic stress disorder), or substance abuse/dependence other than nicotine dependence (which was allowed). Stable medications were permitted in ADHD participants (discussed below); any self-reported psychoactive medication use by controls was an exclusion factor. Symptom severity in patients was assessed with the Adult ADHD Clinical Diagnostic Scale (ACDS), which provides a quantitative assessment of how current Inattention and Hyperactivity symptoms impact patient functioning (Goodman, 2009; Kessler et al., 2010).
2.2 Procedure
Participants completed a tracking version of the Stop-Signal task, which enabled isolation of activation associated with the inhibition of an already-initiated motor response, and calculation of an individualized measure of inhibitory control (Stop-Signal reaction time, SSRT). On the testing day, participants first received training on the Stop-Signal task in the form of one initial demonstration, before completing two experimental runs (one run outside of the scanner and one while inside of the scanner). A complete description of the fMRI acquisition and preprocessing steps is presented in Supplementary Materials.
2.2.1 Stop-Signal task
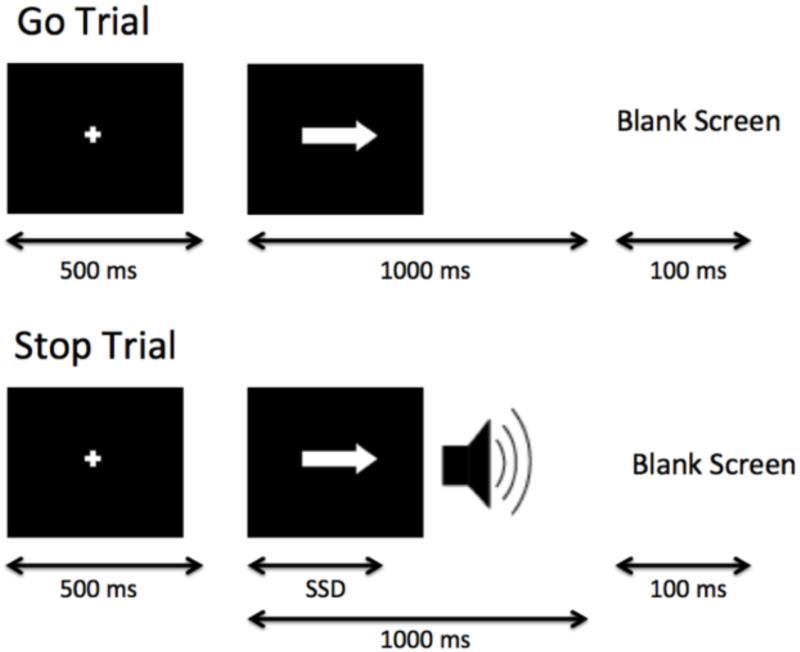
Participants were instructed to respond quickly when a “go” stimulus was presented on the computer screen, except on the subset of trials where the “go” stimulus was paired with a “stop” signal (Fig. 1). Specifically, participants were shown a series of go stimuli (left- and right-wards pointing arrows), to which participants were told to respond with left and right button presses, respectively (Go trials). On a subset of trials (25%), a stop signal (a 500-Hz tone presented through headphones) was presented a short delay after the go stimulus appeared and lasted for 250 ms (Stop trials). Participants were instructed to respond as quickly and accurately as possible on all trials, but to withhold their response on Stop trials (on trials with the tone). They also were instructed that stopping and going were equally important.
Fig. 1.
Schematic of the Stop-Signal task. Participants were shown a series of go stimuli (left- and right-wards pointing arrows), to which participants were told to respond with left and right button presses, respectively (Go trials); on a subset of trials, a stop-signal (a 500-Hz tone presented through headphones) was presented at a variable delay after the onset of the go stimulus (duration indicated by stop-signal delay (SSD)) and lasted for 250 ms (Stop trials), indicating that participants should withhold the go response.
On Stop trials, the delay of the onset of the stop signal, or stop-signal delay (SSD), was varied, such that it was increased after the participant successfully inhibited in response to a stop-signal (making the next stop trial more difficult), and decreased after the participant failed to inhibit in response to a stop-signal (making the next stop trial less difficult). Each SSD increase or decrease was in 50-ms intervals. The SSD values were drawn from two interleaved staircases per block, resulting in 16 trials from each staircase for a total of 32 Stop trials per block. In the first task run completed outside of the scanner, SSD values started at 250 and 350 ms for staircase 1 and 2, respectively. At the end of the behavioral run, the last SSD time from each staircase was then carried over to be the initial SSD for the scan run. This one-up/one-down tracking procedure ensured that subjects successfully inhibited on approximately 50% of inhibition trials. Also as a result, difficulty level is individualized across subjects and both behavioral performance and numbers of successful stop trials are equated across subjects.
Each experiment run contained 128 trials, 96 of which were Go trials and 32 of which were Stop trials, each presented randomly. All trials were preceded by a 500 ms fixation cross in the center of the screen, then each trial began with the appearance of an arrow and ended after 1000 ms, followed by the null period. Jittered null events separated every trial (with a blank screen), with the duration of null events sampled from an exponential distribution (null events ranged from 0.5 to 4 s, with a mean of 1 s). Stimulus presentation and timing of all stimuli and response events were achieved using Matlab (Mathworks) and the Psychtoolbox (www.psychtoolbox.org, Brainard, 1997) on an Apple Powerbook. For the experiment run administered in the scanner, each participant viewed the task through MRI-compatible goggles, responded with his or her right hand on an MR-compatible button box in the scanner.
2.2.2 SSRT calculation
Stop-signal task data were analyzed following the race-model (Logan and Cowan, 1984), as has been previously reported (Congdon et al., 2010; Congdon et al., 2012), in order to estimate SSRT, our primary measure of inhibitory control. The mean and standard deviation of reaction time (RT) on Go trials were calculated only for Go trials in which participants correctly responded. Stop successful trials included only Stop trials on which participants successfully inhibited a response, and Stop unsuccessful trials included only Stop trials on which participants responded. Average SSD was calculated from SSD values across both staircases. SSRT was estimated using the quantile method, which does not require an assumption of 50% inhibition (Band et al., 2003). In order to calculate SSRT according to this method, all RTs on Go trials were arranged in ascending order, and the RT corresponding to the proportion of failed inhibition was selected. The average SSD was then subtracted from this quantile RT, providing an estimate of SSRT, with longer SSRT values reflecting poorer inhibitory control and shorter SSRT values reflecting better inhibitory control.
2.3. Behavioral data analysis
In addition to SSRT, we also examined mean and standard deviation of RT on Go trials, percent inhibition on Stop trials, and percent correct on Go trials. The distribution of each variable was inspected prior to analysis to ensure normality and, in the case of percent accuracy on Go trials, a square root transformation was made. All behavioral analyses were performed using R statistical software (R 2.10.1) (http://www.r-project.org).
First, multiple linear regression models were used to test the relationship between Stop-signal task performance and demographics, including age, gender, education (defined by years of school completed), ethnicity (Hispanic/Latino vs. not Hispanic/Latino), and primary language (English vs. Spanish). Second, one-way analyses of covariance models were used to examine differences in performance as a function of diagnostic status (control vs. ADHD), while controlling for demographic measures. Then, patients were grouped according to self-reported current psychostimulant use and follow-up tests were conducted to compare these subgroups of participants in symptom severity and performance.
2.4. fMRI data analysis
Analyses were performed using tools from the FMRIB software library (Smith et al., 2004), and preprocessing steps are described in Supplementary Materials. For each subject, StopInhibit-Go and StopRespond-StopInhibit contrasts were computed, and the output from the subject-specific analyses was then analyzed using a mixed-effects model with FLAME for between-group comparisons. All group-level statistics images were thresholded with a cluster-forming threshold of z > 2.3 and a cluster probability of p < 0.05, corrected for whole-brain multiple comparisons using Gaussian random field theory. Brain regions were identified using the Harvard-Oxford cortical and subcortical probabilistic atlases (Desikan et al., 2006) (http://www.cma.mgh.harvard.edu/fsl_atlas.html), and all activations are reported in MNI coordinates. For reporting of clusters, we used the cluster command in FSL. Anatomical localization within each cluster was obtained by searching within maximum likelihood regions from the FSL Harvard-Oxford probabilistic atlas to obtain the maximum z-statistic and MNI coordinates within each anatomical region contained within a cluster. For visualization of results, statistical maps were projected onto an average cortical surface with the use of multifiducial mapping using CARET software (Van Essen, 2005) (http://brainvis.wustl.edu/wiki/index.php/Caret:Download).
Similar to behavioral analyses, in order to test the effect of psychostimulant medication status and symptom severity, a whole-brain regression analysis of data from all ADHD participants was conducted, including psychostimulant status (with ADHD participants coded as either On or Off psychostimulants), ACDS Inattention severity scores, and ACDS Hyperactivity severity scores, as covariates of interest. This allowed for examination of the relationship between activation and current symptoms, while controlling for current psychostimulant use, and vice versa. We conducted two follow-up tests comparing controls to ADHD participants On psychostimulants and controls to ADHD participants Off psychostimulants on our primary contrast of interest. Final follow-up analyses were conducted after removing ADHD participants taking any medication other than psychostimulants (results presented in Supplementary Materials), and to compare males and female ADHD participants.
3. Results
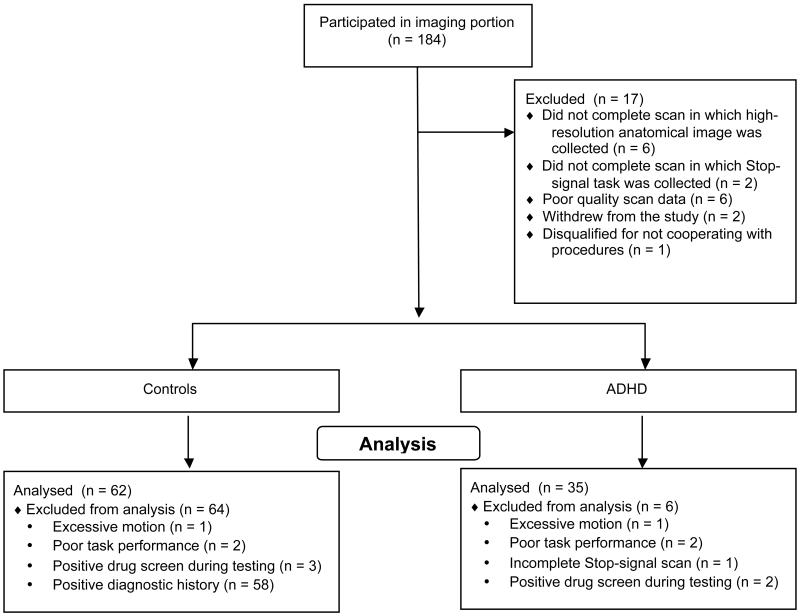
Our final analyses are based on data from 97 subjects with complete, usable Stop-signal fMRI data, including data from 62 healthy participants and 35 adult participants with ADHD. See Fig. 2 for an illustration of subjects excluded at various stages of analysis. There was no difference in any of our demographic measures between healthy participants and adult ADHD participants.
Fig. 2.
Consort diagram of data collection and exclusion.
Stable medications were permitted in ADHD participants; psychostimulants were used most often, with 10 of 35 (29%) participants reporting psychostimulant use (Table 1). Psychostimulant medications taken included preparations containing amphetamine (Adderall XR®, amphetamine and dextroamphetamine mixed salts; or dextroamphetamine sulfate, prescribed either as a generic formulation or as Dexedrine®), lisdexamfetamine dimesylate (Vyvance®), an amphetamine prodrug) or methylphenidate (Concerta® or Metadate™, both extended-release preparations). To examine the potential effects of current medication use, analyses were conducted comparing ADHD participants who were taking a stable dose of psychostimulant medication with those who were not.
Table 1. Descriptive statistics of Stop-signal task performance in healthy control and adult ADHD samples.
| Variable | Controls N = 62 Mean (SD) |
ADHD N = 35 Mean (SD) |
ADHD No Psychostimulants N = 25 Mean (SD) |
ADHD Psychostimulants Used N = 10 Mean (SD) |
Group Comparisons |
|---|---|---|---|---|---|
| Agea | 30.82 (8.97) | 30.86 (10.01) | 31.24(10.37) | 29.90 (9.48) | t(64.42) = −0.02, p 0.05b |
| Gender (%F) | 55% | 46% | 44% | 50% | χ2(l) = 0.42,p > 0.05b |
| Ethnicity (% Hispanic/Latino) |
35% | 17% | 20% | 10% | χ2(1) = 2.83,p > 0.05b |
| Language(% English) |
92% | 100% | 100% | 100% | χ2(1) = 1.55,p > 0.05b |
| Education | 15.10(1.75) | 14.69(1.83) | 14.28 (1.74) | 15.70 (1.70) | t(68.23) = −1.08, p > 0.05b |
| SSRT | 186.38 (52.64) | 198.85 (65.50) | 195.21 (70.80) | 207.95 (52.09) | F(1,90) = 1.09, p > 0.05b |
| Mean Go RT | 494.06 (106.06) | 500.73 (90.29) | 513.46(100.18) | 468.91 (49.57) | F(l,90) = 0.11,p > 0.05b |
| SD Go RT | 111.89 (44.62) | 109.12(39.73) | 112.28 (42.03) | 101.20 (33.98) | F(1,90) = 0.10,p 0.05b |
| Percent correct responses—Go trials |
99.18 (1.76) | 98.18 (5.46) | 97.79 (6.38) | 99.17 (1.61) | F(l,90) = 1.78,p > 0.05b |
| Percent inhibition— Stop trials |
49.45 (5.70) | 49.20 (8.69) | 49.38 (9.46) | 48.75 (6.78) | F(l,90) = 0.03,p > 0.05b |
| ACDS Inattention | NA | 33.94 (2.24) | 33.88 (2.15) | 34.10 (2.56) | t(14.36) = −0.24,p > 0.05c |
| ACDS Hyperactivity | NA | 29.49 (4.49) | 29.60 (4.32) | 29.20 (5.14) | t(14.38) = −0.22,p > 0.05c |
Each sample included participants in the range of 18-50.
Controls (N = 60) vs. all ADHD participants (N = 35).
ADHD participants not currently taking psychostimulant medication (N = 25) vs. ADHD participants currently taking psychostimulant medication (N = 10). ADHD No Psychostimulants = ADHD participants not currently taking psychostimulant medication; ADHD Psychostimulants Used = ADHD participants currently taking psychostimulant medication. %F = percent of each sample comprised of women; % Hispanic/Latino = percent of Hispanic/Latino ethnicity vs. not Hispanic/Latino; % English = percent whose primary language is English vs. Spanish; Education = number of school years competed. SSRT = Stop-signal reaction time; SD = standard deviation. ACDS = ADHD Clinical Diagnostic Scale. Square root transformation of percent correct on Go trials used in ANCOVA, but raw values presented here. Psychostimulant medications taken included preparations containing amphetamine (Adderall XR®, amphetamine and dextroamphetamine mixed salts; or dextroamphetamine sulfate, prescribed either as a generic formulation or as Dexedrine®), lisdexamfetamine dimesylate (Vyvance®), an amphetamine prodrug) or methylphenidate (Con certa® or Metadate™, both extended-release preparations).
3.1. Behavioral results
Behavioral data collected during performance of the Stop-Signal task from all control and ADHD participants included in the present analysis (N = 97) are presented in Table 1; ADHD participants are presented together, as well as separated according to current psychostimulant medication use. Multiple linear regression analyses revealed significant relationships between age and SSRT (β = 1.40, 95% confidence interval (CI), 0.10, 2.70, p = 0.04), and age and mean RT on Go trials (β = 2.81, 95% CI, 0.60, 5.01, p = 0.01), but no significant relationships between task performance and gender, ethnicity, education or language.
When controlling for demographic measures, there were no significant differences between controls and adult ADHD participants for any measure of performance. As shown in Table 1, although the mean SSRT was longer in the ADHD group than in controls (d = 0.21), suggesting poorer inhibitory control, this difference was not significant. These results suggest comparable performance between healthy controls and adult ADHD participants.
Within the ADHD sample, there was no difference in Stop-Signal task performance between ADHD participants On (N = 25) vs. Off (N = 10) psychostimulant medication, and there were no differences between these subgroups in symptom severity, for either the ACDS Inattention or Hyperactivity symptom scores (p > 0.05).
3.2. fMRI results
3.2.1 Inhibition-related activation
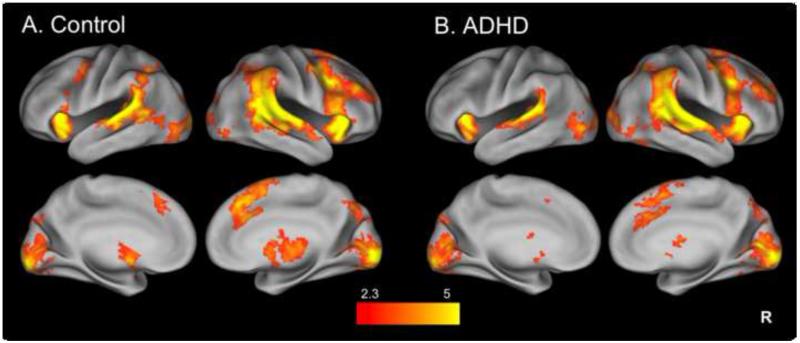
Our primary contrast of interest was StopInhibit-Go, which isolates successful stopping-related activation. We conducted a two-sample comparison of the StopInhibit-Go contrast in order to identify group differences in stopping-related activation; there was no significant difference in activation between controls and adult ADHD participants, in either direction. Similarly, there was no significant difference between controls and ADHD participants, in either direction, when examining activation isolated with the StopRespond-StopInhibit contrast, which identified brain regions with greater activation during inhibition failures as compared to successful inhibition trials between groups.
For illustration, whole-brain activation maps are presented for each group separately (with details provided in Table 2), with Fig. 3A representing controls and Fig. 3B the ADHD sample. Both groups show activation in the set of regions commonly engaged during SST-related inhibition, including bilateral VLPFC, striatum, thalamus, and a cluster spreading through the preSMA/SMA, SFG and cingulate, as well as additional posterior parietal regions. Although the activation seen in the control sample (Fig. 3A) appeared more robust and extensive than in the ADHD sample (Fig. 3B), this apparent difference was not statistically confirmed.
Table 2. Clusters of activation during response inhibition.
| Brain region | Hemisphere | Voxels | Max z-stat | X | y | z |
|---|---|---|---|---|---|---|
| Clusters of Stoplnhibit-Go in Controls alone | ||||||
| Superior temporal gyrus, middle temporal gyrus, IFG (R), insula (R), frontal orbital cortex (R), precentral gyrus (R), MFG, SFG, preSMA, paracingulate/ACC, supramarginal gyms, lateral occipital cortex/occipital pole |
R/L | 33,539 | 7.90 | 64 | −16 | 2 |
| Insula, IFG, frontal orbital cortex, precentral gyrus |
L | 1,691 | 7.20 | −32 | 20 | 4 |
| Caudate, pallidum, putamen, thalamus | R | 784 | 4.22 | 12 | 8 | 4 |
| Caudate, putamen, thalamus | L | 551 | 4.27 | −8 | 2 | −6 |
| Clusters of Stoplnhibit-Go in ADHD alone | ||||||
| Superior temporal gyrus, middle temporal gyrus, IFC, insula, frontal orbital cortex, precentral gyrus, MFG, SFG, preSMA, paracingulate/ACC (R/L), lateral occipital cortex/occipital pole (R/L), supramarginal gyrus |
R | 25,171 | 8.18 | 64 | −16 | 2 |
| Superior temporal gyrus, middle temporal gyrus, insula, frontal orbital cortex, supramarginal gyrus |
L | 4,197 | 7.05 | −42 | −28 | 6 |
| Caudate, putamen (L), thalamus (R) | R/L | 544 | 3.70 | −10 | 8 | −4 |
Voxels: number of activated voxels per cluster; z-stat: maximum z-statistic for each cluster; x, y, and z are MNI coordinates for the peak of each cluster. R = right; L = left; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus; preSMA = pre-supplementary motor area; ACC = anterior cingulate cortex.
Fig. 3.
Separate group maps of StopInhibit-Go activation. Stopping-related activation in controls (A) and adult ADHD (B) groups alone. Statistical maps are corrected for whole-brain multiple comparisons and were projected onto an average cortical surface using CARET (R = Right). The color represents the z-score.
3.2.2 Effect of symptom severity and medication on inhibition-related activation
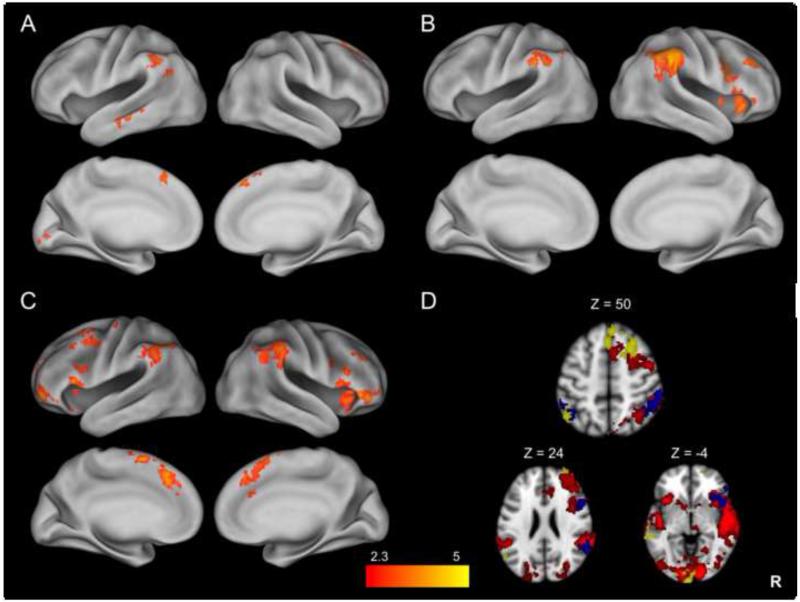
In order to examine the relationship between stopping-related activation and current symptom severity, in follow-up analyses we added current Inattention and Hyperactivity scores, along with current psychostimulant use, to a regression model, which tests the relationship between stopping-related activation and symptom severity while controlling for psychostimulant use, and vice versa. While there was no correlation between stopping-related activation and Inattention symptom scores, Hyperactivity symptom scores were significantly positively correlated with activation in a number of regions, including the right SFG, MFG, and paracingulate gyrus, right anterior frontal pole, left middle temporal gyrus, and left supramarginal gyrus (Fig. 4A), while controlling for psychostimulant use.
Fig. 4.
Differences in StopInhibit-Go activation as a function of symptom severity and medication use. While controlling for psychostimulant use, positive correlation between StopInhibit-Go activation and Hyperactivity symptoms in ADHD participants alone (A). While controlling for Hyperactivity symptoms, greater stopping-related activation seen in ADHD participants Off vs. On psychostimulant medication (B) and in controls vs. ADHD participants On psychostimulant medication (C). Multiple contrasts overlaid on a single image to illustrate overlap (D), with ADHD group mean in red, greater stopping-related activation seen in ADHD participants Off vs. On psychostimulant medication in blue, and the positive correlation between stopping-related activation and Hyperactivity symptoms in yellow. Statistical maps are corrected for whole-brain multiple comparisons and were projected onto an average cortical surface using CARET; in D, axial slices are included to illustrate the overlap of activation with coordinated in MNI space (R = Right). The color represents the z-score.
There was a significant difference in stopping-related activation as a function of current psychostimulant medication use, while controlling for either Inattention or Hyperactivity scores. ADHD participants not currently taking psychostimulants had significantly greater activation in the right IFC extending up through the precentral gyrus and down through the insula, as well as the bilateral supramarginal and angular gyri as compared to ADHD participants currently taking psychostimulants (Fig. 4B, Table 3). There was no greater activation in ADHD participants taking, as compared to those not taking, psychostimulants.
Table 3. Activation differences as a function of psychostimulant medication status and symptom severity during response inhibition.
| Brain region | Hemisphere | Voxels | Max z-stat | X | y | z |
|---|---|---|---|---|---|---|
|
Clusters of Stoplnhibit-Go activation positively correlated
with ACDS Hyperactivity symptoms in ADHD alonea | ||||||
| SFG, MFG, paracingulate (R/L) | R | 996 | 4.16 | 26 | 24 | 46 |
| Middle temporal gyrus, planum polare | L | 447 | 3.81 | −64 | −14 | −12 |
| Occipital pole | (R/L) | 402 | 3.80 | −4 | −102 | −6 |
| Frontal pole | R | 371 | 3.15 | 22 | 62 | −10 |
| Lateral occipital cortex, supramarginal gyrus, angular gyrus |
L | 358 | 3.20 | −48 | −60 | 50 |
| Clusters of Stoplnhibit-Go where ADHD Off> On Psychostimulant medicationb | ||||||
| Supramarginal gyrus, angular gyrus | R | 1,821 | 4.45 | 58 | −38 | 38 |
| IFG, precentral gyrus, insula | R | 1,293 | 4.00 | 54 | 14 | 24 |
| Supramarginal gyrus | L | 445 | 3.72 | −60 | −38 | 36 |
| Clusters of Stoplnhibit-Go where Controls > ADHD On Psychostimulant medication | ||||||
| IFG, insula, frontal orbital cortex | R | 1,639 | 4.31 | 44 | 16 | −10 |
| preSMA, paracingulate, SFG | R/L | 1,314 | 4.18 | −2 | 8 | 60 |
| Angular gyrus, supramarginal gyrus | R | 1,182 | 4.25 | 62 | −50 | 34 |
| Supramarginal gyrus | L | 930 | 4.01 | −46 | −46 | 40 |
| Cerebellum | R | 893 | 4.48 | 34 | −56 | −34 |
| Precentral gyrus, MFG, IFG | L | 752 | 4.06 | −44 | −16 | 66 |
| Frontal pole | L | 621 | 3.99 | −42 | 54 | −6 |
| IFG, insula, frontal orbital cortex | L | 565 | 4.31 | −46 | 16 | −2 |
Controlling for Inattention symptoms and current psychostimulant use;
Controlling for Hyperactivity and Inattention symptoms. Voxels: number of activated voxels per cluster; z-stat: maximum z-statistic for each cluster; x, y, and z are MNI coordinates for the peak of each cluster. ACDS, Adult ADHD Clinical Diagnostic Scale. R = right; L = left; IFG = inferior frontal gyrus; preSMA = pre-supplementary motor area; SFG = superior frontal gyrus; MFG = middle frontal gyrus.
To further examine differences in stopping-related activation as a function of psychostimulant status, we conducted two follow-up tests. Controls showed significantly greater activation in a number of key stopping-related regions as compared to ADHD participants taking psychostimulants. These included the bilateral VLPFC, the preSMA extending through the paracingulate gyrus, and bilateral supramarginal and angular gyri (Fig. 4C, Table 3). In contrast, there was no difference in activation between controls and ADHD participants not taking psychostimulants.
To summarize, overall ADHD participants engaged a set of brain regions commonly recruited during response inhibition. Within the ADHD sample, participants not currently taking psychostimulants showed more activation in a subset of key response inhibition-related regions, whereas those participants with more severe symptoms of Hyperactivity, whether on or off psychostimulant medication, showed greater activation in additional right frontal, temporal and parietal regions. To further illustrate these differences in patterns of activation, the 1) group mean of the ADHD participants (red), 2) the difference between participants not currently taking vs. currently taking psychostimulants (blue) and 3) the positive correlation with Hyperactivity scores (yellow) are overlaid on a single image in Figure 4D. While not a statistical comparison, this illustrates where the separate contrasts differ; there is more overlap between the group mean (red) and the difference as a function of psychostimulant use (blue) than overlap between the group mean (red) and the correlation with Hyperactivity symptoms (yellow), suggesting that the medication effect is specific to response inhibition-related brain activation, whereas – independent of this medication effect – participants suffering from more severe Hyperactivity symptoms recruit additional regions when needing to inhibit a prepotent response.
3.2.3 Ruling out additional medication on inhibition-related activation
As eight ADHD participants reported taking additional medications (including antidepressants, antipsychotics, an anticonvulsant-mood stabilizer, and hormone medication), to rule out any effect of additional medication, follow-up tests excluding these participants were conducted. Of those ADHD participants not currently taking any medication, we do not know the duration of time off medication.
Of the 10 ADHD participants who were taking psychostimulants, four reported current use of an additional medication, and four of the 25 ADHD participants who were not taking psychostimulants reported current use of a medication other than psychostimulants. We re-ran the primary analyses excluding these eight ADHD participants, and the results did not change except in two instances. First, the positive correlation between Hyperactivity symptoms and stopping-related activation was no longer significant; second, the difference between control and ADHD participants taking psychostimulants was no longer significant. In addition, while the difference between ADHD participants not taking psychostimulants and those who were taking psychostimulants was maintained, it was restricted to the right supramarginal gyrus in the smaller sample.
3.2.4 Gender differences on inhibition-related activation in ADHD adult participants
There were no significant differences in stopping-related activation when comparing male and female ADHD participants.
4. Discussion
We examined the pattern of neural activation associated with response inhibition in a sample of 35 adult individuals with ADHD, using the Stop-Signal task, a task requiring participants to inhibit an already-initiated response. There were no differences in SSRT or neural activation between adults with ADHD and healthy controls. However, in follow-up tests, when ADHD participants were stratified according to current psychostimulant medication use, significant differences in stopping-related neural activation that were not accounted for by differences in symptom severity were observed between participants taking psychostimulants and those who were not. Compared with controls and ADHD participants not taking psychostimulant medication, adult ADHD participants currently taking psychostimulants showed less activation in key stopping-related regions. Independent of this, there was a positive relationship between Hyperactivity symptoms and activation in cortical regions outside of the stopping-related network, suggesting that additional regions are recruited in order to achieve comparable inhibition. Thus, both symptom severity and current psychostimulant medication seem to be associated with the degree of response inhibition-related activity within this heterogeneous adult ADHD sample. These findings may help to account for some of the disparities in the existing literature.
Although a deficit in response inhibition is thought to be central to ADHD, much of the relevant literature concerns children and adolescents. Several meta-analyses assessing response inhibition using behavioral assays (Stop-Signal or Go/No-Go tasks) provide convergent evidence for a modest effect size of deficient response inhibition in ADHD, with some evidence that inhibitory deficits persist in adulthood. The most comprehensive of these meta-analyses, focused on SSRT, involved 68 studies of both adults and children, and a weighted mean effect size of 0.62 was reported (Lipszyc and Schachar, 2010). While effect sizes in adult ADHD samples were smaller, age was not a significant moderator of SSRT effect size across all samples. In contrast, we observed no significant differences in SSRT between ADHD participants and controls, a finding that may reflect our recruitment of adults only over the age of 21, features of our inclusion/exclusion criteria, or inclusion of only ADHD participants with sufficient inhibitory control to complete multiple testing sessions. An examination of the mean SSRT in our sample of 35 ADHD participants (198.85) in comparison to the weighted mean SSRT in adult ADHD samples (240.97) reported in a previous meta-analysis (Lijffijt et al., 2005) supports the latter explanation.
There are a number of factors contributing to mixed findings in fMRI studies of response inhibition, including small sample sizes. One of the most comprehensive meta-analyses of fMRI studies in ADHD (Cortese et al., 2012) identified just 16 studies that included adult ADHD patients; by our count, only five of these studies included tasks assessing response inhibition (with ADHD sample sizes varying from 8 to 23) (Banich et al., 2009; Cubillo et al., 2010; Dibbets et al., 2009; Dillo et al., 2010; Karch et al., 2010). A more recent meta-analysis (Hart et al., 2013) included just one additional adult sample investigating motor response inhibition (Kooistra et al., 2010). In our review of the literature, we have identified a total of 10 studies that have investigated neural correlates of response inhibition using either a Go/NoGo or Stop-Signal task and fMRI in adult ADHD participants (Carmona et al., 2012; Cubillo et al., 2010; Dibbets et al., 2009; Epstein et al., 2007; Karch et al., 2010; Kooistra et al., 2010; Mulligan et al., 2011; Schneider et al., 2010; Sebastian et al., 2012). We have summarized these 10 studies in Table 4 in order to highlight differences between them, and to compare their results with those presented here. Some of the differences across studies, which may help to explain discrepancies in results, include inconsistencies in task paradigms, analysis methods, and sample composition.
Table 4. Comparison of response inhibition functional magnetic resonance imaging studies in adult ADHD samples.
| Sample sizes (Mean (SD) Age) |
Sample composition | Response Inhibition Task (contrast) |
Between-group analyses |
Results | |
|---|---|---|---|---|---|
| Carmona et al. (2012) |
|
|
Event-related Go/NoGo (NoGo vs. Go trials) |
|
|
| Cubillo et al. (2010) |
|
|
Tracking Stop-signal task (successful Stop vs. Go trials) |
Whole-brain analyses |
Less activation in bilateral inferior prefrontal cortex, caudate, and thalamus in ADHD vs. controls |
| Dibbets et al. (2009) |
|
|
Modified event- related Go/NoGo task (correct NoGo vs. Go trials) |
Whole-brain analyses |
No differences in activation between for correct NoGo/Go |
| Dillo et al. (2010) |
|
|
Block Go/NoGo task (NoGo vs. Go blocks) |
Whole-brain and ROI-based analysis |
|
| Epstein et al. (2007) |
|
|
Event-related Go/NoGo task (NoGo vs. Go trials) |
ROI-based analyses of striatum, frontal gyri, ACC, posterior parietal gyrus, and cerebellum |
|
| Karch et al. (2010) |
|
|
Modified event- related Go/NoGo task ([Voluntary inhibition vs. Voluntary selection] – [NoGo vs. Go] trials) |
Fixed-effects group analyses |
Less activation in medial and lateral PFC in ADHD subjects vs. controls |
| Kooistra et al. (2010) |
|
|
Modified event- related Go/NoGo task (Fast event NoGo vs. Go trials; Slow even NoGo vs. Go trials) |
Whole-brain analyses |
|
| Mulligan et al. (2011) |
|
|
Event-related Go/NoGo task (NoGo vs. baseline trials) |
Whole-brain and ROI-based analysis |
|
| Schneider et al. (2010) |
|
|
Event-related Go/NoGo task (NoGo vs. NR) |
Whole-brain analysis |
|
| Sebastian et al. (2012) |
|
|
|
Whole-brain analyses |
|
ROI = region of interest; NR = not reported; IFG = inferior frontal gyrus; ACC = anterior cingulate cortex; PFC = prefrontal cortex; SMA = supplementary motor area; SFG = superior frontal gyrus.
While Stop-Signal and Go/No-Go tasks are the most commonly employed measures of response inhibition, and contribute to the same underlying “prepotent response inhibition” construct (Aichert et al., 2012), the Stop-Signal task measures the ability to inhibit or cancel a response that has already been initiated, whereas the Go/No-Go task measures the ability to withhold a response (Schachar et al., 2007), and a quantitative meta-analysis has shown that Stop-Signal and Go/No-Go tasks engage overlapping but distinct brain regions (Swick et al., 2011). As these different task demands may be differentially sensitive to ADHD and corresponding neural disturbances, the use of variable tasks across studies potentially contributes to differences in reported findings, as is clear when comparing across studies listed in Table 4. For example, the following three studies have used an event-related Go/NoGo design similar enough for comparison: 1) Carmona et al. (2012) conducted a primary ROI-based analysis in 19 medication-naïve ADHD males and found no difference in IFG (and exploratory whole-brain analyses) as compared with controls, 2) Epstein et al. (2007) conducted a region-of-interest based analysis in nine ADHD males and females with a mix of medication histories and reported less activation in bilateral IFG and left caudate in the ADHD participants as compared with controls; and 3) (Sebastian et al., 2012) found less activation in the right caudate in 20 medication-naïve ADHD male and female participants as compared with controls. While the majority of these studies report less activation in stopping-related regions in ADHD adult participants as compared to controls, each study continues to differ in additional factors, including sample composition and analysis methods, which may account for the reported findings. Overall, there are still too few studies investigating neural correlates of response inhibition, using either the Go/NoGo or Stop-Signal tasks, to draw conclusions about consistent patterns of inhibition-related activation in adult ADHD participants.
Small sample size has also prevented a complete characterization of how symptom severity or medication affects inhibition-related activation. This is clearly reflected in three recent meta-analyses of the ADHD fMRI literature. While Cortese et al. (2012) report a pattern of ADHD-related hypoactivation in fronto-striatal regions when collapsing across age groups and tasks, the small sample sizes among the included studies could not support contrasts between ages, task types, medication history, or psychiatric comorbidity. Indeed, as the authors report, there were too few published adult ADHD studies using response inhibition tasks to compare against studies in children and adolescents, despite the large body of literature demonstrating a central deficit in younger samples. More recently, Hart et al. (2013) directly investigated age effects by conducting a whole-brain meta-analysis of published data and found that the pattern of differences between patients and controls differed slightly as a function of age, with ADHD children showing less activation in the left putamen, right caudate, SMA and ACC relative to controls, and ADHD adults showing less activation in the right IFC and thalamus relative to controls. Furthermore, while they reported an effect of long-term medication use on attention-related neural activation in their meta-regression analysis, they reported no effect of long-term medication use on inhibition-related activation. These negative findings may be explained by a third recent meta-analysis conducted: while Rubia et al. (2013) were able to conduct a meta-analysis on the effect of psychostimulant function on brain activation in children and adolescents with ADHD, they were unable to identify any studies with adult participants that met their inclusion criteria, which highlights the relative lack of information about medication effects on inhibition-related activation in adult ADHD. Thus, although such meta-analyses are able to provide convergent evidence of an inhibition-related deficit in neural activation in adult ADHD participants, additional empirical studies that systematically examine the influence of age, medication use, and symptom severity are needed.
We attempted to examine differences as a function of psychostimulant medication status in exploratory follow-up analyses and, although our results suggest that differences in medication status may contribute to discrepant findings in the literature on adult ADHD participants, our findings are unexpected. We found less stopping-related activation in 10 ADHD adult participants currently taking psychostimulants as compared to 25 ADHD participants not currently taking psychostimulants. This is in contrast to the majority of studies, which report an increase in neural activation following psychostimulant administration in ADHD youth (Vaidya et al., 1998; Shafritz et al., 2004; Epstein et al., 2007; Prehn-Kristensen et al., 2011; Rubia et al., 2011a; Rubia et al., 2011b). Long-term medication use is thought to normalize an underactive stopping-related response in ADHD youth (Rubia et al., 2013; Schweren et al., 2013) and this has been demonstrated in other domains, including attention-related (Hart et al., 2013) and timing-related (Hart et al., 2012) neural activation. However, as illustrated by a recent meta-analysis, there are insufficient data on the long-term effect of psychostimulants on stopping-related neural activation in adult ADHD participants (Rubia et al., 2013). Our findings of less stopping-related activation in ADHD participants currently taking psychostimulants are in line with previous reports of the effects of medication exposure on inhibition-related activation in healthy adults (Chamberlain et al., 2009; Costa et al., 2012), and with a report of a decrease in fronto-cingulate activation during Go/No-Go task performance in ADHD adolescents treated with methylphenidate (Schulz et al., 2012). Notably, in a randomized treatment study, Schulz et al. (2012) reported that while symptom improvements following methylphenidate (a stimulant) and atomoxetine (a non-stimulant) administration were associated with decreased activation in the bilateral motor cortex, symptom improvement following methylphenidate administration was associated with a decrease in right IFC, ACC/SMA, and posterior cingulate activation while symptom improvement following atomoxetine administration was associated with an increase in these same regions in ADHD youth. However, our cross-sectional results cannot entirely address medication history, illness duration, or illness severity, or the interaction of these factors with developmental processes; less activation as a function of psychostimulant use and/or symptom severity on the background of ADHD that persists in adulthood may not have the same functional implications that it does in ADHD youth. It is clear that additional research is warranted to compare the effects of long-term psychostimulant medication on stopping-related neural activation in adult ADHD participants.
There are additional factors that may explain discrepant findings across fMRI studies of response inhibition in adult ADHD participants, which may also account for our unexpected findings of less activation in ADHD adult participants currently taking psychostimulants as compared with those not currently taking psychostimulants. The heterogeneity of the adult ADHD population is reflected in the varying sample compositions, as reflected in Table 4: gender distribution, ADHD subtype, current medication status as well as medication history, and illness severity (indexed by the method of patient ascertainment) have varied across studies. While we did not find any differences in stopping-related activation as a function of gender in additional follow-up analyses, we did not have enough power to conduct additional analyses examining differences between groups divided by gender and medication status. When we did attempt to rule out additional medication effects on inhibition-related activation, our findings changed in the direction of no longer being significant, which is likely the result of being underpowered to test differences between small follow-up samples. We can speculate further that individual differences in dopaminergic function contribute to variations in the effect of psychostimulant medication on stopping-related activation in ADHD (Ghahremani et al., 2012), and that this interaction may vary as a function of age, given that long-term psychostimulants induce changes in brain structure (Shaw et al., 2009) and function (Rubia et al., 2013). Indeed, the effect of medication history may be a critical factor in explaining mixed findings. While we found no difference in activation between 35 adult male and female ADHD participants with a mix of medication histories and current use, as compared to controls, the two published Stop-signal studies report less activation in medication naïve adults: 1) Cubillo et al. (2010) reported less activation in the bilateral inferior prefrontal cortex, caudate and thalamus in 11 medication-naïve ADHD male adults; and 2) Sebastian et al. (2012) reported less activation in the right pallidum and (at a reduced threshold) the left IFG in 20 medication naïve adult male and female ADHD participants. Motivated by the high rates of ADHD persistence in adulthood, longitudinal investigations are needed to address the influence of psychostimulant use – over time – on the neural correlates of response inhibition in adult ADHD.
Independent of current psychostimulant use, we also found a positive association between Hyperactivity symptoms, as measured with the ACDS, and brain activation in a number of regions, including the right frontal pole, right SFG, paracingulate gyrus, in addition to left temporal and parietal regions. Perhaps not surprisingly, given the variety of task demands and medication status, studies are mixed in terms of whether they report a positive or negative association between stopping-related activation and current symptoms. Carmona et al. (2012) found no association between right IFG activation during inhibition and current symptoms, while Cubillo et al. (2010) reported a negative association between Attention/Hyperactivity symptoms and brain activation in extended frontal, parietal, and temporal regions, as well as in the anterior cingulate, caudate, putamen, thalamus and cerebellum. Our results also differ somewhat with those of Schneider et al. (2010), who found a negative association between Hyperactivity scores, as measured with a self-rating scale for ADHD, and activation in the right SFG, left MFG, left precentral gyrus, and left superior lobe, and a positive association between Hyperactivity scores and activation in the anterior insula, right inferior temporal gyrus, and left lingual gyrus. However, their finding of both increased and decreased activation associated with current Hyperactivity symptoms suggests that, similar to our results, altered patterns of activation are required in patients with more severe symptoms. In additional follow-up analyses, when we excluded eight participants taking medications other than psychostimulants, this positive correlation with Hyperactivity symptoms was no longer significant, although this is likely due to the resulting loss of power. We argue that a finding of a positive correlation between activation in these regions and Hyperactivity scores reflects compensatory activation in those subjects with more severe symptoms, as these regions tend to fall outside the set of regions significantly active in the group. This is illustrated in Fig. 4D, where we have overlaid findings demonstrating an effect of current psychostimulant use (blue), an association between activation and Hyperactivity symptoms (yellow), and the ADHD group mean (red).
Although our study represents one of the largest fMRI investigations of Stop-signal related inhibition in adult ADHD participants, our sample may still be underpowered to detect subtle differences in performance or activation between adult ADHD participants and controls, or differences within the ADHD sample. In particular, although we were able to examine differences with ADHD adult participants as a function of current psychostimulant use, our finding of less activation in 10 ADHD participants currently taking psychostimulants as compared to either 25 ADHD participants not currently taking psychostimulants or 62 controls may be explained by a lack of power to detect activation in this sample. Second, our sample of ADHD participants is heterogeneous with respect to treatments received and, as a result, we examined effects of symptom severity and medication in a series of exploratory follow-up analyses. Although current symptom severity as measured by the ACDS did not account for differences between ADHD participants currently taking vs. not currently taking psychostimulants, there may be additional unmeasured factors that account for these differences in activation amongst ADHD participants—including long-term medication use. Overall, these results concerning differences as a function of current psychostimulant use should be considered preliminary and require replication. Third, we did not find significant differences in Stop-Signal task performance or stopping-related brain activation between the ADHD and control samples, despite widespread evidence supporting such a difference. An advantage of the Stop-Signal task is its ability to isolate neural activation underlying successful response inhibition; however, the tracking-design titrates the parameters of the task to fit each individual’s level of inhibitory control, which ensures that all participants inhibit on approximately 50% of trials. In doing so, the task obscures differences in the number of commission errors that might otherwise be detected using a non-tracking inhibition task, such as the Go/No-Go task. An outstanding question is whether these facets of impulsive behavior (inhibitory control vs. commission errors) are differentially sensitive to adult ADHD pathology. Randomizing drug-naïve ADHD participants to psychostimulant medication or placebo treatment conditions and assessing changes in response inhibition, as measured both behaviorally and with fMRI, using both Stop-Signal and Go/No-Go tasks, would directly address these outstanding questions, and help to elucidate whether deficient response inhibition-related neural activation is an age-independent marker of ADHD pathology.
The results contribute to an understanding of neuroanatomical alterations present in adult ADHD. While deficits in response inhibition and stopping-related hypoactivation are widely reported in samples of children and adolescents with ADHD, the findings in samples of adult ADHD participants are inconsistent. We report no differences in Stop-Signal task performance or associated neural activation between a relatively large sample of adult ADHD participants and controls. However, exploratory follow-up analyses reveal that whether or not adult ADHD participants exhibit less activation in a subset of stopping-related regions, as compared with healthy controls, depends on current psychostimulant use. According to our follow-up tests, this effect of psychostimulant medication use was not solely driven by current symptom severity. Given the sample size, however, our results must be considered preliminary and follow-up studies are warranted. Our results help to explain some of the discrepancies in the literature and lend support to the notion that these deficits persist into adulthood in a subset of individuals with ADHD. Further research is needed to understand why those individuals currently taking psychostimulants show these persistent deficits.
Supplementary Material
Acknowledgments
We thank Angelica Bato and Eric Miller for their assistance with data collection. This work was supported by the Consortium for Neuropsychiatric Phenomics (NIH Roadmap for Medical Research grants UL1-DE019580 (Bilder, PI), RL1MH083268 (Freimer, PI), RL1MH083269 (Cannon, PI), RL1DA024853 (London, PI), and PL1MH083271 (Bilder, PI)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Altshuler has received past and potential future funding from Takeda Pharmaceuticals North America, Inc., and H. Lundbeck A/S (advisory board honoraria, October 2012); and Sunovion Pharmaceuticals Inc. (advisory board honoraria, Jan 2013). Dr. Ventura has received funding from Genentech, Inc., Janssen Scientific Affairs, LLC, and Brain Plasticity, Inc.; he has served as a consultant to Brain Plasticity, Inc. Dr. McGough receives grants or research support from NeuroSigma Inc., Shionogi Pharmaceuticals, and Supernus Pharmaceuticals; he serves as a consultant to Alexa Pharmaceuticals, Targacept, and Theravance; he has provided expert testimony for Shire Pharmaceuticals. These sources of support did not present a conflict of interest in the conduct of this study or presentation of results. Drs. Congdon, Mumford, Karlsgodt, Sabb, London, Cannon, Bilder, and Poldrack reported no biomedical financial interests of potential conflicts of interest.
References
- Aichert DS, Wostmann NM, Costa A, Macare C, Wenig JR, Moller HJ, Rubia K, Ettinger U. Associations between trait impulsivity and prepotent response inhibition. Journal of Clinical and Experimental Neuropsychology. 2012;34:1016–1032. doi: 10.1080/13803395.2012.706261. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Banich MT, Burgess GC, Depue BE, Ruzic L, Bidwell LC, Hitt-Laustsen S, Du YP, Willcutt EG. The neural basis of sustained and transient attentional control in young adults with ADHD. Neuropsychologia. 2009;47:3095–3104. doi: 10.1016/j.neuropsychologia.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 2005;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Ramos-Quiroga JA, Richarte V, Canals C, Bosch R, Rovira M, Soliva JC, Bulbena A, Tobena A, Casas M, Vilarroya O. Response inhibition and reward anticipation in medication-naive adults with attention-deficit/hyperactivity disorder: a within-subject case-control neuroimaging study. Human Brain Mapping. 2012;33:2350–2361. doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biological Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Frontiers in Psychology. 2012;3:37. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. American Journal of Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Riedel M, Pogarell O, Menzel-Zelnitschek F, Schwarz M, Reiser M, Moller HJ, Rubia K, Meindl T, Ettinger U. Methylphenidate effects on neural activity during response inhibition in healthy humans. Cerebral Cortex. 2012;23:1179–1189. doi: 10.1093/cercor/bhs107. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with attention deficit hyperactivity disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dibbets P, Evers L, Hurks P, Marchetta N, Jolles J. Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain and Cognition. 2009;70:73–83. doi: 10.1016/j.bandc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dillo W, Göke A, Prox-Vagedes V, Szycik GR, Roy M, Donnerstag F, Emrich HM, Ohlmeier MD. Neuronal correlates of ADHD in adults with evidence for compensation strategies--a functional MRI study with a Go/No-Go paradigm. German Medical Science. 2010;8 doi: 10.3205/000098. Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. Journal of Child Psychology and Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-IP) Biometrics Research, New York State Psychiatric Institute; New York: 2004. [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. Journal of Neuroscience. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman DW. ADHD in Adults: update for clinicians on diagnosis and assessment. Primary Psychiatry. 2009;16:38–47. [Google Scholar]
- Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neuroscience and Biobehavioral Reviews. 2012;36:2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Karch S, Thalmeier T, Lutz J, Cerovecki A, Opgen-Rhein M, Hock B, Leicht G, Hennig-Fast K, Meindl T, Riedel M, Mulert C, Pogarell O. Neural correlates (ERP/fMRI) of voluntary selection in adult ADHD patients. European Archives of Psychiatry and Clinical Neuroscience. 2010;260:427–440. doi: 10.1007/s00406-009-0089-y. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Green JG, Adler LA, Barkley RA, Chatterji S, Faraone SV, Finkelman M, Greenhill LL, Gruber MJ, Jewell M, Russo LJ, Sampson NA, Van Brunt DL. Structure and diagnosis of adult attention-deficit/hyperactivity disorder: analysis of expanded symptom criteria from the Adult ADHD Clinical Diagnostic Scale. Archives of General Psychiatry. 2010;67:1168–1178. doi: 10.1001/archgenpsychiatry.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra L, van der Meere JJ, Edwards JD, Kaplan BJ, Crawford S, Goodyear BG. Preliminary fMRI findings on the effects of event rate in adults with ADHD. Journal of Neural Transmission. 2010;117:655–662. doi: 10.1007/s00702-010-0374-y. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society. 2010;16:1064–1076. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychological Review. 1984;91:295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Moulton JL., 3rd Persistence of attention-deficit/hyperactivity disorder into adulthood: what have we learned from the prospective follow-up studies? Journal of Attention Disorders. 2003;7:93–100. doi: 10.1177/108705470300700203. [DOI] [PubMed] [Google Scholar]
- Mulligan RC, Knopik VS, Sweet LH, Fischer M, Seidenberg M, Rao SM. Neural correlates of inhibitory control in adult attention deficit/hyperactivity disorder: evidence from the Milwaukee longitudinal sample. Psychiatry Research: Neuroimaging. 2011;194:119–129. doi: 10.1016/j.pscychresns.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Krauel K, Hinrichs H, Fischer J, Malecki U, Schuetze H, Wolff S, Jansen O, Duezel E, Baving L. Methylphenidate does not improve interference control during a working memory task in young patients with attention-deficit hyperactivity disorder. Brain Research. 2011;1388:56–68. doi: 10.1016/j.brainres.2011.02.075. [DOI] [PubMed] [Google Scholar]
- Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.016. 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Smith AB, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naive boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011a;36:1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Mohammad AM, Taylor E, Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011b;70:255–262. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar R, Logan GD, Robaey P, Chen S, Ickowicz A, Barr C. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Krick CM, Retz W, Hengesch G, Retz-Junginger P, Reith W, Rosler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults – a functional magnetic resonance imaging (fMRI) study. Psychiatry Research: Neuroimaging. 2010;183:75–84. doi: 10.1016/j.pscychresns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Bedard AC, Clerkin SM, Ivanov I, Tang CY, Halperin JM, Newcorn JH. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2012;69:952–961. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PubMed] [Google Scholar]
- Schweren LJ, de Zeeuw P, Durston S. MR imaging of the effects of methylphenidate on brain structure and function in attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2013;23:1151–1164. doi: 10.1016/j.euroneuro.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Gerdes B, Feige B, Kloppel S, Lange T, Philipsen A, Tebartz van Elst L, Lieb K, Tuscher O. Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Research: Neuroimaging. 2012;202:132–141. doi: 10.1016/j.pscychresns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.