Abstract
The transposon Tam3 from Antirrhinum majus can transpose in a heterologous host (Nicotiana tabacum); thus the element is autonomous, probably encoding the specific information required for its own transposition. In transgenic tobacco Tam3 rapidly becomes methylated at its ends whilst adjacent flanking sequences remain hypomethylated. This methylation may account for our failure to detect Tam3 transposition in the progeny of transgenic tobacco. Treatment with the inhibitor of cytosine methylation, 5 aza-cytosine appeared to induce transposon related activity at a low level. In Antirrhinum methylation also appears to be associated with inactivation of Tam3 copies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Alleman M., Freeling M. The Mu transposable elements of maize: evidence for transposition and copy number regulation during development. Genetics. 1986 Jan;112(1):107–119. doi: 10.1093/genetics/112.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B., Schell J., Lörz H., Fedoroff N. Transposition of the maize controlling element "Activator" in tobacco. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4844–4848. doi: 10.1073/pnas.83.13.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Walbot V. DNA modification of a maize transposable element correlates with loss of activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1767–1771. doi: 10.1073/pnas.83.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V., Rivin C., Walbot V. Stable non-mutator stocks of maize have sequences homologous to the Mu1 transposable element. Genetics. 1986 Nov;114(3):1007–1021. doi: 10.1093/genetics/114.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Greenblatt I. M., Dellaporta S. L. Transposition of Ac from the P locus of maize into unreplicated chromosomal sites. Genetics. 1987 Sep;117(1):109–116. doi: 10.1093/genetics/117.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomet P. S., Wessler S., Dellaporta S. L. Inactivation of the maize transposable element Activator (Ac) is associated with its DNA modification. EMBO J. 1987 Feb;6(2):295–302. doi: 10.1002/j.1460-2075.1987.tb04753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
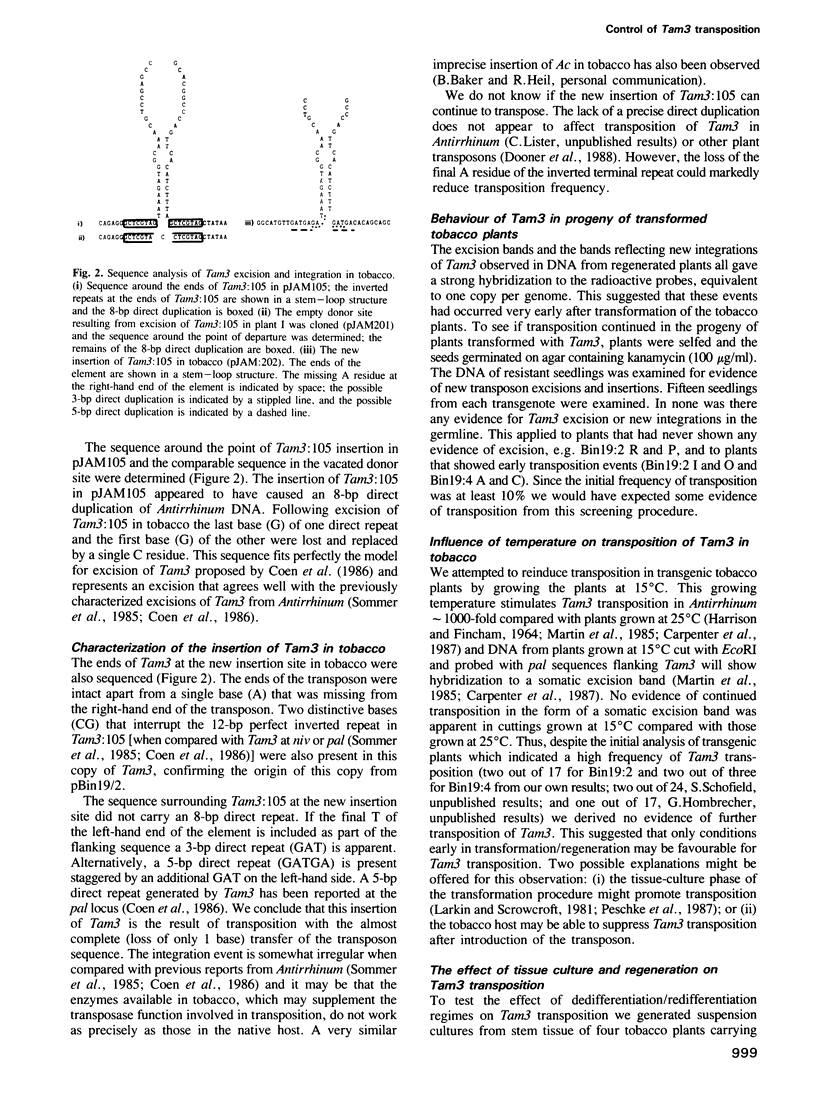
- Coen E. S., Carpenter R., Martin C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell. 1986 Oct 24;47(2):285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Hain R., Baker B., Wirtz U. Studies of the structure and functional organization of foreign DNA integrated into the genome of Nicotiana tabacum. DNA. 1986 Dec;5(6):473–482. doi: 10.1089/dna.1.1986.5.473. [DOI] [PubMed] [Google Scholar]
- Dooner H. K., English J., Ralston E. J. The frequency of transposition of the maize element Activator is not affected by an adjacent deletion. Mol Gen Genet. 1988 Mar;211(3):485–491. doi: 10.1007/BF00425705. [DOI] [PubMed] [Google Scholar]
- Hepburn A. G., White J., Pearson L., Maunders M. J., Clarke L. E., Prescott A. G., Blundy K. S. The use of pNJ5000 as an intermediate vector for the genetic manipulation of Agrobacterium Ti-plasmids. J Gen Microbiol. 1985 Nov;131(11):2961–2969. doi: 10.1099/00221287-131-11-2961. [DOI] [PubMed] [Google Scholar]
- Martin C., Carpenter R., Sommer H., Saedler H., Coen E. S. Molecular analysis of instability in flower pigmentation of Antirrhinum majus, following isolation of the pallida locus by transposon tagging. EMBO J. 1985 Jul;4(7):1625–1630. doi: 10.1002/j.1460-2075.1985.tb03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Mackay S., Carpenter R. Large-scale chromosomal restructuring is induced by the transposable element tam3 at the nivea locus of antirrhinum majus. Genetics. 1988 May;119(1):171–184. doi: 10.1093/genetics/119.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P., Surosky R., Kingsbury J. A., Fedoroff N. V. Genetic and molecular analysis of the Spm-dependent a-m2 alleles of the maize a locus. Genetics. 1987 Sep;117(1):117–137. doi: 10.1093/genetics/117.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCLINTOCK B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci U S A. 1950 Jun;36(6):344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer M. G. Stability of the suppressor element in two mutator systems at the a(1) locus in maize. Genetics. 1966 Mar;53(3):541–549. doi: 10.1093/genetics/53.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G., Bakker A., Molendijk L., Wullems G. J., Gordon M. P., Nester E. W., Schilperoort R. A. T-DNA organization in homogeneous and heterogeneous octopine-type crown gall tissues of Nicotiana tabacum. Cell. 1982 Sep;30(2):589–597. doi: 10.1016/0092-8674(82)90255-0. [DOI] [PubMed] [Google Scholar]
- Peschke V. M., Phillips R. L., Gengenbach B. G. Discovery of transposable element activity among progeny of tissue culture--derived maize plants. Science. 1987 Nov 6;238(4828):804–807. doi: 10.1126/science.238.4828.804. [DOI] [PubMed] [Google Scholar]
- Peterson P. A. Phase variation of regulatory elements in maize. Genetics. 1966 Jul;54(1):249–266. doi: 10.1093/genetics/54.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. W., Raboy V., Fedoroff N. V., Nelson O. E., Jr Deletions within a defective suppressor-mutator element in maize affect the frequency and developmental timing of its excision from the bronze locus. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4783–4787. doi: 10.1073/pnas.82.14.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Berndtgen R., Saedler H. Sequence comparison of 'states' of a1-m1 suggests a model of Spm (En) action. EMBO J. 1985 Oct;4(10):2439–2443. doi: 10.1002/j.1460-2075.1985.tb03953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Lijsebettens M., Inzé D., Schell J., Van Montagu M. Transformed cell clones as a tool to study T-DNA integration mediated by Agrobacterium tumefaciens. J Mol Biol. 1986 Mar 20;188(2):129–145. doi: 10.1016/0022-2836(86)90299-8. [DOI] [PubMed] [Google Scholar]
- Van Sluys M. A., Tempé J., Fedoroff N. Studies on the introduction and mobility of the maize Activator element in Arabidopsis thaliana and Daucus carota. EMBO J. 1987 Dec 20;6(13):3881–3889. doi: 10.1002/j.1460-2075.1987.tb02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot V. Inheritance of mutator activity in Zea mays as assayed by somatic instability of the bz2-mu1 allele. Genetics. 1986 Dec;114(4):1293–1312. doi: 10.1093/genetics/114.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walbot V., Warren C. Regulation of Mu element copy number in maize lines with an active or inactive Mutator transposable element system. Mol Gen Genet. 1988 Jan;211(1):27–34. doi: 10.1007/BF00338389. [DOI] [PubMed] [Google Scholar]