Abstract
Encapsulation of therapeutic agents in polymer particles has been successfully used in the development of new drug carriers. A number of design parameters that govern the functional behavior of carriers, including the choice of polymer, particle size and surface chemistry, have been tuned to optimize their performance in vivo. However, particle shape, which may also have a strong impact on carrier performance, has not been investigated. This is perhaps due to the limited availability of techniques to produce non-spherical polymer particles. In recent years, a number of reports have emerged to directly address this bottleneck and initial studies have indeed confirmed that particle shape can significantly impact the performance of polymer drug carriers. This article provides a review of this field with respect to methods of particle preparation and the role of particle shape in drug delivery.
Keywords: Nanoparticle, Nanotechnology, Morphology, Drug Delivery, Biomaterials
Introduction
Encapsulation of therapeutic agents into polymeric particulate carriers provides several benefits over traditional formulations [1]. Prior to release, the polymer protects the drug from degradation or premature metabolism. Release of the therapeutic agent is sustained over days to months, thereby maintaining plasma drug concentrations at therapeutic levels for longer periods of time. This reduces the frequency of administration and increases patient compliance. Polymeric particles are versatile and can be used to deliver drugs via intravascular, subcutaneous, pulmonary and oral routes, each with different design requirements.
Polymer drug carriers have been made from a variety of biodegradable polymers [2, 3]. Poly(esters) are among the most studied and best characterized of the biodegradable polymers. They include poly(lactic acid) (PLA), poly(glycolic acid) (PGA), their copolymers poly(lactic acid-co-glycolic acid) (PLGA) and poly(ε-caprolactone) [2]. Other biodegradable polymers include poly(orthoesters), poly(anhydrides), poly(amides), poly(phosphazenes) and poly(phosphoesters), as well as their various copolymers [2]. Copolymers of these materials with poly(ethylene glycol) (PEG), poly(ethylene oxide) (PEO) or poly(propylene oxide) (PPO) have also been used to make drug delivery particles [2, 3]. The choice of polymer(s) impacts several aspects of the carrier including the types of drug or protein that can be encapsulated, the mode of degradation and drug release, biocompatibility and physical properties [3]. A variety of methods including single and double emulsification [4, 5], spray drying [6] and drop break-up of a liquid stream [7] have been used to produce spherical polymer particles. Collectively, these methods offer control over basic parameters such as particle diameter, encapsulation efficiency and polydispersity, as well as advanced parameters such as porosity and drug compartmentalization [8, 9].
In addition to polymer chemistry, other important particle parameters include surface chemistry, size and shape. Surface chemistry primarily influences the interactions of particles with cells and tissues in the body. Towards that end, significant attention has been paid to chemical modifications of the particle surface so as to minimize recognition by the components of the immune system [10]. The most commonly used strategy involves modification of the surface with a hydrophilic polymer brush to reduce opsonization, the adsorption of antibodies and complement on the particle surface and the first step in recognition by the immune system. Localization of hydrophilic polymers on the particle surface has been achieved either by physical or chemical adsorption or by incorporation of the stealth polymer into the carrier at the polymer synthesis stage [11]. For example, di-block co-polymers of PLGA with PEG have been used to fabricate particles enriched with hydrophilic PEG at the surface and hydrophobic PLGA at the core [12]. Surface modification has also been actively used to target carriers to specific tissues. A plethora of targeting ligands including small molecules, antibodies, carbohydrates and peptides, have been used to target various tissues including brain, liver and tumors [13–15].
The impact of particle size on carrier function has also been actively investigated. Particle diameter has been controlled through the physical properties of the materials, such as polymer and surfactant concentration, or through the experimental parameters of the fabrication method, for example mixing method (vortexing, sonication, stirring) and speed, nozzle/capillary diameter and material flow rate [4–7]. Size influences almost every aspect of particle function including degradation, flow properties, clearance and uptake mechanisms [10, 16–23]. Degradation of particles is size dependent, though experiments disagree on the relationship between initial degradation rate and size [16, 17]. The conflicting results arise from two competing effects of size. Large particle size reduces surface area available for water penetration. At the same time, it also reduces clearance of degradation products from the particle, which catalyzes further degradation. The effect of particle size on transport in vivo may seem obvious, but nonetheless is crucial to administration, circulation and destination of particles. Diameter of particles administered in blood vessels, airways or gastro-intestinal tract dictates their velocity, diffusion and adhesion to walls [18–20]. Movement of particles in tissues, whether arriving by migration or injection, is also limited by size due to steric hindrance in the extracellular matrix. The pathway of particle migration in the body directly impacts the final destination, whether it be internalized in tumor cells or cleared from the body by macrophages in the liver. In the vascular compartment, there exist a variety of size-based clearance mechanisms. Microparticles (~1–5 µm) typically are trapped in the liver and phagocytosed by Kupffer cells, whereas larger microparticles are typically trapped in the capillary beds [22, 23]. Nanoparticles larger than 200 nm are mechanically filtered in the spleen while those smaller than 100 nm leave the blood vessels through fenestrations in the endothelial lining [10, 23]. For pulmonary administration, 3 µm particles deposit deep in the alveolar region while larger particles accumulate in the upper airways and smaller particles are exhaled [8]. Regardless of the method of administration, particles larger than 500 nm can be phagocytosed by macrophages and smaller particles can be endocytosed by phagocytic or non-phagocytic cells [24, 25]. Internalization by targeted non-phagocytic cells is desirable for localized delivery, but phagocytosis or uptake by macrophages clears particles from the body and is usually undesirable. Size, along with surface chemistry, is also believed to affect opsonization based on the relationship between particle size and curvature for spheres [21].
In contrast to significant research focused on investigating the effect of particle size and surface chemistry on in vivo performance, there is a striking lack of research on understanding the effect of particle shape. Recently, this void has become apparent and inspired new research in the fields of drug delivery and material science. This document describes fabrication methods of non-spherical polymer particles and recent results that demonstrate the significance of particle shape in drug delivery.
Methods of Fabricating Non-Spherical Polymeric Particles
As with many biomaterials, several methods for producing non-spherical particles were inspired by applications outside drug delivery. These methods can be classified into two main groups; ab initio synthesis of non-spherical particles or manipulation of previously fabricated spherical particles into non-spherical geometries. Often the ab initio synthesis routes are more difficult but produce a broader range of shapes. Spherical manipulation techniques, on the other hand, can be simpler but usually cannot produce as diverse a range of shapes.
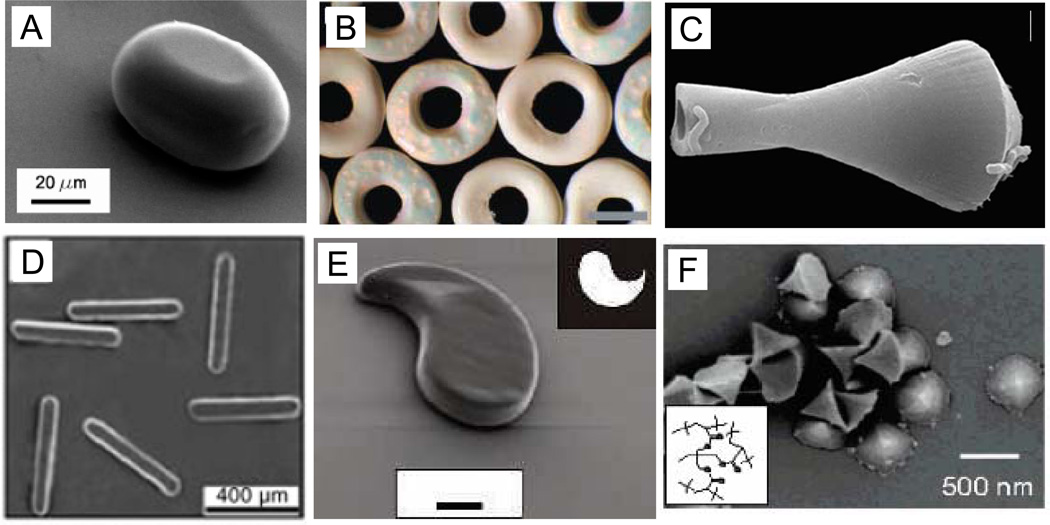
Synthetic methods for generating non-spherical particles make use of techniques such as lithography [26–29], microfluidics [26–28] and photopolymerization [26–28], often in combination (Figure 1). Dendukuri et al. combined microscope projection photolithography and microfluidics to continuously form PEG and poly(acrylate) particles with various morphologies [28]. Rolland et al. employ conventional soft lithographic molding methods, but were able to create isolated PEG, PLA, and poly(pyrrole) particles of various shapes by using a non-wetting PFPE mold instead of traditional PDMS molds [29]. Xu et al. and Dendukuri et al. formed liquid droplets with a microfluidic device, shaped the droplets in a microchannel and polymerized them to form solid particles of several non-spherical geometries [26, 27]. In a departure from the previous methods, Sozzani et al. developed a direct replica method that creates poly(styrene) (PS) and poly(methylmethacrylate) (PMMA) particles of various shapes from mesoporous silica shapes [30]. Velev et al. produce disks and toroids by self-assembly of latex particles in aqueous solutions suspended on fluorinated oil [31].
Figure 1.
(A) Plug-shaped particle made of optical adhesive polymer by Dendukuri et al. with microfluidics [27]. (B) Toroidal PS particles prepared via self-assembly by Velev et al. (scale 500 µm) [31]. (C) PS vase-shaped particle made Sozzani et al. by direct replication (scale 1 µm) [30]. (D) PolyTPGDA rods made by Xu et al. with microfluidics [26]. (E) Curved PEG particle made by Dendukuri et al. via microscope projection photolithography (scale 10 µm) [28]. (F) Conical PEG particles made by Rolland et al. with a non-wetting mold [29].
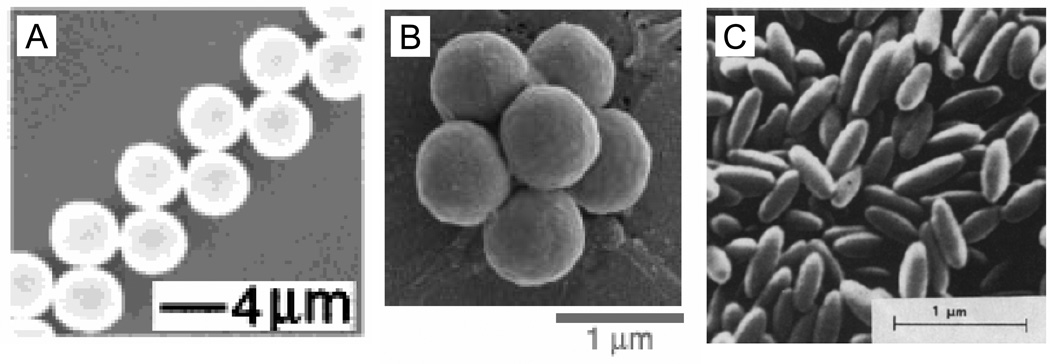
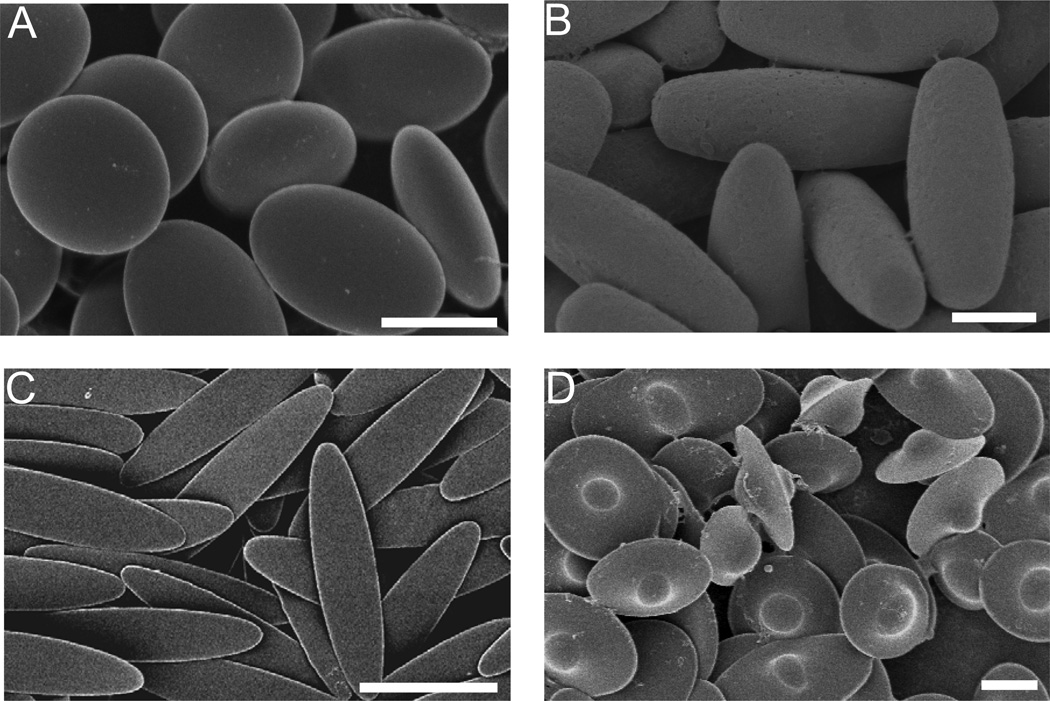
The second type of fabrication technique uses spherical particles as starting materials and manipulates them into different morphologies (Figure 2). Manoharan et al. utilized self-assembly of PS spheres on the surface of an emulsion droplet to form clusters of spheres containing 2 to 15 particles [32]. Yin et al. use template-assisted self-assembly to form clusters and chains of PS spheres by trapping spheres in molded cavities of various shapes and sizes [33]. Alternately, Ho et al. stretch spherical PS particles that have been embedded in a polymer film to create ellipsoidal particles [34]. We have shown that simple, yet significant, modifications of the stretching protocol can produce over twenty different three dimensional shapes using spheres as small as 200 nm, four of which are shown in Figure 3 [35]. In this method, PS spheres are embedded in a poly(vinyl alcohol) (PVA) film and liquefied with heat or solvent. The film is then stretched, which in turn stretches the particles due to hydrogen bonding-mediated adhesion between the particles and film. Diversity of shapes originates from adjusting parameters such as the aspect ratio of stretching, the method of liquefaction, the thickness of the film, and stretching the film prior to particle liquefaction.
Figure 2.
(A) Double layered zigzag chain made by Yin et al. with 4.3 µm PS beads by template-assisted self-assembly [33]. (B) Pentagonal bipyramid containing seven 844 nm PS particles self-assembled by Manoharan et al. [32]. (C) Ellipsoidal PS particles stretched by Ho et al. from 208 nm spheres [34].
Figure 3.
Scanning electron micrographs of polystyrene particles created by stretching method: (A) oblate ellipsoids, (B) prolate ellipsoids, (C) elliptical disks and (D) UFOs [35]. Scale bars = 5 µm.
Each fabrication method discussed here possesses unique advantages and limitations. The primary limitation is in the types of shapes each method can produce. Microfluidic methods are suitable for generating two dimensional shapes and are limited by microchannel geometry. Further, these methods have been used to fabricate particles in the size range of 10–1000 µm. Generation of features smaller than 10 µm has yet to be demonstrated. Self-assembly methods rely on shape formation through controlled self-assembly and cannot be engineered as precisely as in the case of other methods. However, since the starting spherical particles can be easily fabricated over a wide size range, micro to nanoscale, non-spherical particles of many sizes can be easily fabricated using this method. Projection photolithography techniques are able to generate a broad array of shapes, dictated by the mask used. However, the limits are that shapes are two dimensional and the smallest feature size is 10 µm, although the thickness of the particles can be independently controlled. Non-wetting molding produces a variety of shapes in two and three dimensions as small as 200 nm. The film-stretching method produces a variety of two and three dimensional shapes from spheres ranging in diameter from 200 nm to 10 µm. Of the methods discussed here, direct replica, self-assembly and film-stretching do not require specialized equipment whereas several of the other methods require soft lithography to make templates, stamps or microfluidics. Projection photolithography also requires a specialized microscope set-up.
In order for any method to be used for producing drug carriers, a variety of requirements must be met. First, the method must be able to accommodate drug delivery polymers such as PLGA, PEG and others mentioned in the introduction. In that regard, non-wetting molding and projection lithography methods have demonstrated use of biodegradable polymers and several of the other methods could theoretically be adapted for biodegradable polymers [27–29]. In our own experience, PLGA particles of various shapes can be prepared by the film stretching method with no major changes from the protocol used to make PS particles. Like PS, PLGA is a hydrophobic polymer and PVA should also exhibit hydrogen-bonding with PLGA. However, the glass transition temperature of PLGA is much lower, 40–60°C depending on the ratio of lactic to glycolic acids, as compared to 90°C for PS. PLGA spheres were prepared by single emulsion solvent evaporation method. 20% v/v PLGA (75:25, Sigma Chemical), dissolved in dichloromethane (50 mg/ml), was emulsified in 1% PVA solution by sonication at 18 W for 1 minute. The resulting emulsion was stirred overnight to evaporate dichloromethane. The particles were collected and embedded in films consisting of 5% PVA (PVA 40–48, Fluka, degree of hydrolysis 86.7%–88.7%) and 2% glycerol. Films were submersed in toluene for 4 hours, dichloromethane for 2 hours, or 85°C oil for 5 minutes and then stretched 2 to 4 times the initial length in one or two directions. The films were dried overnight, incubated with isopropanol to remove residual toluene and dissolved in water at room temperature for 3 hours. Particles were collected by centrifugation at 10,000 rpm for 15 minutes and washed with ice cold water 10 times. To verify particle morphology, particles were coated with palladium (Hummer 6.2 Sputtering System, Anatech Ltd., Union City, CA) and imaged with the Sirion 400 Scanning Electron Microscope (FEI Company, Hillsboro, OR) at 5 eV. Figure 4 shows PLGA elliptical disks made with this method.
Figure 4.
Scanning electron micrograph of poly(lactic acid-co-glycolic acid) elliptical disks created by film stretching method. Polydispersity in size is due to polydispersity in original spherical particles. Scale bar = 1 µm.
Another requirement for particle fabrication methods is that the method must allow for incorporation of therapeutic drug molecules and not require any conditions which could inactivate the drug molecules. Rolland et al. have encapsulated DNA, protein and therapeutic small molecules in non-spherical particles with non-wetting molding [29]. In the film stretching method, therapeutic agents are encapsulated in particles prior to stretching. Therapeutic molecules are incorporated in either organic or aqueous phases, depending upon their solubility and stability, during the sphere fabrication process using current technology. As an example, 66 kDa FITC-labeled bovine serum albumin (BSA) was encapsulated in PLGA by the double emulsion solvent evaporation method. 2.4% v/v of FITC-labeled BSA, in 50 mM PBS (50 mg/ml), was emulsified in a solution of PLGA in dichloromethane (50mg/ml) by sonication at 18W for 1 minute. The secondary emulsion was prepared by one minute of sonication of 25% v/v of the primary emulsion with 1% PVA solution. The final emulsion was stirred overnight, particles were collected, centrifuged, embedded in the film and stretched as described above. No leaching of BSA occurred during the stretching process due to its insolubility in the solvents. However, if the encapsulated material is soluble and is small enough to diffuse through the PVA film, leaching may occur. To prevent leaching, encapsulated material could be dissolved in solvent to eliminate the concentration gradient, or stretched films may be exposed to a highly concentrated solution of the encapsulated material in solvent. In order to stabilize encapsulated proteins, co-encapsulation of amphiphilic molecules, which are preferentially adsorbed on the aqueous organic interface while shielding vulnerable therapeutic protein molecules, may be employed. Since some shapes are formed using solvent and others using heat, shape selection should also take into account the method of liquefaction. In the case of heat, the glass transition temperature of PLGA is relatively low and it can be controlled from 40–60°C by altering the ratio of lactic to glycolic acids.
The shape fabrication method should also not inhibit post-production processing, such as tethering of targeting ligands or stealth moieties to the particle surface. This has been verified with the BSA-encapsulated PLGA ellipses. After shape fabrication, fluorescent IgG was physically adsorbed on the surface of the particles and confirmed with fluorescence microscopy. The final requirement for non-spherical drug delivery particle production is scalability for high throughput production for experimental and therapeutic needs. For bench-scale production, non-continuous methods have produced more particles to date than continuous methods; self-assembly of spheres has produced 108–1010 per batch of clusters containing 2–5 particles [32], template-assisted self-assembly has created 105 per template [33] and the film-stretching method has made 108–1010 particles per stretching apparatus, depending on size. The batch methods can be scaled up simply by increasing the amount of starting materials or the size or number of molds/templates/films. For example, throughput of the stretching method can be dramatically increased by utilizing thicker films or by using a stack of films at a single time. Projection lithography and microfluidics have produced 100 and 250 particles per second, respectively, which translates to 106–107 per day if continuous 24 hour operation is possible [26, 28].
Role of Particle Shape in Drug Delivery
The precise role of particle shape in drug delivery has not been fully elucidated, most likely due to the lack of easy-to-use methods available to control particle shape. Certainly, shape, along with size and chemistry, is a critical feature of drug delivery particles. Particle size, measured simply by diameter for spheres, must be redefined since non-spherical particles may have two or more different length scales. Depending on the orientation of the particle, one length scale may dominate the others.
There is already evidence that the most basic function of particles, degradation to release therapeutic drug, will depend on particle shape [36]. Zero-order release, the goal of many sustained release devices, was achieved with a hemi-spherical particle that only allowed degradation on the face. The particles, however, were of millimeter size and therefore not viable for most in vivo applications. Spherical degradation demonstrated the significance of surface area and diameter [16, 17], which will also be dictated by shape. Non-spherical particles that have areas of different thicknesses could offer unique degradation profiles, as the shape of the particle will change over time.
Transport of particles in the body, regardless of the mode of administration, will be affected by particle shape. Just as diameter dictates particle velocity, diffusion and adhesion to walls in blood vessels, airways and intestine, shape will also affect these properties but in more complex ways. Movement of spheres is easier to predict due to their inherent symmetry, but non-spherical particles may align or tumble in the presence of flow. Alignment or tumbling issues will be compounded when particles flow through filtering organs, such as the liver or spleen, or when bifurcations in the vessels are encountered. For example, filtering units in the spleen are described as slits, implying that they are asymmetric [21]. Spherical particles must be less than 200 nm in diameter to pass through the spleen, but disk-shaped, flexible red blood cells with diameters of ~10 µm routinely pass through the spleen. This indicates that shape, orientation and mechanical stiffness are also important, in addition to size. The same could be true when particles move through the tortuous pathways in liver or through the extracellular space in tissues. Particle extravasation through fenestrations between the endothelial cells, which is predominant in leaky tumor vasculature [21], will also depend on the flow properties of particles, especially orientation and contact with vessel walls, which should be significantly affected by shape.
Shape of particles will also influence their targeting ability. Not only is the overall surface area available for targeting ligands important, but the local curvature also affects ligand and opsonin adsorption and the degree to which particles fit the contours of target cell membranes. Once attached, shear induced by blood flow on the protruding particle could detach it from the desired location. Particle shape, in particular the profile extending away from the cell into the flow, will determine the longevity of the targeted attachment. Internalization of targeted particles, whether intended or undesired, could also be dictated by particle shape. Due to the limits on size of particle uptake by non-phagocytic cells, particle orientation could prove to be important. Particle shape could affect the cells’ ability not only to internalize successfully, but also the transport and sorting of the particle once inside the cell. Internalized particles are encapsulated in intracellular vesicles (endosomes, lysosomes or phagosomes) and are actively transported along microtubules and actin filaments to be processed inside the cell [37]. It is not clear whether these vesicles will take on the shape of the particle. If they do, shape will have an intriguing effect on intracellular particle trafficking.
Unlike targeted uptake, macrophage phagocytosis, the actin-dependent uptake of particles by immune cells, is usually undesirable. Phagocytosis is a critical interaction between particles and phagocytic immune cells. Internalization of particles by macrophages prevents delivery of drugs to required tissues and is one of the primary obstacles of particulate drug delivery. Phagocytosis is an actin-dependent process that internalizes foreign particles of diameters of 500 nm and larger [25]. Incubation of PS shapes with rat alveolar macrophages revealed a crucial, but unexpected role of shape in phagocytosis. The local shape of the particle at the point where the cell attached, not the overall shape, dictated whether or not a macrophage began internalization [35]. For example, a macrophage attached to an ellipse at the pointed end internalized it in a few minutes while a macrophage attached to a flat region of the same ellipse did not internalize the particle for over 12 hours (Figure 5). Spherical particles were internalized from any point of attachment, due to their symmetry. Polymerization of actin into coordinated structures is necessary to push the membrane around the particle to begin and complete phagocytosis. These structures were not present when cells attached to a particle in an orientation that could not be phagocytosed. These results were independent of particle size over particle volumes ranging from 0.1% to 100% of the volume of a macrophage. Particle size only played a role in completion of phagocytosis if the particle volume was larger than the cell volume.
Figure 5.
Colored scanning electron micrographs of alveolar macrophages (brown) interacting with PS particles (purple). (A) The cell body can be seen at the end of an opsonized elliptical disk and the membrane has progressed down the length of the particle. Scale bar = 10 µm. (B) A cell has attached to the flat side of an opsonized elliptical disk and has spread on the particle. Scale bar = 5 µm. (C) An opsonized spherical particle has attached to the top of a cell and the membrane has progressed over approximately half the particle. Scale bar = 5 µm.
Summary and Future Directions
The results and overview presented here leave no doubt that shape is a critical parameter for drug delivery particles. The successful fabrication of non-spherical, biodegradable drug delivery particles eliminates the primary obstacle in determining the actual role of shape in every aspect of particle performance. With the shapes that are now available, effort can be focused on uncovering the effect of shape in degradation, transport, targeting, internalization and possibly other areas of drug delivery. Though each of these areas can be studied separately, ultimately optimization must be performed to identify the shape or shapes that give the best performance in all categories. The effect of shape on biological interactions is not as clear as physical behavior (degradation, flow), as evidenced by the intriguing dependence of phagocytosis on shape. These observations demonstrate the complexity of cell-particle interactions and reveal the ability of cells to sense, identify and respond to particle shape. The fabrication methods described here create a group of particles where important properties such as volume, shape, dimensions, surface area, curvature and surface chemistry can be systematically and independently varied. This is critical in determining exactly what aspect of shape influences particle behavior and also the interplay between shape, size and surface chemistry. Currently available non-spherical particles can not only be used as tools in determining the role of shape, but also as guides in the design of even more new shapes that exhibit the best performance for a given application. The most exciting conclusion of this work is the reality that shape is now a useful tool in the toolbox for effective drug delivery particle design. Current drug delivery literature is already beginning to reflect this growing realization of the importance of shape even though its many roles have not yet been uncovered [29, 38]. In studying the effect of shape on certain aspects of drug delivery, such as splenic filtration, other important particle properties have come to light. Mechanical properties, such as stiffness or flexibility, seem to be important as red blood cells pass unhindered through the spleen despite their large size. Beningo et al. have revealed that particle stiffness affects phagocytosis and macrophages are less able to internalize soft particles [39]. These results offer yet another tool to further expand the possibilities for drug delivery particle design. There is obviously a great deal of work to be done in this field and contributions from materials, engineering, biology, immunology, anatomy, pharmaceutics and medicine will all be required.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R. New Methods of Drug Delivery. Science. 1990;249(4976):1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 2.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem. Rev. 1999;99(11):3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 3.Pillai O, Panchagnula R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001;5(4):447–451. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 4.Park TG, Lee HY, Nam YS. A new preparation method for protein loaded poly(D,L-lactic-co-glycolic acid) microspheres and protein release mechanism study. J. Control. Release. 1998;55(2–3):181–191. doi: 10.1016/s0168-3659(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 5.Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E, Alonso MJ, Labrude P, Vigneron C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Release. 1998;50(1–3):31–40. doi: 10.1016/s0168-3659(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 6.Wang FJ, Wang CH. Sustained release of etanidazole from spray dried microspheres prepared by non-halogenated solvents. J. Control. Release. 2002;81(3):263–280. doi: 10.1016/s0168-3659(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 7.Berkland C, Kim KK, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J. Control. Release. 2001;73(1):59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 8.Edwards DA, Hanes J, Caponetti G, Hrkach J, BenJebria A, Eskew ML, Mintzes J, Deaver D, Lotan N, Langer R. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868–1871. doi: 10.1126/science.276.5320.1868. [DOI] [PubMed] [Google Scholar]
- 9.Roh KH, Martin DC, Lahann J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 2005;4(10):759–763. doi: 10.1038/nmat1486. [DOI] [PubMed] [Google Scholar]
- 10.Stolnik S, Illum L, Davis SS. Long Circulating Microparticulate Drug Carriers. Adv. Drug Deliv. Rev. 1995;16(2–3):195–214. [Google Scholar]
- 11.Storm G, Belliot SO, Daemen T, Lasic DD. Surface Modification of Nanoparticles to Oppose Uptake by the Mononuclear Phagocyte System. Adv. Drug Deliv. Rev. 1995;17(1):31–48. [Google Scholar]
- 12.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable Long-Circulating Polymeric Nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 13.Poste G, Kirsh R. Site-Specific (Targeted) Drug Delivery in Cancer-Therapy. Bio-Technology. 1983;1(10):869–878. [Google Scholar]
- 14.Reddy GR, Bhojani MS, McConville P, Moody J, Moffat BA, Hall DE, Kim G, Koo YEL, Woolliscroft MJ, Sugai JV, Johnson TD, Philbert MA, Kopelman R, Rehemtulla A, Ross BD. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006;12(22):6677–6686. doi: 10.1158/1078-0432.CCR-06-0946. [DOI] [PubMed] [Google Scholar]
- 15.Liang HF, Chen CT, Chen SC, Kulkarni AR, Chiu YL, Chen MC, Sung HW. Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials. 2006;27(9):2051–2059. doi: 10.1016/j.biomaterials.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Panyam J, Dali MA, Sahoo SK, Ma WX, Chakravarthi SS, Amidon GL, Levy RJ, Labhasetwar V. Polymer degradation and in vitro release of a model protein from poly(D,L-lactide-co-glycolide) nano- and microparticles. J. Control. Release. 2003;92(1–2):173–187. doi: 10.1016/s0168-3659(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 17.Dunne M, Corrigan OI, Ramtoola Z. Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials. 2000;21(16):1659–1668. doi: 10.1016/s0142-9612(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 18.Goldsmith HL, Turitto VT. Rheological Aspects of Thrombosis and Hemostasis - Basic Principles and Applications - Icth-Report - Subcommittee on Rheology of the International Committee on Thrombosis and Hemostasis. Thromb. Haemost. 1986;55(3):415–435. [PubMed] [Google Scholar]
- 19.Patil VRS, Campbell CJ, Yun YH, Slack SM, Goetz DJ. Particle Diameter Influences Adhesion under Flow. Biophys. J. 2001;80(4):1733–1743. doi: 10.1016/s0006-3495(01)76144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18(6):788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 21.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharm. Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 22.Illum L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood Clearance and Organ Deposition of Intravenously Administered Colloidal Particles - the Effects of Particle-Size, Nature and Shape. Int. J. Pharm. 1982;12(2–3):135–146. [Google Scholar]
- 23.Tabata Y, Ikada Y. Phagocytosis of Polymer Microspheres by Macrophages. Adv. Polym. Sci. 1990;94:107–141. [Google Scholar]
- 24.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J. Cell Sci. 2001;114(6):1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- 26.Xu SQ, Nie ZH, Seo M, Lewis P, Kumacheva E, Stone HA, Garstecki P, Weibel DB, Gitlin I, Whitesides GM. Generation of monodisperse particles by using microfluidics: Control over size, shape, and composition. Angew. Chem. Int. Ed. Engl. 2005;44(5):724–728. doi: 10.1002/anie.200462226. [DOI] [PubMed] [Google Scholar]
- 27.Dendukuri D, Tsoi K, Hatton TA, Doyle PS. Controlled synthesis of nonspherical microparticles using microfluidics. Langmuir. 2005;21(6):2113–2116. doi: 10.1021/la047368k. [DOI] [PubMed] [Google Scholar]
- 28.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater. 2006;5(5):365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 29.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 30.Sozzani P, Bracco S, Comotti A, Simonutti R, Valsesia P, Sakamoto Y, Terasaki O. Complete shape retention in the transformation of silica to polymer micro-objects. Nat. Mater. 2006;5(7):545–551. doi: 10.1038/nmat1659. [DOI] [PubMed] [Google Scholar]
- 31.Velev OD, Lenhoff AM, Kaler EW. A class of microstructured particles through colloidal crystallization. Science. 2000;287(5461):2240–2243. doi: 10.1126/science.287.5461.2240. [DOI] [PubMed] [Google Scholar]
- 32.Manoharan VN, Elsesser MT, Pine DJ. Dense packing and symmetry in small clusters of microspheres. Science. 2003;301(5632):483–487. doi: 10.1126/science.1086189. [DOI] [PubMed] [Google Scholar]
- 33.Yin YD, Xia YN. Self-assembly of monodispersed spherical colloids into complex aggregates with well-defined sizes, shapes, and structures. Adv. Mater. 2001;13(4):267–271. doi: 10.1021/ja011048v. [DOI] [PubMed] [Google Scholar]
- 34.Ho CC, Keller A, Odell JA, Ottewill RH. Preparation of Monodisperse Ellipsoidal Polystyrene Particles. Colloid Polym. Sci. 1993;271(5):469–479. [Google Scholar]
- 35.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh DST, Rhine WD, Langer R. Zero-Order Controlled-Release Polymer Matrices for Micromolecules and Macromolecules. J. Pharm. Sci. 1983;72(1):17–22. doi: 10.1002/jps.2600720105. [DOI] [PubMed] [Google Scholar]
- 37.Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr. Opin. Cell Biol. 2000;12(1):63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 38.Dalhaimer P, Engler AJ, Parthasarathy R, Discher DE. Targeted worm micelles. Biomacromolecules. 2004;5(5):1714–1719. doi: 10.1021/bm049884v. [DOI] [PubMed] [Google Scholar]
- 39.Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 2002;115(4):849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]