Abstract
Purpose
The aim of this study was to compare the effect of resistance exercise with three different methods on integrated electromyography (IEMG) and metabolic responses in recreational athletes.
Methods
Twenty four males (mean 23.59±0.87 years) were randomly assigned to three experimental groups. Participants performed knee extension exercises: Slow (SL: 3-3, 3s for each concentric and eccentric action with 50% of 1 RM), Normal (NH: 1-1, 1 s for each concentric and eccentric action 80% of 1 RM) and Traditional (TH: 2-4, 2s for concentric and 4s for eccentric action with 80% of 1 RM). Plasma lactate, glucose and triglyceride concentration and IEMG was measured before and immediately after performing four sets of resistance exercise.
Results
Each method significantly decreased IEMG (P<0.05), but there was no significant difference between groups. Lactate was increased following TH and NH more than SL method (P<0.05). Each method significantly increased plasma glucose (P<0.05). Work considering time under tension (workTUT) was higher (P<0.05) during TH method than the other methods and during SL it was higher than NH method (P<0.05). Volume load was higher (P<0.05) during NH than the other two methods and during TH it was higher than SL method (P<0.05).
Conclusion
These results indicate that exercise intensity during the resistance exercise is important for the enhancement of lactate responses, but the slow resistance exercise method could induce acute neuromuscular response as much as high intensity methods. It seems that this method will be advantageous for those who want to increase acute neuromuscular changes with low exercise intensity and volume.
Keywords: Lactate, Recruitment, Motor Unit, Resistance Exercise, Metabolic Stress Response
INTRODUCTION
Although resistance training is recognized beneficial for health and athlete performance, the most appropriate method is still not defined [1–3]. Resistance training programs are specific to training goals of individuals and their daily physical demands [3–5]. A resistance training program can be described with many acute training variables such as training intensity, volume, repetition numbers, rest intervals, contraction speed and other variables [1–6].
It is regarded that to achieve optimal muscle hypertrophy and strength, exercise intensity should be middle to high (≥65% of 1 RM) [7–9], these authors suggested that mechanical stimuli are essential and represent the major determinant of resistance training adaptation [3, 8]. For this reason, some middle-high intensity resistance exercises like traditional and normal methods with different concentric and eccentric duration time have been introduced [9–11]. In contrast, some researchers have claimed metabolic and hormonal changes are important for hypertrophy and strength gain as well [9–14]. For this claim, several resistance training methods have been developed like slow, super slow and modified vascular occlusion to compare metabolic and hormonal changes [9–12, 15, 16]. The similarity between these methods is the training intensity being low to middle (20-50% of 1 RM).
Recent studies indicate that low intensity resistance exercise induces muscle hypertrophy and strength when exercise combined with partial vascular occlusion or performed at slow velocity. Slow training methods are more practical in the weight room because neither tourniquet nor monitoring is required [9, 10, 12, 14]. [Using tourniquet or garrote during resistance exercises is not usual particularly in study. But, in clubs could increase artificial ischemia and caus more anabolic and metabolic responses. In this regard, scientists reported that these responses are essential for hypertrophy.] Goto et al compared hormonal and metabolic changes between low intensity with slow method (SL, 50% 1 RM, 3s for each concentric and eccentric phase) and high intensity with normal method (NH, 80% 1 RM, 1s for each concentric and eccentric action), these authors concluded that circulating catecholamine and anabolic hormones increased more after SL than NH method [16]. Although training intensity and pressure on joints was lower in SL method than NH method because of the different intensity and volume which was used [9, 11, 12, 17].
Furthermore, Tanmito and Ishii and Westcott reported that slow resistance training ends in considerable hypertrophy and strength [9]. Goto et al found that slow method increased the serum concentration of growth hormone (GH) and free testosterone whereas the anabolic response after NH method was lower than SL method. Their study's results revealed that extending time under tension (TUT) is an important factor that can stimulate anabolic hormone responses in resistance exercise [12]. Mazetti et al revealed slow resistance exercise as the most fatiguing method. This fatigue might prompt hormonal and metabolic changes that are important for acute and chronic adaptation like hypertrophy and strength gain [18].
Insight into training protocols may be initially gained by monitoring the acute muscle fatigue because of its association with strength enhancement. The reduction of integrated electromyography (IEMG) immediately following the training protocols could present development of central fatigue [4]. Authors assumed that slow method might be the cause for lower neural activation (IEMG) with prolonged period of muscle tension, but no authors have not examined this hypothesis so far [12]. Furthermore, several researchers have compared slow method with normal and traditional methods regarding hormonal and metabolic changes [9, 10, 12, 17–20], but neuromuscular responses were not assessed [4, 12].
The purpose of the current study was to assess neuromuscular and metabolic responses to slow, traditional and normal resistance exercise methods, this study compared acute responses among three methods and we expected that the observed results may provide some possible insights into chronic training adaptations.
METHODS AND SUBJECTS
Subjects
Twenty four male recreational athletes voluntarily participated in this study (of mean ± standard deviation: age 23.59 ± 0.87 years, height 172.75 ± 5.80 cm, body mass 68.73 ± 7.42 kg) participated in this study, all the subjects were physically active and familiar with resistance training, but they had not participated in a regular resistance training program for six months before the beginning the study. None of the participants was taking medical treatment or nutritional supplementation. They were informed about the potential risks involved and their subscription forms were received. This study was approved by the ethic committee for human experiments at faculty of physical education and sport science in University of Guilan.
Anthropometric and 1 RM tests
Body mass and height were assessed to the nearest 0.1 kg and 0.1 cm, respectively. Body fat percentage was estimated using the three-site skinfold procedure according to standards with a Lafayette caliper (model 01127, USA) [21–23].
A week before the main testing session, subjects were recruited to 1 RM test. Before testing, subjects performed three warm-up sets on the knee extension machine using low-moderate weights. The subject’s 1RM was determined by allowing three attempts to lift the heaviest weight by trial and error procedure. Range of motion was from 90° to 0° (0° at full extension). Their upper body was fixed with a comfortable and special belt. Subjects used their natural concentric and eccentric repetition speed to perform all warm up and 1 RM attempts and rested 3 minutes between sets. Exercise protocols and 1 RM test were performed between 06.00 and 08.00 A.M to control diurnal physiological variation.
Familiarization
A day after the 1 RM test session, subjects were asked to come to the university lab to become familiar with the protocol of tests. Before they carry out 1RM test and exercise protocols, all of them were divided into three groups randomly. After general (5 min cycling on ergometer with low intensity) and specific warm-up (two warm-up sets: 10 repetitions, with 10% to 30% of 1RM), each group was allowed to do its protocol (NH, TH and SL methods), similar to the same way that were asked to complete protocol and maximal voluntary isometric contraction (MVC) test.
Experimental protocols
The subjects’ completed protocols included four sets of lower body resistance exercises. All the experimental protocols were conducted in 2 separate days. The subjects fasted for 12 h and slept for 8 h prior to each morning blood sample and experimental protocol. participants performed their special protocols using bilateral knee extension exercise: 1-High intensity exercise with normal speed (NH, 1-1 method, 1s for CON and 1s for ECC action); 2- high intensity exercise with traditional speed (TH, 2-4 method, 2 s for CON and 4 s for ECC action); 3- Low intensity exercise with slow speed (SL, 3-3 method, 3s for CON and 3s for ECC action) [12].
Each method consisted of four sets, with one minute rest intervals between sets. Exercise intensity was 50% in SL and 80% of 1 RM in TH and NH methods at first set, after that training load was reduced by 10% of 1 RM from sets two to four, subjects performed each set until voluntary exhaustion. Volume load was calculated by load x repetition × set number. However, in this research we also compared work considering time under tension (volume load x time). Contraction speed has been monitored with the aid of metronome at a defined constant velocity during each method and an experienced assistant who announced the end of CON and ECC action. Range of motion (ROM) was from 90° to 0° (0° at full extension).
Electromyography
Surface electromyography of rectus femoris was measured before and immediately after each protocol by MVC in knee extension which was performed on a Biodex isokinetic dynamometer (Biodex corporation, Shirley, NY, United States). Participants carried out an MVC test before and immediately after the exercise. All MVC attempts were 3s in duration. The subjects were in a seated position with restraining straps across the chest and over the pelvis, in accordance to Biodex recommendation [24–26].
Hair was removed with a razor and skin was thoroughly prepared via sanding of the designated area and cleaned with alcohol pad before electrode placement. Electrode area was marked by non-permanent ink and subjects were asked to redraw the marks when they appeared to fade. Two surface electrodes (silver-silver chloride, 10 mm in diameter) with 20 mm inter electrode distance were placed over the motor point (mid belly) of rectus femoris muscle (50% of the anterior spinal iliac superior to the superior part of the patella). A ground electrode was placed on the knee cap, Signals were recorded with a MegaWin ME3000 device (MEGA Electronics, Ltd., Kuopio, Finland), sampling rate of 1000 Hz, and was rectified and integrated by MegaWin 2.06 EMG analysis software [27, 28].
Plasma metabolites measurement
Venous blood samples were obtained from antecubital vein for measurement of plasma lactate, glucose and triglyceride concentration before and immediately after protocol, when the subjects sat in a slightly reclined position. Blood samples were allowed to flow into a brand NH4 heparinized capillary tube. These samples were then placed in refrigeration at approximately 4°C to be transported to laboratory and then put in refrigerator. The serum was stored at -20°C until analyzed. Plasma lactate was determined enzymatically using the YSI 1500 lactate analyzer (Yellow springs, OH) and plasma triglyceride and glucose using an enzymatic method by automated analyzer (Hitachi, ltd., Japan).
Statistics
All values are expressed as means ± SD. Before parametric analyses were done, the normality of distribution of the data was assessed with Kolmogrov–Smirnov tests. A two-way (groups × time) analysis of variance (ANOVA) with repeated measures was conducted to determine the interaction and main effects. Where a significant main effect and/or interaction was observed, one-way ANOVA of pre-intervention to post-intervention differences (absolute values) was applied to determine the source of significance. Effect size (ES) was determined and reported. To compare volume load, repetition, time under tension and maximal strength data, a one-way ANOVA was applied. When ANOVA revealed significant, Bonferroni method was used for post-hoc comparisons. For all tests, P<0.05 was considered as significant. All statistical tests were performed using SPSS software, version 16.
RESULTS
General characteristics of subjects
Descriptive characteristics of the subjects are shown in Table 1. The number of repetitions during exercises was greater in NH method in comparison to the SL and TH methods, but this difference was not statistically significant (P>0.05). Time under tension was higher (P<0.05) during TH method than the other methods and during SL than NH method (P<0.05). Volume load was higher (P<0.05) during NH than the other methods and during TH higher than SL method (P<0.05). All data has been shown in Table 2.
Table 1.
General characteristics [means (SD)] of subjects
| Variable | Groups | ||
|---|---|---|---|
| Normal Method | Traditional Methods | Slow method | |
| Age (years) | 23.87(0.83) | 23.63(1.06) | 23.38(0.91) |
| Height (cm) | 173.25(7.44) | 174.62(6.2) | 172(4.40) |
| Weight (kg) | 69.12(8.57) | 70. 62(8.1) | 68.2(6.4) |
| Body Mass Index (kg / m 2 ) | 22.95(1.32) | 22.91(1.47) | 23.1(1.38) |
| Body fat (%) | 12.77(1.46) | 13.31(0.79) | 12.87(1.09) |
Number of repetitions, volume load and work considering time under tension (work TUT)
Table 2.
Values of Number of repetitions, volume load and work considering time under tension among groups
| Groups | Slow method Mead (SD) | Normal Method Mead (SD) | Traditional Methods Mead (SD) | F ratio | P Value |
|---|---|---|---|---|---|
| Number of repetition(n) | 32.75 (1.03) | 33. 37 (3.46) | 29 (4.7) | 2.48 | 0.1 |
| Volume load* (kg) | 571.5 (130.6) | 1145.5 (188.43) | 825.62 (131.15) | 18.83 | 0.001 |
| Time under tension (s.kg) | 3429 (380.92) | 2291 (376.87) | 4953 (379.33) | 16.12 | 0.001 |
SD: Standard Deviation; The volume load is calculated by number of repetition × number of sets × work load; Time under tension is calculated by each repetition time ×number of repetitions × sets × load
Blood metabolites
Pre exercise blood lactate and glucose concentration were similar among groups (Fig. 1 and 2). Triglyceride baseline was a little lower in SL group than the other groups, but this difference was not statistically significant (P>0.05). Plasma lactate concentration was elevated after exercise in all groups (P<0.05), but after the SL, it was lower (P<0.05) than the other high intensity methods (TH and NH method) and estimated ES was 0.89. The plasma glucose concentration increased (P<0.05) after exercise in each method and was similar among the groups (P>0.05) (Fig. 3) and estimated ES was 0.54. The plasma triglyceride concentration increased from pre to post level, but this increase was not statistically significant (P>0.05), there was no statistical difference among groups (P>0.05) and estimated ES was 0.27.
Fig. 1.
Plasma lactate (mmol/L) concentration responses to exercises among groups.
*Significantly higher than pretest. Ŧ significantly lower than NH and TH groups
Fig. 2.
Plasma glucose (mg/dl) concentration responses to exercises among groups.
*Significantly higher than pre test
Fig. 3.
Plasma triglyceride (mg/dl) concentration responses to exercises among groups.
*Significantly lower than pre test
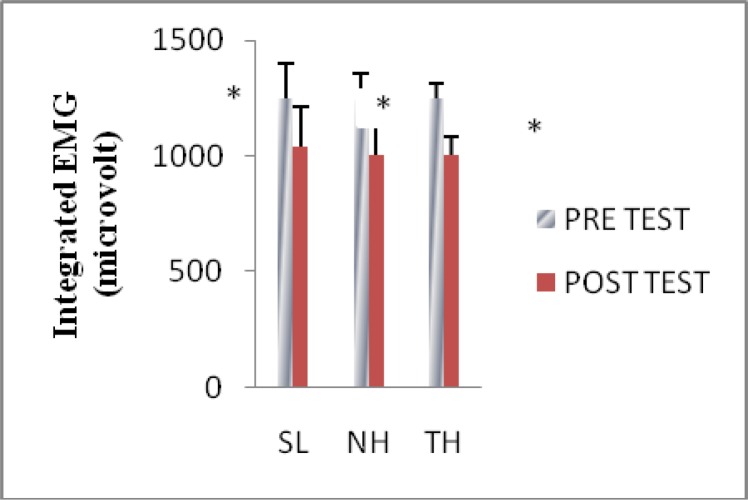
Integrated electromyography (IEMG)
All methods resulted in significant (P<0.05) decrease in IEMG activity from pre to post values. However, no significant differences occurred between the protocols (P>0.05). This finding has appeared in Fig. 4.
Fig. 4.
Integrated electromyography (micro volt) responses to exercise among groups.
*Significantly lower than pre test
DISCUSSION
The purpose of this study was to assess neuromuscular and metabolic responses to slow, traditional and normal resistance exercise methods. The main findings of this study were that high intensity resistance exercises (TH and NH methods) induced greater lactate response compared to low intensity method (SL). Additionally, number of repetitions and neuromuscular response in SL method was similar to other two types of resistance exercise methods.
The acute changes in the neuromuscular system that selectively optimize chronic adaptation of the nervous system are not clearly known [26, 29, 30]. Electromyography represents the electrical properties of the muscle and is often used to monitor central drive because of the relationship between the amplitude of the surface EMG and the net motor activity [27–29, 31]. Fatigue protocols result in decrease in IEMG [26, 29, 30], previous researches have observed that the magnitude and source of such fatigue may vary when different contraction type, intensity, repetition, speed and rest period are manipulated in resistance exercise protocols [29–31].
High intensity resistance exercise has appeared to elicit strength gain and increase muscle unit activation (MUA). Also, it is known to stimulate type II muscle fibers. Therefore, high intensity resistance exercise optimizes nervous system adaptation [39]. On the other hand, low intensity resistance exercise with slow or modified ischemic manner perhaps prompts peripheral fatigue and increases strength through muscle morphology and cross sectional area and anabolic hormone changes [11, 29].
Considering the literature, it is hypothesized that high intensity method may result in greater fatigue due to central origin [12, 29]. In this research, all protocols resulted in significant decrease in IEMG but not significantly different from each other. The results of IEMG suggest that temporary decline in voluntary muscle activation may be caused by central fatigue. However, it was not specific to any protocols. Therefore, in this study it was revealed that central fatigue is not influenced by manipulating TUT or exercise intensity. In our findings it is assumed that although, total volume and load in SL method was lower than NH and TH methods, perhaps increase in concentric and eccentric action time (TUT) compensate for the low intensity and little volume load in SL method and could make acute neuromuscular response similar to high intensity methods (NH and TH).
The current data on IEMG are in agreement with previous studies that have elicited neuromuscular fatigue by using IEMG as a fatigue index [26, 29, 30]. But caution should be advised because some authors suggested that median frequency (MF) is a better index than IEMG for neuromuscular fatigue [26, 30]. Above all, some investigators used IEMG as acute neuromuscular response and somewhere as fatigue index [30–32].
In the present study, we observed that plasma lactate concentration increased significantly following all methods, but this increment was lower in low-intensity slow (SL) group than the other two high intensity groups (NH and TH). There are contradictory findings about lactate changes following SL and high intensity methods (NH and TH) [9, 11, 12, 18]. Goto et al (2009) and Hunter et al (2003) found that high intensity (TH and NH) exercises increased blood lactate concentration more than SL method [12]. Their results are in agreement with our findings. In contrast, some authors like Tanmito et al (2005) found no significant differences between TH and SL methods on lactate responses [9, 10]. Furthermore Mazzeti et al (2007) found that SL increased blood lactate concentration significantly more than high speed resistance exercise [18]. The discrepancy between the present results and other studies could be due to methodological differences. For instance, Tanmito et al (2005) used three sets, but there are four sets in the current study. Or Mazzeti et al (2007) used higher intensity in SL method (60% of 1 RM).
The higher lactate production response to high-intensity exercise (NH and TH), may reflect more peripheral fatigue which is caused following TH and NH methods than SL method. Although lactate may not be a direct promoter of muscle adaptations, it may be used as marker of metabolic stress. This assumption was based on previous findings that reported elevated responses of lactate and other metabolites involved that stimulating increase in muscle size and strength [33–35]. In this study it was revealed that NH and TH methods could make a higher metabolic stress than SL method.
Results indicate that plasma glucose concentration increased following each method, but between-group difference was not seen. To our knowledge, just one study compared blood glucose response to SL and high intensity (NH) forms of exercises [12], its results agree with our findings.
As mentioned in the introduction, training volume and intensity are the most important variables in resistance training program design [1–3, 5, 36, 37]. Scientists have suggested a different definition of training volume [6, 12, 18, 29, 37]. It is most commonly calculated by load x repetition number and expressed as volume load. Also, it is computed by the cumulative time that a muscle group is under tension or all contraction time during a training session that is named TUT. In this research we compared volume load (sets x repetition x load) and work considering time under tension (volume load x repetition time). Training volume is one of the major determinants of hypertrophy and strength gain. Each set in this study was carried out to failure point, furthermore, training intensity was different in each method, so training volume was different in three protocols [12, 18].
Tran et al (2007) concluded that training volume is a more important stimulus than TUT in resistance training and they suggested when training volume is equated, TUT is the major determinant of neuromuscular changes [34]. According to the present findings, the NH method provided a greater volume load than SL and TH methods, and the TH and SL method provided greater work considering time under tension than NH method. This could be caused by the higher TUT achieved with SL and TH when compared with lower duration methods (NH). We concluded that an increase in repetition duration (contraction time) declines volume load that can be displaced. The findings of Goto et al (2009) and Hatfield et al (2006) would support this suggestion [12, 13, 19].
The other finding of this research was that SL method could decrease total number of repetition as much as NH and TH methods, although exercise intensity was lower in SL rather than the other two methods (50% of 1 RM and 80% of 1 RM respectively). This may be because of TUT that makes resistance exercise more fatiguing, as some researchers proved this finding [9, 10, 12, 18]. They concluded that increasing TUT could decrease total number of repetition as much as high intensity method in a fast manner. Hatfiled et al (2006) resulted that the more time under tension, the less volume load can be displaced and Mazzetti et al (2007) reported that SL method made greater fatigue than high intensity methods [18]. Perhaps, this is why some authors suggest that the slow method must be performed with low intensity (<50% of 1 RM) [11, 12, 38, 39].
Practical applications
According to the stimulus-tension theory, the intensity and duration of the muscular tension are responsible for neural and morphological adaptations [11]. The interesting finding of this study is that SL method could stimulate IEMG as much as NH and TH methods. Although, training intensity, volume and pressure on joints was lower. The other interesting finding of this research is that SL method could decrease total number of repetition as much as NH and TH methods, even though exercise intensity was lower in SL. This is maybe because of more fatigue caused by SL than high speed methods. These results indicate that high intensity resistance exercise causes higher lactate response, but slow resistance exercise method could induce acute neuromuscular response as much as high intensity methods. It seems that this method will be advantageous for those who want to increase acute neuromuscular changes with low exercise intensity and volume.
CONCLUSION
This study showed that the SL method caused similar IEMG responses when compared to the two other methods, but high intensity methods increased more metabolic response (lactate) than SL method. Longitudinal studies are needed to evaluate the chronic adaptations of SL, TH and NH to test if the acute differences in selected physiological parameters reflect on increase in muscle size and strength.
ACKNOWLEDGMENTS
The authors would like to gratefully thank the Physical Education staff at University of Guilan for their close interactions, and subjects for their willing participation in this study.
Conflict of interests: None
REFERENCES
- 1.Brayant CX, Peterson JA, Graves JE. Muscular strength and endurance. In: American College of Sport Medicine, editor. ACSM's resource manual for guidelines for exercise testing and prescription. Baltimore (MA): Williams & Wilkins; 1998. pp. 448–55. [Google Scholar]
- 2.Kraemer WJ, Ratamess NA. Fundamentals of Resistance Training: Progression and Exercise Prescription. Med Sci Sports Exerc. 2004:674–88. doi: 10.1249/01.mss.0000121945.36635.61. [DOI] [PubMed] [Google Scholar]
- 3.Rippetoe M, Kilgore L. 3rd ed. USA: Aasgaard Company; 2008. Practical Programming for Strength Training. [Google Scholar]
- 4.Tan B. Manipulating Resistance Training Program Variables to Optimize Maximum Strength in Men: A Review. J Strength Cond Res. 1999;13:289–304. [Google Scholar]
- 5.Ratamess NA, Adams Kent. Progression models in resistance training for healthy adults. By the American College of Sports Medicine. 2009 doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 6.Fisher J, Steele J, Bruce-Low S, Smith D. Evidence-Based resistance training recommendations. 2011;15(3):147–162. [Google Scholar]
- 7.Izquierdo M, Ibanez J, Gonzalez-Baddilo JJ, et al. Differential effects of strength training leading to failure versus not to failure on hormonal responses, strength and muscle power gains. J App Physiol. 2006;100:1647–56. doi: 10.1152/japplphysiol.01400.2005. [DOI] [PubMed] [Google Scholar]
- 8.McDonagh MJ, Davies CT. Adaptive response of mammalian skeletal muscle to exercise with high loads. EUR J Appl physiol Occup Physiol. 1984;52:139–55. doi: 10.1007/BF00433384. [DOI] [PubMed] [Google Scholar]
- 9.Tanimoto M, Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Applied Physiol(1985) 2006;100:1150–75. doi: 10.1152/japplphysiol.00741.2005. [DOI] [PubMed] [Google Scholar]
- 10.Tanimoto M, Madarame H, Ishii N. Muscle oxygenation and plasma growth hormone concentration during and after resistance exercise: comparison between kaatsu and other types of regimen. Int J Kaatsu Training Res. 2005;1:51–6. [Google Scholar]
- 11.Gentil PE, Bottaro OM. Time under tension and blood lactate response during Four different resistance training methods. J Physiol Anthropol. 2006;25:339–44. doi: 10.2114/jpa2.25.339. [DOI] [PubMed] [Google Scholar]
- 12.Goto K, Ishii N, Kizuka T, et al. Hormonal and metabolic responses to slow movement resistanse exercise with different durations of concentric and eccentric actions. Eur J Appl Physiol. 2009;106:731–9. doi: 10.1007/s00421-009-1075-9. [DOI] [PubMed] [Google Scholar]
- 13.Goto K, Nagasawa M, Yanagisawa O, et al. Muscular Adaptations to combinations of high and low intensity resistance exercises. J Strength Cond Res. 2004;18:730–7. doi: 10.1519/R-13603.1. [DOI] [PubMed] [Google Scholar]
- 14.Takarada Y, Takazawa H, Sato Y, et al. Effects of resistance exercise combined with moderate vascular occlusion on muscular functions in humans. J Appl Physiol. 2000;88:2097–106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 15.Neils CM, Udermann BE, Brice GA, et al. Influence of contraction velocity in untrained individuals over the initial early phase of resistance training. J Strength Cond Res. 2005;19:883–7. doi: 10.1519/R-15794.1. [DOI] [PubMed] [Google Scholar]
- 16.Goto K, Takahashi K, Yamamoto M, Takamatsu K. Hormone and recovery responses to resistance exercises with slow movement. J Physiol Sci. 2008;58:7–14. doi: 10.2170/physiolsci.RP003107. [DOI] [PubMed] [Google Scholar]
- 17.Westcott WL, Winett RA, Anderson ES, et al. Effects of regular and slow speed training on muscle strength. J Sports Med Phys Fitness. 2001;41:154–8. [PubMed] [Google Scholar]
- 18.Mazzetti S, Douglass M, Yocum A, Harber M. Effect of explosive versus slow contraction and exercise intencity on energy expenditure. Med Sci Sports Exerc. 2007;39:1291–301. doi: 10.1249/mss.0b013e318058a603. [DOI] [PubMed] [Google Scholar]
- 19.Hatfield D, Kraemer WJ, Spring BA, et al. The impact of velocity of movement on performance factors in resistance exercise. J Strength Cond Res. 2006;20:760–6. doi: 10.1519/R-155552.1. [DOI] [PubMed] [Google Scholar]
- 20.Headley SA, Henry K, Nindl BC, et al. Effects of lifting tempo on one repetition maximum and hormonal responses to a bench press protocol. J Strength Cond Res. 2011;25:406–13. doi: 10.1519/JSC.0b013e3181bf053b. [DOI] [PubMed] [Google Scholar]
- 21.Brozek J, Grande F, Anderson JT, Keys A. Densitometric analysis of body composition: Revision of some quantitative assumptions. ANN NY Acad Sci. 1963;110:113–40. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 22.Jackson AS, Pollock ML. Generalized equations for predicting body density for men. J Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AS, Pollock ML. Practical assessment of body composition. Phys Sports med. 1985;113:76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Li Y, Wang R. Evaluation of exercise-induced muscle damage by surface electromyography. J Electromyogr Kinesiol. 2011;21:356–62. doi: 10.1016/j.jelekin.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Kornard P. The ABC of EMG, a practical introduction to kinesiological electromyography. Ver 1:0; 2005. [Google Scholar]
- 26.Grant O. McCaulley, Jeffrey M, et al. Acute hormonal and neuromuscular responses to hypertrophy, strength and power type resistance exercise. Eur J Appl Physiol. 2009;105:695–704. doi: 10.1007/s00421-008-0951-z. [DOI] [PubMed] [Google Scholar]
- 27.Staudenman D, Roeleveld K, Stegeman DF, Van-dieen JH. Methodological aspects of SEMG recordings for force estimation: A tutorial and review. J Electromyogr Kinesiol. 2010;20:375–87. doi: 10.1016/j.jelekin.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Merletti R, Parker P. New York: Wiley & Sons, Inc; 2004. Electromyography, Physiology, Engineering and Non-Invasive Applications. [Google Scholar]
- 29.Tran Q, Docherty D. Dynamic training volume: A construct of both time under tension and volume load. J Sport Sci Med. 2007;5:707–13. [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrie DA, Kamen G, Frost G. Neural adaptations to resistive exercise. Sports Med. 2006;36:133–49. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Nordlund MM, Thorstensson A, Cresswell AG. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol. 2004;96:218–25. doi: 10.1152/japplphysiol.00650.2003. [DOI] [PubMed] [Google Scholar]
- 32.Hakkinen K, Komi PV. Training induced changes in neuromuscular performance under voluntary and reflex conditions. Eur J Appl Physiol. 1986;55:147–55. doi: 10.1007/BF00714997. [DOI] [PubMed] [Google Scholar]
- 33.Schott J, Mccully K, Rutherford OM. The role of metabolites in strength training, short versus long isometric contractions, II. Eur J Appl Physiol. 1995;71:337–41. doi: 10.1007/BF00240414. [DOI] [PubMed] [Google Scholar]
- 34.Smith RC, Rutherford OM. The role of metabolites in strength training, I, the role of metabolites in strength training, I, A comparison of eccentric and concentric contractions. Eur J Appl Physiol. 1995;71:332–6. doi: 10.1007/BF00240413. [DOI] [PubMed] [Google Scholar]
- 35.Kavada S. What phenomena do occur in blood flow-restricted muscle? Int KAATSU training research. 2005;1:37–44. [Google Scholar]
- 36.Hakkinen K, Pakarinen A, Kallinen MK. Neuromuscular adaptations and serum hormones in women during short term intensive strength training. Eur J Appl Physiol. 1992;64:106–11. doi: 10.1007/BF00717946. [DOI] [PubMed] [Google Scholar]
- 37.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand: Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 38.Keeler LK, Finkelestein LH, Miller W, Fernhall B. Early-phase adaptation of traditional-speed resistance training on strength and aerobic capacity in sedentary individuals. J Strength Cond Res. 2001;15:309–14. [PubMed] [Google Scholar]
- 39.Hunter GR, Seelhorst D, Snyder S. Comparison of metabolic and heart rate responses to super slow vs. traditional resistance training. J Strength Cond Res. 2003;17:76–81. doi: 10.1519/1533-4287(2003)017<0076:comahr>2.0.co;2. [DOI] [PubMed] [Google Scholar]