Abstract
Background
Numerous in vitro reports suggest that Low Level Laser Therapy (LLLT) affects cellular processes by biostimulation, however most of them emphasize on using visible light lasers which have low penetration. The aim of this study was to determine the effect of infrared laser light (which is more useful in clinic because of its higher penetration) on secretion of Fibroblast Growth Factor (FGF), Platelet Derived Growth Factor (PDGF) and Vascular Endothelial Growth Factor (VEGF), as important growth factors in wound healing.
Methods
Fibroblasts were extracted from the skin of 7 diabetic and 7 nondiabetic mice and cultured. Cell cultures of experimental group were irradiated with single dose of LLLT (energy density of 1 J/cm 2) using an 810 nm continuous wave laser and the control group was not irradiated. Secretion of growth factors by skin fibroblasts were quantified through real time poly-merase chain reaction.
Results
Diabetic irradiated group showed significant increase in FGF (p = 0.017) expression, although PDGF increased and VEGF decreased in both diabetic and nondiabetic irradiated groups, but these variations were not statistically significant.
Conclusion
These results suggest that LLLT may play an important role in wound healing by stimulating the fibroblasts.
Keywords: Biostimulation, Cell culture, Low level laser therapy, Techniques, Wound healing
Introduction
During the past three decades, lasers have been broadly used in medical fields. Since then, numerous scientific studies have been carried out to evaluate laser effects on various cells including fibroblasts (1–3). One of the most important functional aspects of laser therapy is photobiostimulation effects of low level lasers, often described as lasers with less than 500 mW average power, on various biological systems especially microcirculation (4–7). There are numerous reports of LLLT effects on wound healing especially diabetic ulcers (6, 8–10), and its effect in increasing new capillary formation through the release of growth factors (11). Among the many physiological mechanisms of LLLT, it is important to recognize that laser may affect the release of various growth factors.
The growth factors play a role in epithelial cell formation, formation of new fibroblasts and collagen and neovascularization. The release of growth factors from injured and inflammatory cells is, therefore, an important part of wound healing (2, 7, 11). The basic Fibroblast Growth Factor (bFGF) stimulates the proliferation of all cell types involved in wound healing both in vitro and in vivo (12). VEGF is an extremely potent angiogenic stimulator (11). PDGF regulates cell growth and division and plays a significant role in angiogenesis (7). In most of these studies, visible red light is used which is suitable for monolayer cell cultures in vitro. However, in vivo, infrared lights with higher penetration are more useful, though some reports show inhibitory effects of infrared lasers on fibroblasts (3, 13). The aim of this study was to evaluate the effects of 810 nm infrared laser (which have very good results in our clinical use in diabetic patients) on in vitro expression of FGF, PDGF and VEGF from isolated skin fibroblasts in diabetic and nondiabetic mice.
Materials and Methods
Skin fibroblasts were isolated from 4 week old male Balb/c mice, obtained from Pasteur Institute of Iran. Mice were sacrificed using chloroform according to the ethics committee of Tehran University of Medical Sciences. After shaving the back of mice, 1 cm 2 of full thickness skin was cut by scalpel and placed into 70% ethanol for 2 min. Then, it was washed with DMEM (Gibco) plus antibiotic (penicillin and streptomycin) three times and was cut into small pieces using scalpel and transferred into 15 ml round bottomed tube. After adding 2 ml of 0.25% trypsin and brief vortexing, it was incubated for 30 min at 37°C in 95% air with 10% CO2 in humidified incubator and vortexed briefly every 10 min. After spinning at 120 g/5 min, supernatant was removed and 4-5 ml of 0.1% collagenase type 2 (invitrogen) was added and incubated for 4 hr at 37°C and was vortexed briefly every 10 min. After spinning at 120 g/5 min, supernatant was transferred to a well of a 6-well plate and the sediment was added into 5 other wells (instead of discarding the supernatant which contained small number of cells, we transferred it to a well to use the cells later) and 1 ml DMEM supplemented with 10% FBS, 2 mM L-glutamine and 25 µg/ml gentamycin was added to each well. Medium was changed to a fresh one every other day. Upon reaching confluency, cells were detached with trypsin and subcultured in two 12-well culture plates. Prior to irradiation, 12-well plates were microscopically verified to guarantee that the cells were confluent and there was no contamination. Following aspiration of 75% DMEM culture medium, irradiation was started. The remaining 25% medium avoided dehydration of the fibroblasts through irradiation. One plate was irradiated (case) and the other plate was non-irradiated (control).
Irradiation source
In this study, we used an infrared GaAlAs Laser (Physio laser, RJ, Germany) with a wavelength of 810 nm (13), with an area of 1 cm 2, power output of 10 mW and in continuous mode. In all experiments, the laser probe was fixed with a delivery arm that permitted precise positioning of the fiber tip 1 cm in front of the cell monolayer that allowed the laser beam width to be 1 cm 2. The laser power of 10 mW was used in all experiments. The cell cultures were irradiated with a single dose for 1 min and 40 s at a power density of 10 mW/cm 2 and energy density of 1 J/cm 2. After irradiation, the remaining medium was removed and new DMEM medium was added. Cells were then incubated for 1 hr prior to RNA extraction.
PCR
Total RNA was isolated from laser treated and untreated fibroblasts using CinnaPure RNA extraction kit (CinnaGen Co., Iran). One µg of RNA was reverse transcribed to cDNA using PrimeScript cDNA Synthesis Kit (Taka-ra) with 2 µl PrimeScript Buffer, 0.5 µl Prime Script RT Enzyme, 0.5 µl Oligo dT Primer, Random Hexamers and 1.5 µl RNase Free dH2O. The amplification through Polymeraze Chain Reaction (PCR) was carried out using 10 µg/µl of cDNA and primer design master mix and primers. The primers sequences are shown in Table 1. PCR amplification was performed using a 48-well tray (ABI) with 20 µl final reaction mixture containing 10 µl real time master mix (primer design), 7 µl ddH2O, 1 µl forward and reverse primer (10 pmol/µl) and 2 µl cDNA. PCR profile consisted of 95°C for 15 min, 95°C for 30 s, 60°C for 55 s, and 72°C for 20 s. Amplification was carried out for 40 cycles. To avoid any contamination of the preparation, PCR set-up was performed in a separate laboratory under sterile conditions. In every run, real time was performed in duplicate for each sample and one negative control for each primer was included to exclude false-positive results.
Table 1.
Primers sequences
| Vegf-f | 5’-CTGCTGTAACGATGAAGCCCTG-3’ | Vegf -r | 5’-GCTGTAGGAAGCTCATCTCTCC-3’ |
| Egf-f | 5’-ACTGGTGTGACACCAAGAGGTC-3’ | Egf -r | 5’-CCACAGGTGATCCTCAAACACG |
| Pdgf-f | 5’-AATGCTGAGCGACCACTCCATC-3’ | Pdgf -r | 5’-TCGGGTCATGTTCAAGTCCAGC-3’ |
| Fgf-2-f | 5’-AAGCGGCTCTACTGCAAGAACG-3’ | Fgf -2-r | 5’-CCTTGATAGACACAACTCCTCTC-3’ |
| Tbp-f | 5’-AAGGGAGAATCATGGACCAGAAC | Tbp-r | 5’-GGTGTTCTGAATAGGCTGTGGAG |
In this study, we used TATA box binding protein (TBP) as the housekeeping gene (internal control). Data was analyzed using ΔΔCT equation. The fold change for PCR products were calculated in laser treated versus non-treated samples in each group of cells. PCR products were resolved in 1% aga-rose gel electrophoresis, and ethidium bromide-stained specific bands were visualized under UV light and photographed (to check if they have correct PCR product size).
Results
Data were analyzed using the SPSS (SPSS Inc., Chicago, IL, 16th version). A p-value less than 0.05 was considered statistically significant. The descriptive statistics were presented as median and standard deviation. All experiments were repeated at least 2 times with 2 parallel measurements (duplicate) in different wells. The statistical analysis was done by calculating ANOVA, post-hoc (LSD).
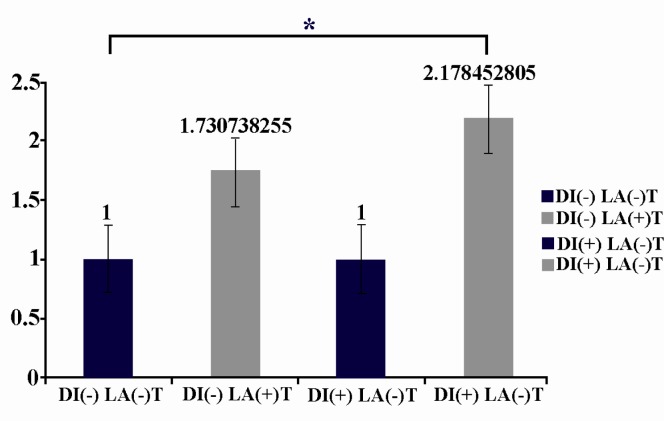
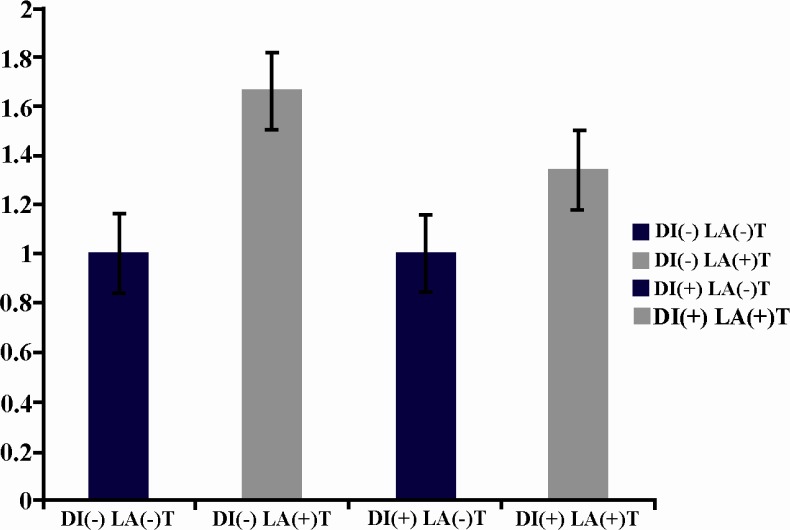
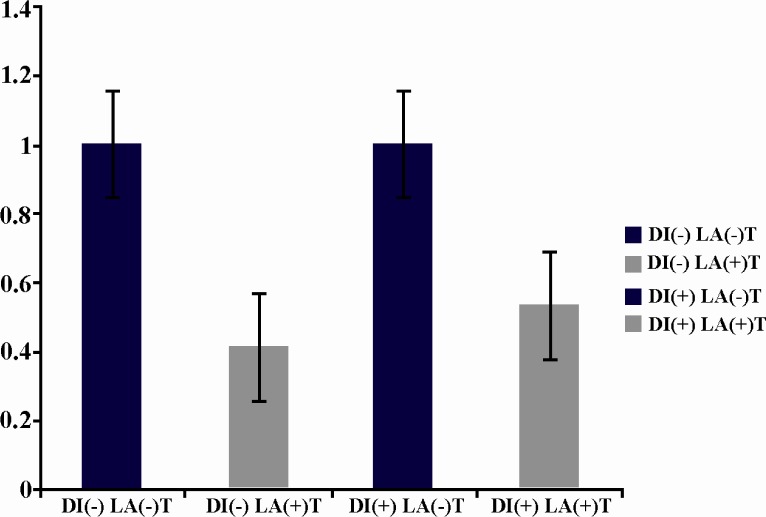
The investigated factors before laser irradiation had no significant difference in diabetic and nondiabetic mice. A significant increase (p = 0.017) was seen in the expression of FGF after irradiation in diabetic mice in comparison with nondiabetic mice after laser irradiation (Figure 1). Although the expression of PDGF also increased in both diabetic and nondiabetic mice after LLLT, it was not statistically significant (Figure 2). The expression of VEGF decreased after LLLT in both diabetic and nondiabetic mice, but it was not statistically significant (Figure 3). The investigated factors after laser irradiation had significant increase in diabetic mice in comparison with nondiabetic mice only for FGF after laser irradiation. P-value of growth factors in diabetic and nondiabetic mice in laser group and control group are shown in Table 2.
Figure 1.
LLLT increases Fgf expression from isolated skin fibroblasts in diabetic mice in comparison with non diabetic mice, significantly [DI(-)LA(-) in comparison with DI(+)LA(+)] The starred column, DI(+)LA(+), shows this significant increase. Fgf expression between diabetic and non-diabetic mice before laser exposure [DI(-)LA(-) and DI(+)LA(-)] shows no significant difference
Figure 2.
LLLT increases Pdgf expression in both diabetic and non diabetic mice, but this increase is not significant [DI(-)LA(-) in comparison with DI(-)LA(+) and DI(+)LA(-) in comparison with DI(+)LA(+)]. Pdgf expression between diabetic and non-diabetic mice before laser exposure [DI(-) LA(-) and DI(+)LA(-)] shows no significant difference. Pdgf expression between diabetic and non-diabetic mice after laser exposure [DI(-)LA(+) and DI(+)LA(+)] shows no significant difference
Figure 3.
LLLT decreases Vegf expression in diabetic and non diabetic mice, but this decrease is not significant [DI(-) LA(-) in comparison with DI(-)LA(+) and DI(+)LA(-) in comparison with DI(+)LA(+)]. Vegf expression between diabetic and non-diabetic mice before laser exposure [DI(-) LA(-) and DI(+)LA(-)] shows no significant difference.
Vegf expression between diabetic and non-diabetic mice after laser exposure [DI(-)LA(+) and DI(+)LA(+)] shows no significant difference
Table 2.
P-value of growth factors comparison in diabetic and non diabetic mice in laser and control groups
| Subject | DI(-) LA(-) | ||
|---|---|---|---|
| Vegf | Fgf | Pdgf | |
| DI(-) LA(-) VS DI(-)LA(+) | 0.381 | o.329 | 0.142 |
| DI(+)LA(-) VS DI(+)LA(+) | 0.127 | 0.017 | 0.456 |
Discussion
There are many clinical and experimental data on the relationship between metabolic disturbance in diabetes and wound healing. Mattin (14), Goldstein (15) and Loots (16) examined skin fibroblasts from diabetics in tissue culture and found that their in vitro replication life span was reduced when compared with the control group. Grazul-Bilska et al suggested a defective FGF receptor or down-regulation of the FGF receptor-mediated cascade in diabetic status (17). Greenhalgh et al showed that PDGF and FGF stimulate wound healing in diabetic patients (18). Recent studies show the potential role of increased VEGF in diabetic retinopathy and nephropathy (19, 20).
Although some studies (1, 2) demonstrate no beneficial effects of low level laser irradiation on fibroblasts, this study showed stimulatory effects of LLLT on some fibroblast growth factors and therefore confirmed previous studies that yielded beneficial stimulating effect on fibroblasts (8, 9, 21, 22). Moore et al (13) reported inhibitory effect of 810 nm infrared laser on fibroblast. Van Breughel et al (3) reported a range of absorption peaks in 420, 445, 470, 560, 630, 690 and 730 nm and a general decrease in absorption at longer wavelengths and concluded that several molecules in fibroblasts serve as photoacceptors and absorb special wavelengths. Karu (23) also showed that the use of appropriate wavelength within the bandwidth of the absorption spectra of photo-acceptor molecules is an important factor. In this study, we observed the inhibitory effect of 810 nm infrared laser only on VEGF expression, but in other measured growth factors, the stimulatory effect was observed, although the increase in PDGF expression was not statistically significant which can be related to the small sample size. In our study, penetration depth can almost be ignored as wavelengths in the visible and infrared spectrum will pass through a monolayer cell culture (24).
Moreover, the irradiance (W/cm2) might have an important influence on the outcome of this study. As other experiments confirmed, lower irradiances are more effective than higher irradiances (3, 10, 24). The weak photo-stimulating effect in this study may be related to short incubation period. Hawkins et al (25) showed that 1-3 hr post irradiation is sufficient to, measure cellular response to laser therapy. In some studies, this incubation period lasts 24-72 hr. Moreover, some authors proposed longer incubation period for having the response although the photobiostimulation influence may be weakened over time due to decreased vitality and untimely cell death in the irradiated cell cultures as a result of reaching confluence(22, 26).
Although some studies demonstrate FGF and PDGF decrease and VEGF increase in diabetic status (17–19), we did not find significant difference between these factors before laser irradiation in diabetic mice in comparison with nondiabetics. It may be due to the small sample size. However, we observed significant increase in FGF expression following LLLT in cultured fibroblasts of diabetic mice in comparison with nonirradiant nondiabetic mice. Previous studies suggest that LLLT is more effective on impaired tissues rather than normal condition (27). In this study, we also found PDGF increase and VEGF decrease following LLLT in diabetic mice compared with nondiabetic mice but it was not statistically significant which can be related to small sample size. These results confirmed our previous studies using LLLT on diabetic patients (28–30) and other studies suggesting beneficial effects of LLLT on fibroblast activity (8, 21, 22, 31).
This study suggests potential beneficial effects of LLL irradiation at the cellular level in clinical studies. LLLT application in cutaneous wounds of human skin may be assumed useful at the applied dosimetric parameters, but future investigation with larger sample size is necessary to explain the exact mechanisms of laser biomodulation. Subsequently, resolving the lack of scientific evidence and nullifying the controversial acknowledgements of the effect of low power lasers can bring about a wide spread acceptance for the use of LLLT in clinical settings.
Acknowledgement
The authors are grateful to Dr. Bonakdar for lab supplies and necessary materials provided for this study and also laboratory workers of Cell Bank of Iran affiliated to Pasteur Institute of Iran, Mr. Mehrjo and Mr. Majidi for providing the culture medium and technical support. We also thank Tehran University of Medical Sciences for financial support by grant number 10415.
References
- 1.Pogrel MA, Chen JW, Zhang K. Effects of low-energy gallium-aluminum-arsenide laser irradiation on cultured fibroblasts and keratinocytes. Lasers Surg Med. 1997;20(4):426–432. doi: 10.1002/(sici)1096-9101(1997)20:4<426::aid-lsm8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Hallman H, Basford J, O'Brien JF, Cummins LA. Does low-energy helium-neon laser irradiation alter “in vitro” replication of human fibroblasts? Lasers Surg Med. 1988;8(2):125–129. doi: 10.1002/lsm.1900080206. [DOI] [PubMed] [Google Scholar]
- 3.Van Breugel HH, Bär P. Power density and exposure time of He-Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med. 1992;12(5):528–537. doi: 10.1002/lsm.1900120512. [DOI] [PubMed] [Google Scholar]
- 4.Lubart R, Wollman Y, Friedmann H, Rochkind S, Laulicht I. Effects of visible and near-infrared lasers on cell cultures. J Photochem Photobiol B. 1992;12(3):305–310. doi: 10.1016/1011-1344(92)85032-p. [DOI] [PubMed] [Google Scholar]
- 5.Passarella S, Casamassima E, Molinari S, Pastore D, Quagliariello E, Catalano IM, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by heliumneon laser. FEBS Lett. 1984;175(1):95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 6.Grossman N, Schneid N, Reuveni H, Halevy S, Lubart R. 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: involvement of reactive oxygen species. Lasers Surg Med. 1998;22(4):212–218. doi: 10.1002/(sici)1096-9101(1998)22:4<212::aid-lsm5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Naim J, Lanzafame R. The effects of photo-irradiation on the secretion of TGF and PDGF from fibroblasts in vitro. Lasers Surg Med Suppl. 1994;6:8. [Google Scholar]
- 8.Reddy GK, Stehno-Bittel L, Enwemeka CS. Laser photostimulation accelerates wound healing in diabetic rats. Wound Repair Regen. 2001;9(3):248–255. doi: 10.1046/j.1524-475x.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- 9.Nemeth AJ. Lasers and wound healing. Dermatol Clin. 1993;11(4):783. [PubMed] [Google Scholar]
- 10.Kana JS, Hutschenreiter G, Haina D, Waidelich W. Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg. 1981;116(3):293. doi: 10.1001/archsurg.1981.01380150021005. [DOI] [PubMed] [Google Scholar]
- 11.Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R, et al. Low power helium: Neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med. 2001;28(4):355–364. doi: 10.1002/lsm.1062. [DOI] [PubMed] [Google Scholar]
- 12.Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med Sci. 2008;23(2):211–215. doi: 10.1007/s10103-007-0477-3. [DOI] [PubMed] [Google Scholar]
- 13.Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD. Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg Med. 2005;36(1):8–12. doi: 10.1002/lsm.20117. [DOI] [PubMed] [Google Scholar]
- 14.Martin G, Sprague C, Epstein C. Replicative life-span of cultivated human cells. Effects of donor's age, tissue and genotype. Lab Invest. 1970;23(1):86–92. [PubMed] [Google Scholar]
- 15.Goldstein S, Littlefield JW, Soeldner JS. Diabetes mellitus and aging: diminished plating efficiency of cultured human fibroblasts. Proc Natl Acad Sci USA. 1969;64(1):155–160. doi: 10.1073/pnas.64.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loots MA, Kenter SB, Au FL, Van Galen W, Middelkoop E, Bos JD, et al. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol. 2002;81(3):153–160. doi: 10.1078/0171-9335-00228. [DOI] [PubMed] [Google Scholar]
- 17.Grazul-Bilska A, Luthra G, Reynolds L, Bilski J, Johnson M, Adbullah SA, et al. Effects of basic fibroblast growth factor (FGF-2) on proliferation of human skin fibroblasts in type II diabetes mellitus. Exp Clin Endocrinol Diabetes. 2002;110(4):176–181. doi: 10.1055/s-2002-32149. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh D, Sprugel K, Murray M, Ross R. PD GF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136(6):1235. [PMC free article] [PubMed] [Google Scholar]
- 19.Hammes H-P, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–406. doi: 10.2337/diabetes.47.3.401. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert RE, Tsalamandris C, Allen TJ, Colville D, Jerums G. Early nephropathy predicts vision-threatening retinal disease in patients with type I diabetes mellitus. J Am Soc Nephrol. 1998;9(1):85–89. doi: 10.1681/ASN.V9185. [DOI] [PubMed] [Google Scholar]
- 21.Whelan HT, Houle JM, Whelan NT, Donohoe DL, Cwiklinski J, et al. The NASA light-emitting diode medical program-progress in space flight and terrestrial applications. Space Tech & App Intl Forum. 2000;504:37–43. [Google Scholar]
- 22.Webb C, Dyson M, Lewis W. Stimulatory effect of 660 nm low level laser energy on hypertrophic scar-derived fibroblasts: possible mechanisms for increase in cell counts. Lasers Surg Med. 1998;22(5):294–301. doi: 10.1002/(sici)1096-9101(1998)22:5<294::aid-lsm6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Karu TI. Amsterdam: Gordon & Breach Science Publishers; 1998. The science of low-power laser therapy. [Google Scholar]
- 24.Boulton M, Marshall J. He-Ne laser stimulation of human fibroblast proliferation and attachment in vitro. Lasers Life Sci. 1986;1:125–134. [Google Scholar]
- 25.Hawkins D, Abrahamse H. How long after laser irradiation should cellular responses be measured to determine the laser effect? J Laser Appl. 2007;19:74. [Google Scholar]
- 26.Pourreau-Schneider N, Ahmed A, Soudry M, Jacquemier J, Kopp F, Franquin JC, et al. Heliumneon laser treatment transforms fibroblasts into myofibroblasts. Am J Pathol. 1990;137(1):171. [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins D, Houreld N, Abrahamse H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann NY Acad Sci. 2005;1056:486–493. doi: 10.1196/annals.1352.040. [DOI] [PubMed] [Google Scholar]
- 28.Kazemi khoo N. Successful treatment of diabetic foot ulcers with low-level laser therapy. Foot. 2006;16:184–187. [Google Scholar]
- 29.Kazemi khoo N, Iravani A, Arjmand M, Vahabi F, Lajevardi M, Akrami SM, et al. A metabolomic study on the effect of intravascular laser blood irradiation on type 2 diabetic patients. Lasers Med Sci. 2013;28(6):1–6. doi: 10.1007/s10103-012-1247-4. [DOI] [PubMed] [Google Scholar]
- 30.Khamseh ME, Kazemi khoo N, Aghili R, Forough B, Lajevardi M, Hashem Dabaghian F, et al. Diabetic distal symmetric polyneuropathy: Effect of low-intensity laser therapy. Lasers Med Sci. 2011;26(6):831–835. doi: 10.1007/s10103-011-0977-z. [DOI] [PubMed] [Google Scholar]
- 31.Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC. Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci. 2003;18(2):95–99. doi: 10.1007/s10103-003-0262-x. [DOI] [PubMed] [Google Scholar]