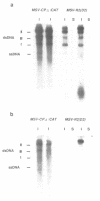
Abstract
The entire genome of single component geminiviruses such as maize streak virus (MSV) consists of a single-stranded circular DNA of ~2.7 kb. Although this size is sufficient to encode only three average sized proteins, the virus is capable of causing severe disease of many monocots with symptoms of chlorosis and stunting. We have identified viral gene functions essential for systemic spread and symptom development during MSV infection. Deletions and gene replacement mutants were created by site-directed mutagenesis and insertion between flanking MSV or reporter gene sequences contained in Agrobacterium T-DNA derived vectors. Following Agrobacterium-mediated inoculation of maize seedlings, the mutated MSV DNAs were excised from these binary vectors by homologous recombination within the flanking sequences. Our analyses show that the capsid gene of MSV, while not required for replication, is essential for systemic spread and subsequent disease development. The `+' strand open reading frame (ORF) located immediately upstream from the capsid ORF and predicted to encode a 10.9 kd protein was also found to be dispensable for replication but essential for systemic spread. By this analysis, MSV sequences that support autonomous replication were localized to a 1.7 kb segment containing the two viral intergenic regions and two overlapping complementary `-' strand ORFs. Despite the inability of the gene replacement mutants to spread systemically, both inoculated and newly developed leaves displayed chlorotic patterns similar to the phenotype observed in certain developmental mutants of maize. The similarity of the MSV mutant phenotype to these developmental mutants is discussed.
Keywords: disease development, geminiviruses, maize streak virus, site-directed mutagenesis, systemic spread
Full text
PDF

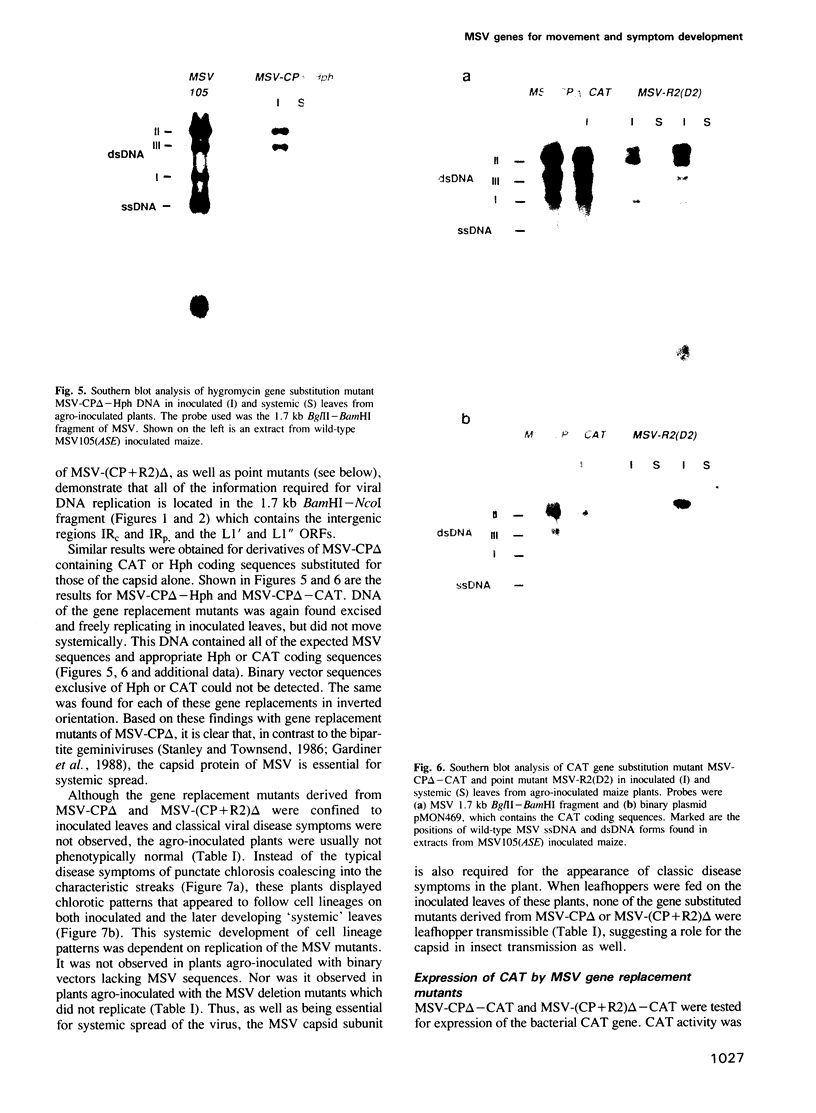





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donson J., Accotto G. P., Boulton M. I., Mullineaux P. M., Davies J. W. The nucleotide sequence of a geminivirus from Digitaria sanguinalis. Virology. 1987 Nov;161(1):160–169. doi: 10.1016/0042-6822(87)90182-6. [DOI] [PubMed] [Google Scholar]
- Elmer J. S., Brand L., Sunter G., Gardiner W. E., Bisaro D. M., Rogers S. G. Genetic analysis of the tomato golden mosaic virus. II. The product of the AL1 coding sequence is required for replication. Nucleic Acids Res. 1988 Jul 25;16(14B):7043–7060. doi: 10.1093/nar/16.14.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etessami P., Callis R., Ellwood S., Stanley J. Delimitation of essential genes of cassava latent virus DNA 2. Nucleic Acids Res. 1988 Jun 10;16(11):4811–4829. doi: 10.1093/nar/16.11.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoll C., Black D. M., Howell S. H. The intergenic region of maize streak virus contains promoter elements involved in rightward transcription of the viral genome. EMBO J. 1988 Jun;7(6):1589–1596. doi: 10.1002/j.1460-2075.1988.tb02984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner W. E., Sunter G., Brand L., Elmer J. S., Rogers S. G., Bisaro D. M. Genetic analysis of tomato golden mosaic virus: the coat protein is not required for systemic spread or symptom development. EMBO J. 1988 Apr;7(4):899–904. doi: 10.1002/j.1460-2075.1988.tb02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R. B., Klee H. J. Rapid assay of foreign gene expression in leaf discs transformed by Agrobacterium tumefaciens: Role of T-DNA borders in the transfer process. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4428–4432. doi: 10.1073/pnas.83.12.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S. H. Physical structure and genetic organisation of the genome of maize streak virus (Kenyan isolate). Nucleic Acids Res. 1984 Oct 11;12(19):7359–7375. doi: 10.1093/nar/12.19.7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenow H., Henningsen I. Selective elimination of the exonuclease activity of the deoxyribonucleic acid polymerase from Escherichia coli B by limited proteolysis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):168–175. doi: 10.1073/pnas.65.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale J. A., Metzler M. C., Nelson T. The argentia mutation delays normal development of photosynthetic cell-types in Zea mays. Dev Biol. 1987 Jul;122(1):243–255. doi: 10.1016/0012-1606(87)90349-6. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G. Infectivity and complete nucleotide sequence of the genome of a South African isolate of maize streak virus. Nucleic Acids Res. 1988 Jan 11;16(1):229–249. doi: 10.1093/nar/16.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDowell S. W., Macdonald H., Hamilton W. D., Coutts R. H., Buck K. W. The nucleotide sequence of cloned wheat dwarf virus DNA. EMBO J. 1985 Sep;4(9):2173–2180. doi: 10.1002/j.1460-2075.1985.tb03912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Krsinich B. A., Mullineaux P. M., Donson J., Boulton M. I., Markham P. G., Short M. N., Davies J. W. Bidirectional transcription of maize streak virus DNA and identification of the coat protein gene. Nucleic Acids Res. 1985 Oct 25;13(20):7237–7256. doi: 10.1093/nar/13.20.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux P. M., Donson J., Morris-Krsinich B. A., Boulton M. I., Davies J. W. The nucleotide sequence of maize streak virus DNA. EMBO J. 1984 Dec 20;3(13):3063–3068. doi: 10.1002/j.1460-2075.1984.tb02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Rogers S. G., Bisaro D. M., Horsch R. B., Fraley R. T., Hoffmann N. L., Brand L., Elmer J. S., Lloyd A. M. Tomato golden mosaic virus A component DNA replicates autonomously in transgenic plants. Cell. 1986 May 23;45(4):593–600. doi: 10.1016/0092-8674(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Stanley J., Townsend R. Infectious mutants of cassava latent virus generated in vivo from intact recombinant DNA clones containing single copies of the genome. Nucleic Acids Res. 1986 Aug 11;14(15):5981–5998. doi: 10.1093/nar/14.15.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Direct selection for gene replacement events in yeast. Gene. 1983 Dec;26(2-3):231–241. doi: 10.1016/0378-1119(83)90193-2. [DOI] [PubMed] [Google Scholar]
- Ward A., Etessami P., Stanley J. Expression of a bacterial gene in plants mediated by infectious geminivirus DNA. EMBO J. 1988 Jun;7(6):1583–1587. doi: 10.1002/j.1460-2075.1988.tb02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]