Abstract
Background
The well documented source for adult multipotent stem cells is Spermatogonial Stem Cells (SSCs). They are the foundation of spermatogenesis in the testis throughout adult life by balancing self-renewal and differentiation. The aim of this study was to assess the effect of percoll density gradient and differential plating on enrichment of undifferentiated type A spermatogonia in dissociated cellular suspension of goat testes. Additionally, we evaluated the separated fractions of the gradients in percoll and samples in differential plating at different times for cell number, viability and purification rate of goat SSCs in culture.
Methods
Testicular cells were successfully isolated from one month old goat testis using two-step enzymatic digestion and followed by two purification protocols, differential plating with different times of culture (3, 4, 5, and 6 hr) and discontinuous percoll density with different gradients (20, 28, 30, and 32%). The difference of percentage of undifferentiated SSCs (PGP9.5 positive) in each method was compared using ANOVA and comparison between the highest percentage of corresponding value between two methods was carried out by t-test using Sigma Stat (ver. 3.5).
Results
The highest PGP9.5 (94.6±0.4) and the lowest c-Kit positive (25.1±0.7) in Percoll method was significantly (p ≤ 0.001) achieved in 32% percoll gradient. While the corresponding rates in differential plating method for the highest PGP9.5 positive cells (81.3±1.1) and lowest c-Kit (17.1±1.4) was achieved after 5 hr culturing (p < 0.001). The enrichment of undifferentiated type A spermatogonia using Percoll was more efficient than differential plating method (p < 0.001).
Conclusion
Percoll density gradient and differential plating were efficient and fast methods for enrichment of type A spermatogonial stem cells from goat testes.
Keywords: C-kit, Differential plating, Goat, Percoll gradient, PGP 9.5, Type A spermatogonia
Introduction
Type A Spermatogonial Stem Cells (SSCs) are small self-renewing subpopulation of un-differentiated spermatogonia that are defined by their unique properties such as prolonged proliferation, self-renewal, generation of differentiated progeny, and maintenance of developmental potential (1). These cells can proliferate rapidly when the testis is damaged by chemicals or radiation, whereas under physiological conditions, these cells divide slowly to produce both stem cells and progenitor cells (2, 3). When transplanted into the seminiferous tubules of an infertile male, they can establish donor-derived spermatogenesis and produce spermatozoa that transmit the donor haplotype to progeny (4). Additionally, when they are cultured in appropriate conditions, they can acquire pluripotency and differentiate into derivatives of the three embryonic germ layers including sperm (5, 6).
A great excitement and expectation in today's biomedical world is the study of stem cells, owing to their ability to exist in an undifferentiated state and their transformation into different tissue types, depending on the ambient conditions (7).
The complexity of the seminiferous epithelium and systemic and testicular microenvironments makes it difficult to study cellular and molecular aspects of the regulation of spermatogonial stem cell behavior (8, 9). This deficiency is largely caused by the small number of SSCs in testis and the inability to isolate a purified SSCs population because of the lack of specific, accurate and inexpensive purification protocol with minimum side effects on spermatogonia (9).
It is valuable to develop an efficient method for SSCs enrichment as well as in vitro culture of purified SSCs and modulate culture conditions that control self-renewal versus differentiation in vitro. The practice would allow us to better understand the various aspects of adult stem cell biology such as the mechanisms that regulate SSC renewal and differentiation, the cell signaling pathways that regulate SSC function, to unravel the cellular and molecular mechanisms driving spermatogenesis, to preserve and manipulate male fertility and tissue regeneration, and to develop more improved techniques for male germline modification and transfection (10–13).
Additionally, due to extremely small population of SSCs as the stem cells that transmit genetic information to subsequent generations (for instance type A spermatogonia comprise 0.02-0.03% of total germ cells) (14–16), the ability to study their self-renewal and their characteristics is limited and further investigation requires larger populations of pure SSCs (17). Several laboratories have established the methods which allow the enrichment of male germline stem cells or improve their culture conditions such as expansion techniques with feeder layers or a combination of growth factors, or serial transplantation procedures (10, 18–20).
In 1970s, the feature of serpmatogonia was known better (20, 21). Bellve et al (1977) first used BSA to isolate spermatogonia from 8 day old mice and obtained a 90% purified fraction of type A spermatogonia (22). Bucci et al (1986) used enzymatic digestion and got 76% purity of spermatogoina from rat testis (23). But these methods either used a large number of experimental animals or resulted in insufficient purity of spermatogonia for experimental analyses.
With improvements in cellular biology approaches during several decades, the technology of isolation and purification of spermatogonia achieved a great breakthrough. Several methods have been employed to isolate SSC in different species including elutriation (23), differential plating (7, 17, 24), velocity sedimentation (10, 22), discontinuous percoll density gradient (25, 26), Magnetic-Activated Cells Sorting (MA CS) (7, 10, 13) and Fluorescence Activated Cells Sorting (FACS) (26, 27).
Considering the inadequate information regarding the effect of purification protocols on goat type A SSCs and the application of transfected SSCs as an efficient tool in production of transgenic animals, the present study was designed to compare the differential plating and percoll density gradient methods in enrichment of undifferentiated spermatogonia in goats.
Undoubtedly, availability of best practical isolation and purification techniques and establishment of stable SSCs in vitro will pave the way for innovative research into stem cell biology, leading to further breakthrough in the understanding of spermatogonial physiology and development of powerful tools for fertility preservation.
Materials and Methods
Except where otherwise indicated, all chemicals were obtained from Sigma (St. Louis, MO, USA).
Collection and evaluation of testis
Following castration of a one month old goat, the testes were transported to the lab in transition media (PBS + antibiotics) at 37°C. After macroscopic evaluation of the testes for any pathologic signs (trauma, cyst, tumor and hematoma), the testes were washed (3 times) with transition media and the tunica albuginea and visible connective tissues were aseptically removed.
Cell isolation and preparation
Single cell suspensions were prepared using a protocol which was recently described (28). Briefly, upon arrival of testes to laboratory, they were washed several times with transition media and tunica albuginea and visible connective tissues were removed. Testes samples were transferred into the Dulbeco Modified Essential Medium (DMEM) supplemented with NaHCO3 (14 mol/l), Hepes (15 mol/l), NEAA (10 µl/ml), penicillin (50 IU/ml) and streptomycin (50 mg/ml) for 5-8 min. Then testis was again washed in sterile transition media and dissection was done aseptically under laminar airflow hood. The SSCs were isolated through two-step digestion methods. The samples were incubated in DMEM containing 1 mg/ml collagenase type I at 38°C in 5% CO2 for 60 min. The samples were examined with an interval of 1 min for determination about disentanglement. After initiation of tissue disentanglement, the somniferous tubules’ fragments were separated from interstitial cells by centrifugation at 100×g for 1 min, and the supernatant, containing mostly the interstitial cells, was discarded. The tubules’ fragments were washed two times with ice-cold DMEM.
In a second digestion step, the tubules’ fragments were incubated in trypsin/EDTA (0.25% /1 mM) for 12-25 min. The dispersed cells were then washed twice in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS; GibcoBRL) to stop the enzymatic digestion and undigested debris was removed by centrifugation at 100×g for 1 min. The supernatant was then processed by sequential filtration through 60 µm nylon mesh (Small part, F062N-08-C). The filtrate was centrifuged at 500×g for 5 min, and the pellet was resuspended in DMEM supplemented with 10% FBS (Gibco) and NEAA. In the final cell suspension, the different testicular cell types including Sertoli cells and spermatogonia with different sizes were identified using light microscope. Total cell number and cell viability were determined after trypan blue staining. The cell suspension was mixed with 0.4% trypan blue (1:1, v/v). The number of live (trypan blue excluding) and dead cells were determined using hemocytometer.
Identification of SSC by histological evaluation
For histological evaluation, a small sample of testes was fixed overnight in Bouin's solution, washed in 70% ethanol, embedded in paraffin and sectioned at 5 µm using standard procedures. The sections were stained with Hematoxylin and Eosin and examined under a light microscope for identification of cell types and their developmental stages.
In cell suspension, after collection of SSCs at each stage, SSCs were identified based on their morphology in phase contrast microscopy.
Enrichment of goat undifferentiated SSCs by discontinuous percoll density gradient
The spermatogonial stem cells were enriched from cell suspension by following the method of Gang et al (29) with minor modification. Briefly, percoll was first sterilized by autoclaving and diluted to gradient concentrations of 20, 28, 30 and 32% with PBS. The gradient was made in 15 ml graduated tube by adding 1 ml of each of percoll solution with different densities in a sequence that the highest density percoll solution came in bottom and that of the lowest in the top of the tube. The newly collected cell suspension was slowly layered on the top of the above gradient and centrifuged at 800×g for 30 min at 18°C. The cells were collected in the interface between different densities of percoll solution, washed twice with PBS and resuspended in culture medium (1 ml). The cell numbers and cells viability were determined by trypan blue staining. The cell suspension was mixed with 0.4% trypan blue (1:1, v/v). The number of live (trypan blue excluding) and dead cells were determined using hemocytometer. The collected cells, in each gradient, were cyto-spun at 400 rpm for 5 min in poly-L-lysine pretreated slides (5×104 cells per each slide). Two immunocytochemical reactions were successively performed using PGP9.5 and c-kit primary antibodies.
Enrichment of undifferentiated SSCs by differential plating
The freshly isolated cells were seeded at a concentration of 2×104/cm 2 in 12-well cell chamber slides (Falcon, USA) in basic culture system consisted of high glucose DMEM (GibcoBRL) supplemented with 10% FBS and 1% penicillin-streptomycin (GibcoBRL). The cells were incubated in atmosphere of 5% CO2 with 85% relative humidity at 37°C for 3, 4, 5 and 6 hr. After that, the culture solutions containing non-adherent cells were removed gently and centrifuged at 1200 rpm for 10 min. The cell numbers and viability rates were determined after trypan blue staining. The cell suspension was mixed with 0.4% trypan blue (1:1, v/v). The collected cells, in each time, were cytospun at 400 rpm for 5 min in poly-L-lysine pretreated slides (5×104 cells per each). Two immunocyto-chemical reactions were successively performed using PGP9.5 and c-kit.
Identification of SSCs and purity detection by immunocytochemical staining
Type A spermatogonia were identified through immunocytochemical staining according to the protocol described by Herdari et al (28). Briefly, the purified cells, 5×104 cells, in each group were placed in each slide and centrifuged at 400 rpm for 5 min, fixed in acetone for 2 min at -20°C and placed at 4°C for 2 min. After drying the slides, cells were immunostained as described. Antigen retrieval was performed on sections through immuno-staining for PGP 9.5 and c-kit by Tween 20 (0.2% in PBS). The first unspecific site blocking was done with avidin/biotin. Therefore, the slides were incubated with avidin for 10 min in dark, and after three times of washing were incubated with biotin for 10 min in the same condition. All sections were exposed to 0.3% H2O2 for 15 min in dark to inhibit endogenous peroxidase and washed in TBS/ BSA (three times, 4 min each).
Another unspecific site blocking was done with 10% sheep serum in PBS for 30 min at room temperature. Subsequently, the slides were incubated with unconjugated primary antibodies including rabbit anti-PGP 9.5 (Dako, Carpinteria, CA, USA) and rabbit anti c-kit (Santa Cruz, Santa Cruz, CA, USA), each used at 1:100 dilution in PBS with 2.5% goat serum (PBS-GS), for 1 hr at room temperature. After washing three times in TBS/ BSA (5 min each), the sections were exposed to secondary antibody (biotinylated sheep anti-rabbit IgG, Avicenna Research Institute, Iran) for 45 min at room temperature and washed in TBS/BSA as described above. The sections were exposed to HRP-conjugated streptavidin (Biosource, USA) with 1:500 dilution for 30 min, and then washed with TBS/ BSA. At the final step, color was developed by the addition of 3, 3’-Diaminobenzidine (DAB; Roche, Germany) for 8-10 min. The slides were rinsed thoroughly in distilled water, counterstained with Harris hematoxylin for 30 s, washed in distilled water, dehydrated in graded alcohols, cleared in xylol, and were then mounted in Entellan (Merck, Germany).
Immunohistochemistry results were quantified by two evaluators blinded to the diagnosis of immunohistochemical reactivity. The percentages of PGP9.5 and c-kit positive and negative cells were evaluated by cell counting of prepared immunocytochemical slides.
Statistical analysis
All data were analyzed using one way ANOVA (Tukey Test). A p < 0.05 was defined as statistical significance.
Results
Histological evaluation of testes
In histological evaluation, the immature Sertoli cells, spermatogonial cells, and a small number of leydig cells were identified by morphological evaluations (Figures 1A and B). The seminiferous tubules had no lumen and different subpopulations of type A spermatogonia were mostly localized in the middle of the somniferous tubules (Figures 1A and B).
Figure 1.
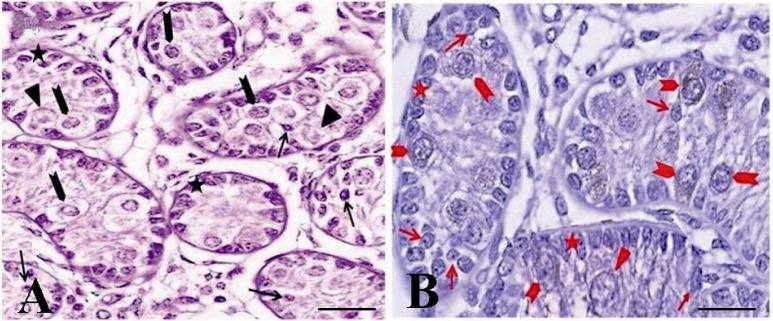
The immunohistochemical evaluation of the testis of a one month old goat using an antibody against c-kit. The presence of three groups of spermatogonia with different sizes was determined: basal (arrow, A, B) aggregated (arrowhead, A, B) and committed (triangle, A, B) type A spermatogonia. Both aggregated and committed spermatogonia were c-kit positive. Note that the strong and weak c-kit immunoreactivity was observed among aggregated (arrowhead, A, B) and committed (triangle, A, B) respectively. Basal spermatogonia (arrow, A, B), surrounding Sertoli and Leydig cells were negative for c-kit
In seminiferous tubules, the presence of three groups of spermatogonia with different sizes was determined (Figures 1A and B). These cells were often round or oval in shape with the following characteristics: normal size of about 10-12 µm in diameter, big spherical nucleus containing one to three irregular nucleoli of about 1-3 µm in diameter, high nucleus, cytoplasm ratio, and cytoplasmic inclusions which were mostly concentrated at one side of the cell (Figures 1 and 2).
Figure 2.
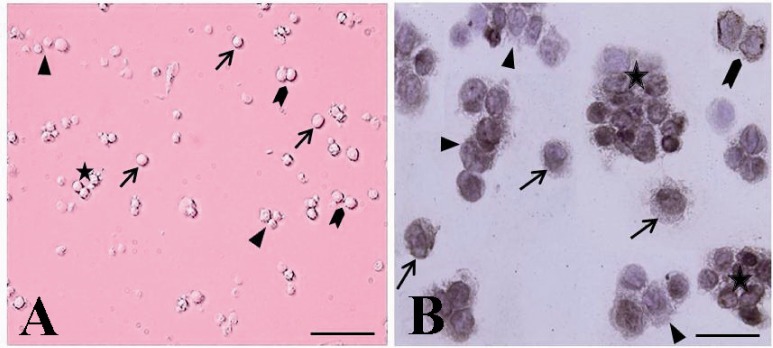
The cytological and immunocytochemical evaluation of spermatogonial stem cells using PGP9.5 immediately after isolation. The spermatogonia were seen as different forms: single (arrow, A, B), paired (arrowhead, A, B) aligned (triangle, A, B) and cluster (asterisk, A, B). The isolated SSCs were round cells with a spherical nucleus, a high nucleus, cytoplasm ratio, and many cytoplasmic inclusions that tended to congregate and form small cell clusters
Cytological analysis of spermatogonial stem cells
After cellular isolation, the phase contrast microscopy was used to identify spermatogonial stem cells in fractions on the basis of SSCs morphology. The cellular suspension was consisted of relatively homogenous population of the cells.
The presence of four groups of undifferentiated type A spermatogonia with different sizes in cell suspension was confirmed (Figure 2). They were observed in different forms as single (Figures 2A), paired (Figure 2B and C), aligned and cluster (Figure 2D) forms. The isolated SSCs, with more than 95% viability tended to congregate and form small cell clusters.
Enrichment of type A spermatogonia by discontinuous percoll gradient
The percentage of type A spermatogonia in suspension was taken to determine the effectiveness of the gradients on enrichment of the undifferentiated spermatogonia.
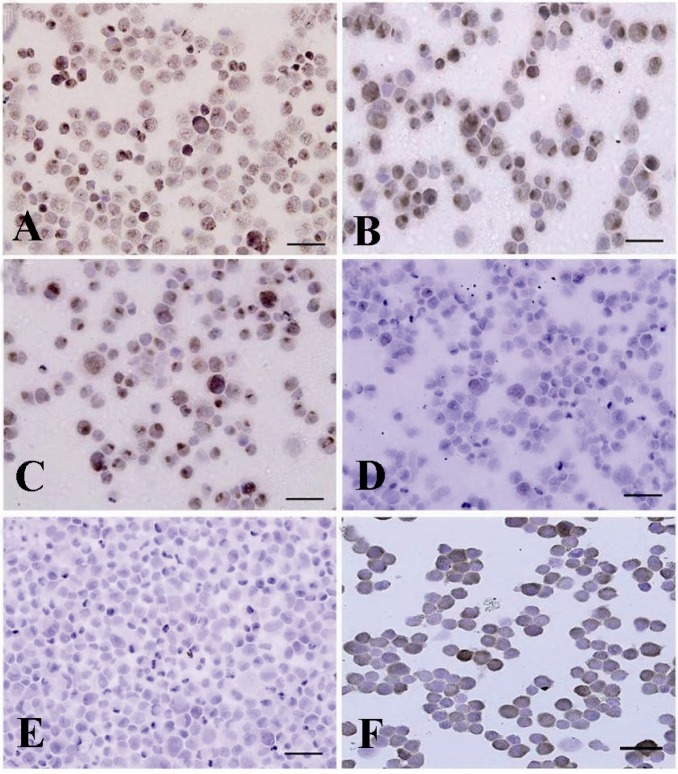
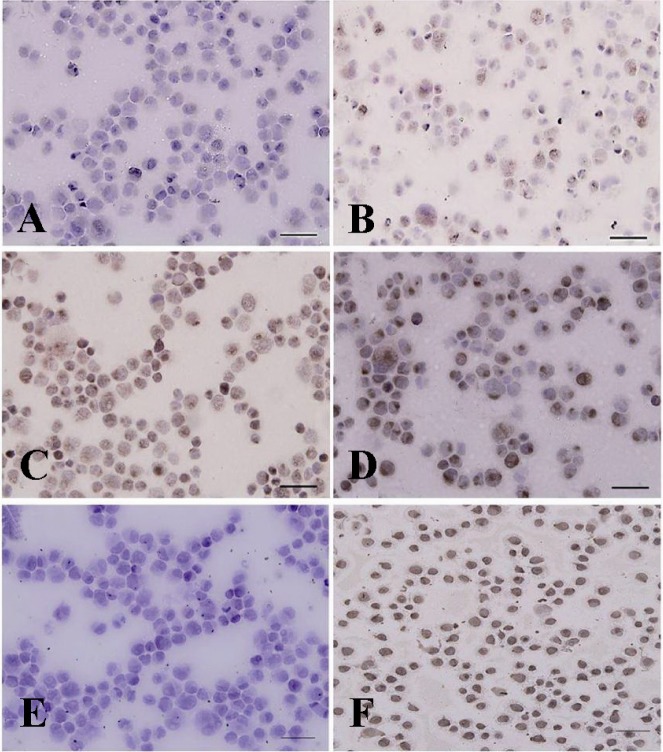
Data indicated the significant differences in the percentage of type A spermatogonia in cell population across the individual gradients (p < 0.001) and the percentage of PGP9.5 positive cells (undifferentiated type A cells) was found to be significantly different among groups. The proportions of undifferentiated spermatogonia in 30 and 32% gradients were significantly higher than 20 and 28% gradients (p < 0.001). The lowest percentage of undifferentiated type A spermatogonia was determined in 20% gradient (Figure 3). Concurrent with the increment of percoll gradient, the number of differentiated type A spermatogonia (c-kit positive cells) decreased (p < 0.001 Figure 4). The highest and lowest percentages of undifferentiated spermatogonia were found in gradients 32 and 20%, respectively (Table 1). Cell viability in all fractions was observed and found to be almost identical (100%).
Figure 3.
Immunocytochemical identification of undifferentiated type A spermatogonia after percoll separation method using an antibody against PGP9.5. Basal spermatogonia (dark brown) and some aggregated spermatogonia (light brown) in gradients A) 32% and B) 30% were significantly higher than C) 28% and D) 20% gradients. Note the intense staining in the cytoplasm, especially in the vicinity of the nucleus, in basal spermatogonia. E) Negative and F) positive controls were detected
Figure 4.
Immunocytochemical evaluation of differentiated type A spermatogonia in percoll separation method using ckit antibody. The proportion of c-kit positive cells (brown cells) in A) 32%; B) 30%; C) 28% and D) 20% gradients were determined. The highest (D) and lowest (A) percentages of differentiated spermatogonia were found in gradients 32 and 20% respectively. Note that the strong and weak c-kit immunoreactivity was observed among the aggregated (Aal) and committed (A1-A4) spermatogonia, respectively. E) Negative and F) positive controls were detected
Table 1.
Two methods of goat SSCs purification using PGP9.5 and c-kit molecular markers
| Molecular marker | Purification method | |||||||
|---|---|---|---|---|---|---|---|---|
| Percoll | Differential plating | |||||||
| 20% | 28% | 30% | 32% | 3 hr | 4 hr | 5 hr | 6 hr | |
| PGP 9.5 (%) | 41.5±3.1a | 85.7±1.2b | 92.7±0.6b,c | 94.6±0.4c,A | 55.5±1.1a | 62.9±0.8b | 81.3±1.1c,B | 74.9±1.3d |
| c-kit (%) | 63.0±0.7a | 33.5±0.7b | 18.1±0.9c | 25.1±0.7d,A | 43.6±1.3a | 27.3±0.9b | 17.1±1.4c,B | 26.3±1.2b |
In each method numbers with different lowercase letters in the same row differ significantly (p < 0.001)
Numbers with different uppercase letters in the same column indicates significant difference between two methods (p < 0.001)
Enrichment of type A spermatogonia by differential plating
According to the expression of PGP9.5 and c-kit molecular markers, the optimum time for adherence of most of somatic cells to plate and having the highest percentage of undifferentiated type A spermatogonia was 5 hr after culture initiation (Table 1).
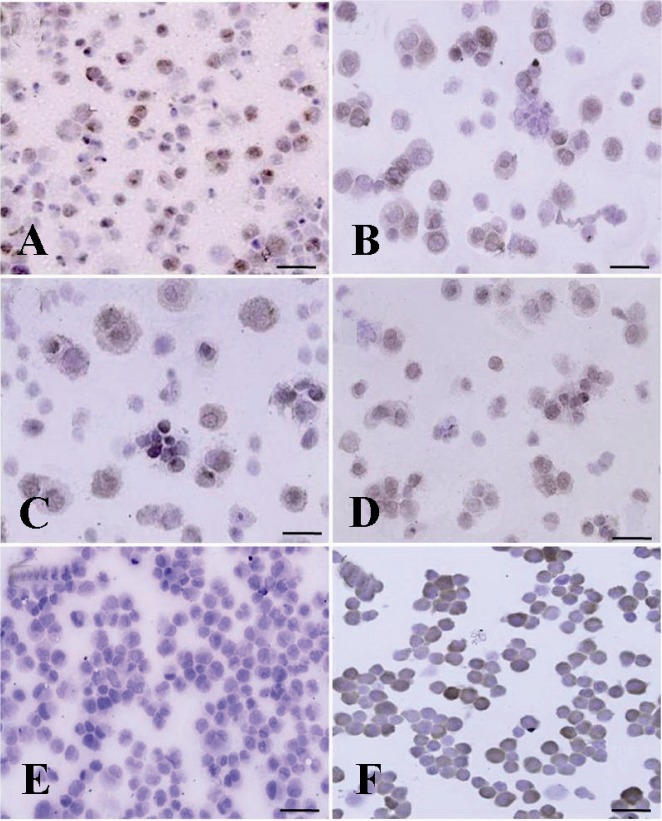
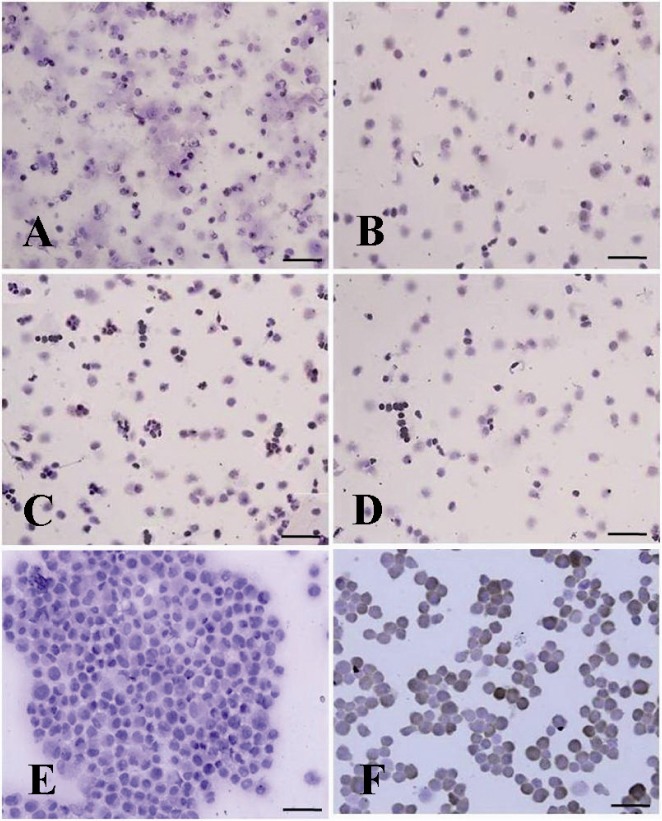
Enrichment of cell suspension, by 5 hr of culturing, resulted in a significant increase in PGP9.5 positive cells and reduced the population of differentiated type A spermatogonia (p < 0.001, Figures 5 and 6).
Figure 5.
Immunocytochemical localization of differentiated type A spermatogonia in differential plating method using c-kit. The percentage of c-kit positive cells (Aggregated spermatogonia, dark brown and committed spermatogonia, light brown) was evaluated; A) 3 hr; B) 4 hr; C) 5 hr and D) 6 hr after culture initiation. The most purified undifferentiated spermatogonia were achieved 5 hr after culture initiation. E) Negative and F) positive controls were detected
Figure 6.
Immunocytochemical identification of undifferentiated type A spermatogonia by differential plating method using PGP9.5. The proportion of PGP9.5 positive cells (basal spermatogonia, dark brown, and some aggregated spermatogonia, light brown) C) 5 hr after culture initiation was significantly higher than; A) 3 hr; B) 4 hr and D) 6 hr after culture. E) Negative and F) positive controls were determined
Discussion
Spermatogonial stem cells are unipotent adult stem cells responsible for maintenance of the fertility throughout the entire life of the males. These cells are the foundation of spermatogenesis (11) and have the potential to self-renew and at the same time generate the cascade of differentiating germ cells that will eventually lead to the formation of sperm (12, 17).
The development of the methods for the isolation and purification of undifferentiated SSCs in vitro will be the key in identification of their biochemical and morphologic properties, and attainment of the great potentials of SSCs in development of novel therapeutic strategies for treatment of infertility (1, 13, 30). The important aspects of spermatogonial stem cells resulted in their widespread use in reproductive technologies such as spermatogonial stem cell transplantation and transgenesis in species wherein embryonic stem cells are not available and somatic cell nuclear transfer and reprogramming pose several problems (16, 30, 31).
Three types of spermatogonia (types A, intermediate, and B) were initially described based on their nuclear morphology (32). spermatogonial stem cells undergo both self-repli-cating proliferation and production of daughter progeny to become type A spermatogonia (16, 17). The main characteristics in analysis of goat type A spermatogonia were morphological characteristics of the cells (nuclear and nucleolus) and the expression level of specific markers (28). In previous study, the presence of three groups of type A spermatogonia with different morphological characteristics in seminiferous tubules by immunostaining techniques was determined. Basal stem cells (As, Ap spermatogonia) were observed as the small round cells with thin rim of cytoplasm, large central nucleolus and one to three irregular nucleoli. Aggregated spermatogonia (Aal) with different sizes from small to large, containing one to two nucleoli and the larger ones committed spermatogonia that consisted of subpopulations of differentiating spermatogonia (A1-A4) (28). The difference in size of the spermatogonia might be related to either the phase of cell cycle (33) or their differentiation status (28, 34).
The testicular suspension isolated from seminiferous tubules is often contaminated by differentiating spermatogonial cells, Sertoli cells, Leydig cells and peritubular myoid cells (11, 13). Among the large population of somatic cells and differentiating germ cells within seminiferous tubules, only a small population of undifferentiated type A spermatogonia resides at the basement membrane of seminiferous tubules (7). Most culture systems are known to contain a mixture of testicular cells with about 1.33% SSCs (11, 12, 17). Therefore, the establishment of an efficient purification technique with the least influence on the SSCs survival and proliferation is necessary.
Among different methods of goat type A spermatogonia purification, in the present study, percoll density gradient and differential plating were chosen because these methods were faster, easier, less costly, safer and had the least negative effects on SSCs viability and their morphological characteristics.
The low viscosity and osmolality, two ideal features for separation of various cell types, has made the percoll ideal for separation of various types of cells (35). Percoll has been applied to separate adult and prepuberal types of spermatogonia cells in chicken (36), porcine (37), bovine (34, 35), and murine (25). The cell viability using percoll in previous studies (25, 38) ranged from 30 to 95% after SSCs separation which was consistent with our results (100%).
In the present study, the maximum type A spermatogonia purification (94.6%±0.4) was achieved by 32% percoll density gradient that was slightly higher than those studies which applied percoll in other species including rat with 80±6.1% SSCs purification in 30%-32% gradient (25), pig with 83.62±4.24% SSCs purification in 30-45% gradient (37), and human with 86.7% SSCs purification in 27-35% gradient (9).
In SSCs purification using differential plating, during cellular incubation, the somatic cells were attached to the bottom of culture plate due to its anchorage dependence so that the fast adherent velocity of the Sertoli and Leydig cells makes it possible for the majority of them to be removed from cell suspension after differential plating. In the present study, the maximum purity of undifferentiated type A spermatogoinia (81.3%) in differential plating was achieved 5 hr after culture initiation which was identical to the report by Kok-kinaki with a purity of 85.7% obtained for mouse testicular tissue (38). The obtained purity, however, in rat (97%) and pig (94%) using this method with 90-95% viability rate (30, 39) was higher than the corresponding rate in the current study. Concerning the time of culturing, the overnight incubation in other species resulted in lower purification rate compared to our results, for instance, 40% in bovine (17) and 75% in ram (40). In above studies, the cellular viability after isolation was also lower than the one in the present study (80-90% vs. 99%).
Conclusion
In our study, apart from more typical morphological characteristics of SSCs and the higher intensity of immunocytochemical reactivity in percoll method compared to differential plating, regarding the superiority of two purification methods in separation of undifferentiated spermatogonia, higher PGP9.5 positive and lower c-kit positive, the maximum purification rate in percoll method (94.6%) was significantly (p < 0.001) higher than the maximum rate in differential plating method (81.3%). The higher purity of undifferentiated spermatogonia using percoll compared to differential plating method in ovine and bovine species (35, 40) was in agreement to the present results.
Acknowledgement
The authors would like to thank Avicenna Research Institute for technical and financialsupports, ACECR, Tehran.
References
- 1.Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: are there differences to those in rodents? Reproduction. 2010;139(3):479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Andro. 2000;21(6):776–798. [PubMed] [Google Scholar]
- 3.Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Akt mediates self-re-newal division of mouse spermatogonial stem cells. Development. 2007;134(10):1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 4.Dobrinski I. Advances and applications of germ cell transplantation. Hum Fertil. 2006;9(1):9–14. doi: 10.1080/14647270500440671. [DOI] [PubMed] [Google Scholar]
- 5.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440(7088):1190–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y, Liu T, Zhao H, Xu H, Wang W, Liu R, et al. Establishment of normal medaka fish spermatogonial cell line capable of sperm production in vitro. Proc Natl Acad Sci USA. 2004;101(21):8011–8016. doi: 10.1073/pnas.0308668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaul G, Kumar Sh, Kumari S. Enrichment of CD9+ spermatogonial stem cells from goat (Capra aegag-rus hircus) testis using magnetic microbeads. Stem Cell Discov. 2012;2(3):92–99. [Google Scholar]
- 8.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141–150. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279(1):114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365(1546):1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113(11-12):727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- 13.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71(3):722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 14.Tegelenbosch RAJ, de Rooij DG. A quantitative study of spermatogonial multiplication and stem-cell renewal in the C3h/101 F1-hybrid mouse. Mutat Res. 1993;290(2):193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 15.Meistrich ML, van Beek MEAB. Spermatogonial stem cells. In: Desjardins C, Ewing L.L, editors. Cell and Molecular Biology of the Testis. New York: Oxford University Press; 1993. pp. 266–295. [Google Scholar]
- 16.Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24(6):1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aponte PM, de Rooij DG. Biomanipulation of bovine spermatogonial stem cells. Anim Reprod. 2008;5(1):16–22. [Google Scholar]
- 18.Hasthorpe S. Clonogenic culture of normal spermatogonia: in vitro regulation of postnatal germ cell proliferation. Biol Reprod. 2003;68(4):1354–1360. doi: 10.1095/biolreprod.102.008458. [DOI] [PubMed] [Google Scholar]
- 19.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Long-term culture of mouse male germline stem cells under serum- or feeder-free conditions. Biol Reprod. 2005;72(4):985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 20.Shinohara T, Brinster RL. Enrichment and transplantation of spermatogonial stem cells. Int J Androl. 2000;23(Suppl 2):89–91. doi: 10.1046/j.1365-2605.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 21.Huckins C. The spermatogonial stem cell population in adult rats, their morphology, proliferation and maturation. Anat Rec. 1971;169(3):533–558. doi: 10.1002/ar.1091690306. [DOI] [PubMed] [Google Scholar]
- 22.Bellvé AR, Cavicchia JC, Millete CF, O'Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. J Cell Biol. 1977;74(1):68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucci LR, Brock WA, Johnson TS, Meistrich Ml. Isolation and biochemical studies of enriched populations of spermatogonia and early primary spermatocytes from rat testes. Bioi Reprod. 1986;34(1):195–206. doi: 10.1095/biolreprod34.1.195. [DOI] [PubMed] [Google Scholar]
- 24.Borjigin U, Davey R, Hutton K, Herrid M. Expression of promyelocytic leukaemia zinc-finger in ovine testis and its application in evaluating the enrichment efficiency of differential plating. Reprod Fertil Dev. 2010;22(5):733–742. doi: 10.1071/RD09237. [DOI] [PubMed] [Google Scholar]
- 25.van Pelt AM, Morena AR, van Dissel-Emiliani FM, Boitani C, Gaemers IC, de Rooij DG, et al. Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod. 1996;55(2):439–444. doi: 10.1095/biolreprod55.2.439. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Megee S, Rathi R, Dobrinski I. Protein gene product 9.5 is a spermatogonia-specific marker in the pig testis: application to enrichment and culture of porcine spermatogonia. Mol Reprod Dev. 2006;73:1531–1540. doi: 10.1002/mrd.20529. [DOI] [PubMed] [Google Scholar]
- 27.Iwanami Y, Kobayashi T, Kato M, Hirabayashi M, Hochi S. Characteristics of rat round spermatids differentiated from spermatogonial cells during co-culture with Sertoli cells, assessed by flow cyto-metry, microinsemination, and RT-PCR. Theriogenology. 2006;65(2):288–298. doi: 10.1016/j.theriogenology.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, et al. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. 2012;29(10):1029–1038. doi: 10.1007/s10815-012-9828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gang B, Yanfeng L, Qiansheng L, Fengshuo J, Yong Z. Isolation and purification of human sperm-atogenous cells. Acta Acad Med Militaris Tertiae. 2005;27:1142–1144. [Google Scholar]
- 30.Han SY, Gupta MK, Uhm SJ, Lee HT. Isolation and in vitro culture of pig spermatogonial stem cell. Asian-Australasian J Anim Sci. 2009;22(2):187–193. [Google Scholar]
- 31.Dobrinski I, Travis AJ. Germ cell transplantation for the propagation of companion animals, nondomestic and endangered species. Reprod Fertil Dev. 2007;19(6):732–739. doi: 10.1071/rd07036. [DOI] [PubMed] [Google Scholar]
- 32.Monesi V. Autoradiographic study of DNA synthesis and the cell cycle in spermatogonia and spermatocytes of mouse testis, using tritiated thymidine. J Cell Biol. 1962;14:1–18. doi: 10.1083/jcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lok D, Weenk D, de Rooij DG. Morphology, proliferation, and differentiation of undifferentiated spermatogonia in the Chinese hamster and the ram. Anat Rec. 1982;203(1):83–99. doi: 10.1002/ar.1092030109. [DOI] [PubMed] [Google Scholar]
- 34.Izadyar F, Matthijs-Rijsenbilt JJ, den Ouden K, Creemers LB, Woelders H, de Rooij DG. Development of a cryopreservation protocol for type A spermatogonia. J Androl. 2002;23(4):537–545. [PubMed] [Google Scholar]
- 35.Izadyar F, Spierenberg G, Creemers L, Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124(1):85–94. [PubMed] [Google Scholar]
- 36.Wu XS, Wu H, Li BC, Zhou GY, Sun SY, Qin J, et al. Isolation, purification and culture of spermatogonia in chicken. J Anim Vet Adv. 2009;8(12):2418–2423. [Google Scholar]
- 37.Marret C, Durand P. Culture of porcine spermatogonia: Effects of purification of the germ cells, extracellular matrix, and fetal calf serum on their survival and multiplication. Reprod Nut Dev. 2000;40(3):305–319. doi: 10.1051/rnd:2000127. [DOI] [PubMed] [Google Scholar]
- 38.Kokkinaki M, Lee TL, He Z, Jiang J, Golestaneh N, Hofmann MC, et al. The molecular signature of spermatogonial stem cells in the 6-day-old mouse testis. Biol Reprod. 2009;80(4):707–717. doi: 10.1095/biolreprod.108.073809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamra FK, Gatlin J, Chapman KM, Grellhes DM, Garcia JV, Hammer RE, et al. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2002;99(23):14931–14936. doi: 10.1073/pnas.222561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Sosa JR, Dobson H, Hahnel A. Isolation and transplantation of spermatogonia in sheep. Theriogenology. 2006;66(9):2091–2103. doi: 10.1016/j.theriogenology.2006.03.039. [DOI] [PubMed] [Google Scholar]