Abstract
The expression of IL-17F is seen in the airway of asthmatics and its level is correlated with disease severity. Several studies have demonstrated that IL-17F plays a pivotal role in allergic airway inflammation and induces several asthma-related molecules such as CCL20. IL-17F-induced CCL20 may attract Th17 cells into the airway resulting in the recruitment of additional Th17 cells to enhance allergic airway inflammation. We have recently identified, for the first time, that bronchial epithelial cells are its novel cell source in response to IL-33 via ST2-ERK1/2-MSK1 signaling pathway. The receptor for IL-17F is the heterodimeric complex of IL-17RA and IL-17RC, and IL-17F activates many signaling pathways. In a case-control study of 867 unrelated Japanese subjects, a His161 to Arg161 (H161R) substitution in the third exon of the IL-17F gene was associated with asthma. In atopic patients with asthma, prebronchodilator baseline FEV1/FVC values showed a significant association with the H161R variant. Moreover, this variant is a natural antagonist for the wild-type IL-17F. Moreover, IL-17F is involved in airway remodeling and steroid resistance. Hence, IL-17F may play an orchestrating role in the pathogenesis of asthma and may provide a valuable therapeutic target for development of novel strategies.
1. Introduction
Asthma is characterized by bronchoconstriction, airway hyperreactivity, inflammation, mucus hypersecretion, and remodeling. These processes are coordinated by a complex cytokine network. The clarification of the modulation of this cytokine network could contribute to the understanding of asthma pathogenesis and development of new therapeutic strategies. IL-17A, the original member of the IL-17 cytokine family, was first identified in 1993 and was initially recognized for its similarity to a sequence belonging to the open reading frame 13 of Herpesvirus saimiri (HVS13) [1, 2]. Moreover, five additional members, IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F, were discovered within a short period of time since 2000 to 2002 [3–9]. Structurally, the IL-17 cytokine family members have no sequence similarity to any other known cytokine or other mammalian proteins [2]. Similarly, the IL-17 receptor family (IL-17RA-RE) is not related to any of the other known cytokine receptors [2]. Thus, the IL-17 cytokine family appears to represent a distinct ligand-receptor signaling system. We and other groups discovered the human IL-17F gene from a human EST sequence, a genomic DNA clone, and T-cell cDNA sequences in 2001 [3, 8, 9]. The gene is localized on the same chromosome at the distance of about 50 kb from telomeric sequences of IL-17A gene, and both genes are in a tail-to-tail orientation [3]. Functional studies have suggested that IL-17F is involved in asthma pathology. Hence, increased understanding of the significance of IL-17F would help to uncover the molecular mechanisms of asthma. In this review, we discuss the finding that IL-17F has a key role in asthma pathology and is a novel drug target for asthma.
2. Structural Features
Among the IL-17 cytokine family members, IL-17F shows the highest amino acid sequence homology (50%) to IL-17A, while only 10–30% sequence identity is seen between IL-17A and the other family members [10]. These cytokines have their greatest similarity within the C-terminal 70 amino acids and have four well-conserved cysteines. The four conserved cysteines in the C-terminal half of the IL-17F sequence are shown to form a cystine knot structural motif in the crystal structure, and, interestingly, this cystine knot structure is similar to a common structural motif seen in several growth factors, such as bone morphogenic proteins (BMPs), TGF-β, nerve growth factor (NGF), and platelet-derived growth factor (PDGF) [9]. Of note, recent reports have demonstrated that IL-17A and IL-17F can be produced as heterodimers termed IL-17A/F [11]. These three cytokines are differentially expressed in activated CD4+ T cells.
3. Cellular Source and Tissue Distribution
IL-17F is expressed in activated CD4+ T cells, basophils, and mast cells, three important cell types involved in allergic airway inflammation [3]. Moreover, IL-17F is also derived from Th17 cells, a CD4+ T-cell lineage distinct from Th1 cells and Th2 cells [12]. However, Th17 cells may not be the major cell source of IL-17F in lung diseases [13]. Recent studies have demonstrated that IL-17F is also produced by many cell types such as memory CD4+ T cells, CD8+ T cells, γδT cells, NKT cells, B cells, and LTi cells [14–17]. These findings suggest that IL-17F is involved in the pathogenesis of a wide range of diseases beyond asthma. However, it remains to be determined which IL-17F-producing cell types contribute to asthma pathogenesis in response to various stimuli in human. So far, IL-17F has been thought to be derived from hematopoietic cells, but not nonhematopoietic cells such as lung structural cells. Recently, we have reported, for the first time, that bronchial epithelial cells are a novel cell source of IL-17F in response to IL-33 [18]. IL-33 is genetically and functionally associated with the pathogenesis of asthma [19, 20]. These findings suggest that bronchial epithelial cells play a central role in asthma, at least partially, as target and effector cells for IL-17F. In addition, IL-17F is detected in a wider range of tissues such as liver, lung, ovary, and fetal liver when compared with IL-17A [3]. This suggests that IL-17F has a more diverse biological function, despite the high degree of sequence homology with IL-17A.
4. Biological Activities
IL-17F has multiple biological activities (Figure 1). IL-17F is able to induce asthma-related cytokines, chemokines, and adhesion molecules in bronchial epithelial cells [3, 20–28]. In addition to bronchial epithelial cells, IL-17F is also able to stimulate lung structural cells such as vein endothelial cells, airway smooth muscle cells, and fibroblasts [7, 8, 21, 22, 29]. Interestingly, a recent report demonstrated that IL-17F acts upon eosinophils, one of the most important inflammatory cells in allergic airway inflammation and remodeling, to induce several cytokines and chemokines such as IL-1β, IL-6, IL-8, GROα, and MIP-1β [30]. These cell types may play crucial roles in asthma in response to IL-17F. IL-17F may develop and amplify allergic airway inflammation by facilitating the activation of inflammatory cells and lung structural cells through the induction of a wide range of molecules. Moreover, Th2 cytokines, IL-4 and IL-13, are able to enhance the biological activities of IL-17F [26–28, 31]. These findings suggest that the interaction of Th2 cytokines and IL-17F augments allergic airway inflammation.
Figure 1.

Biological activities of IL-17F. IL-17F has multiple biological activities. IL-17F is produced by several cell types including Th17 cells and bronchial epithelial cells. IL-17F can induce various asthma-related cytokines, chemokines, and adhesion molecules in bronchial epithelial cells, eosinophils, fibroblasts, airway smooth muscle cells, and vein endothelial cells, and thereby contributes to the pathogenesis of asthma.
5. Receptor and Signaling Pathway
Our understanding of the signaling pathway of IL-17F has gradually become clearer (Figure 2). Similar to IL-17A, the receptor for IL-17F is the heterodimeric complex of IL-17RA and IL-17RC [32]. Both IL-17RA and IL-17RC are necessary for the biological activity of IL-17F. Although human IL-17RA binds IL-17A effectively, it binds IL-17F with ~1000-fold lower affinity [33]. The relative binding affinity of IL-17F to IL-17RC is much stronger than to IL-17RA. Activation of the receptor by IL-17F leads to an interaction with Act-1 via the similar expression to fibroblast growth factor genes, IL-17 receptors, and TIR (SEFIR) domain [34]. This sequentially mediates activation of TNF receptor-associated factor- (TRAF-) 6, leading to the activation of TGFβ activated kinase (TAK) 1 [34, 35]. We have reported that the Raf1-MEK1/2-ERK1/2 pathway is a central upstream signaling pathway for IL-17F-induced cytokine and chemokine expression in bronchial epithelial cells and vein endothelial cells [21–28, 31]. In the downstream signaling pathway, we have also identified that mitogen- and stress-activated protein kinase1- (MSK1-) cyclic AMP response element binding protein (CREB) and p90 ribosomal S6 kinase- (p90RSK-) CREB are critical downstream signaling pathways [25–28, 31]. These pathways are located downstream of the Raf1-MEK1/2-ERK1/2 kinase cascade and are essential for cytokine expression by IL-17F. Further, IL-17F also activates transcriptional factors such as C/EBPβ, C/EBPγ, and NF-κB [34]. On the other hand, little is known about the signaling mechanisms of IL-17F expression. As shown in Figure 3, we have recently reported that bronchial epithelial cells are a novel cell source of IL-17F, and epithelial IL-17F expression is mediated via the activation of ST2-ERK1/2-MSK1 signaling pathway in response to IL-33 [18]. ST2 is a receptor for IL-33 [36]. However, other signaling molecules including transcriptional factors for IL-17F expression still remain undiscovered. These findings suggest that these signaling pathways are potential pharmacological targets in the IL-17F-mediated airway inflammation.
Figure 2.
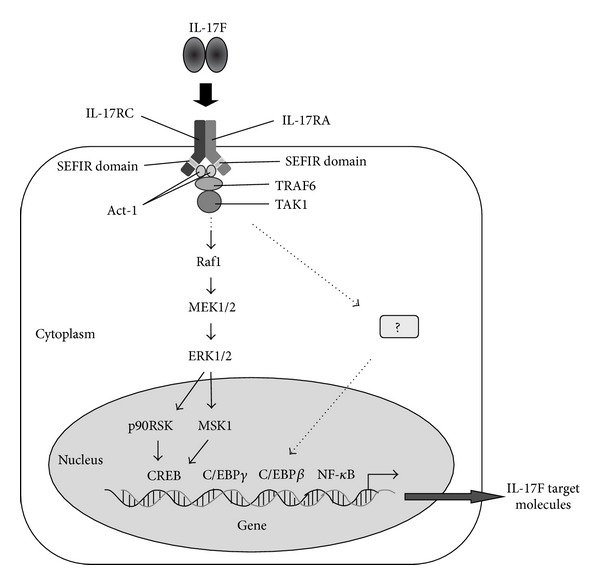
Signaling pathways induced by IL-17F. IL-17F utilizes a heterodimer of IL-17RA and IL-17RC as its receptor, and then IL-17RA engages the SEFIR domain-containing Act-1. Act-1 is required for recruitment of TRAF6, leading to the activation of TAK1. The Raf1-MEK1/2-ERK1/2-p90RSK/MSK1-CREB is the pivotal signaling pathway. On the other hand, IL-17F also activates transcriptional factors such as C/EBPβ, C/EBPγ, and NF-κB. Activation of these pathways leads to the expression of various inflammatory molecules.
Figure 3.
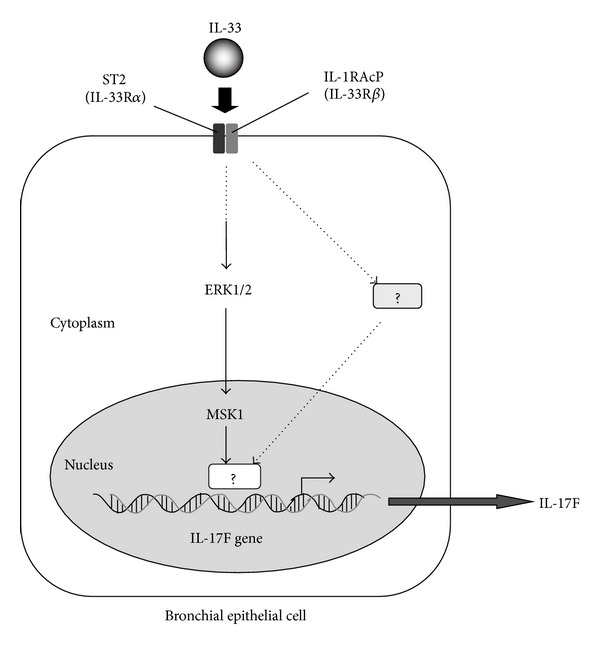
Signaling mechanism of IL-17F expression in bronchial epithelial cells. Bronchial epithelial cells are a novel cell source of IL-17F. The expression of IL-17F is induced by IL-33. IL-33 binds to its receptor, ST2, and then the ERK1/2-MSK1 signaling pathway is activated. However, other stimuli inducing IL-17F and their signaling pathways are largely unknown.
6. Recruitment of Th17 Cells
Th17 cells play a pivotal role in a diverse group of immune-mediated diseases and host defense mechanisms [12]. Emerging evidence suggests that the Th17 cells provide a new insight into the molecular mechanisms of asthma [37]. Th17 cells have been isolated from bronchial tissues taken from patients during acute episodes of severe asthma [38]. Another study demonstrated that the percentages of Th17 cells in PBMCs are higher in allergic asthmatics than those in healthy subjects and show a tendency to increase with the disease severity [39]. However, it is unclear how Th17 cells migrate into the airway of asthmatics. We demonstrated that IL-17F induces CCL20 in bronchial epithelial cells [31]. Human Th17 cells predominantly express CCR6 [40]. This implies that its ligand, CCL20, is able to attract Th17 cells into the site of airway inflammation via CCR6. Taken together, it is possible that IL-17F-induced epithelial CCL20 attracts Th17 cells into the airway, and accumulated Th17 cells establish a positive feedback loop resulting in the recruitment of additional Th17 cells via the induction of IL-17F. Hence, IL-17F-producing cells may exert an effect on bronchial epithelial cells to induce CCL20 and attract Th17 cells via CCR6. Although in vivo study is needed to clarify this hypothesis, the IL-17F/CCL20 axis might be especially important in the pathophysiologic events of allergic airway inflammation.
7. Airway Neutrophilia
IL-17F is involved in neutrophilic inflammation in the airway [41, 42]. Although airway neutrophilia is one of the hallmarks of severe asthma, its mechanism is not well understood. In a mouse model of study, overexpression of IL-17F using an adenoviral gene transfer strategy in the mouse airways also leads to an increased number of neutrophils in bronchoalveolar lavage fluid (BALF) [41]. Another study using a different model has revealed that overexpression of IL-17F through intratracheal delivery of the IL-17F gene results in an increase in the number of neutrophils and macrophages in the airways [42]. Moreover, IL-17F-deficient mice have revealed that IL-17F is more critical than IL-17A in inducing airway neutrophilic inflammation to Aspergillus oryzae [34]. Specific inhibition of CD4+ T cells, either with a CD4 Ab or an IL-2R Ab, prevents allergen-induced recruitment of both eosinophils and neutrophils in animal models, suggesting CD4+ T cells regulate airway neutrophilia [43, 44]. However, little is known about how CD4+ T cells elicit neutrophil accumulation into the airway. IL-17F may be one of the key regulators for airway neutrophilia induced by CD4+ T cells such as Th17 cells. Of interest is the finding that lung tissues from IL-17F gene transduced mice show substantial increases in the level of various inflammatory cytokines and chemokines, including IL-1β, IL-6, KC, and MIP-2 [41, 42]. These molecules are known to be involved in chemotaxis and activation for neutrophils. Additionally, in vitro studies have demonstrated that IL-17F is able to induce C-X-C chemokines, such as IL-8, ENA-78, and GROα, which are potent chemoattractants for neutrophils [3, 21, 22]. Neutrophil recruitment into the airway may be regulated through, at least partially, IL-17F-induced C-X-C chemokines. In contrast, C-C chemokines, such as eotaxin and RANTES, which are potent chemoattractants for eosinophils, are not produced by IL-17F, suggesting a selective role of IL-17F in neutrophil recruitment and activation in the airway [3].
8. Mucus Hypersecration and Airway Hyperreactivity
Asthma is characterized by mucus hypersecretion (goblet cell hyperplasia/metaplasia) and airway hyperreactivity that are consistently linked to asthma symptoms and morbidity. IL-17F may be involved in these pathological processes. Overexpression of IL-17F in the airway of mice resulted in the induction of goblet cell hyperplasia and the gene expression of MUC5AC, but only when the mice are challenged with antigen, and increased goblet cell hyperplasia is seen only in the small airways [42]. These results suggest that in addition to IL-13, IL-17F may also be an important contributor to mucus hypersecretion in asthma. Moreover, a significant increase in airway hyperreactivity was also noted in mice overexpressing IL-17F following Ag challenge, when compared to that of mice receiving mock control [42]. These findings suggest that IL-17F has an additive or enhancing effect on antigen-induced allergic inflammatory responses.
9. Airway Remodeling
We have reported that IL-17F induces profibrotic cytokines, IL-11 and IGF-I, in bronchial epithelial cells [27, 28]. IL-11 elicits subepithelial fibrosis, accumulation of fibroblasts, myofibroblasts and myocytes, and deposition of types I and III collagen [45]. IGF-I is able to induce collagen synthesis and smooth muscle hyperplasia and is also a potent mitogen for fibroblasts and smooth muscle cells [46–48]. The blockade of IGF-I inhibited the elevation of airway resistance, airway inflammation, increase in airway wall thickening, and the expression of ICAM-1. In humans, the expression of IGF-I is significantly increased within the airways of subjects with severe asthma when compared with those with mild asthma [49]. Its expression was inversely correlated to collagen thickening and the number of fibroblasts. Moreover, treatment with beclomethasone dipropionate significantly decreased the expression of IGF-I with reduction of the thickness of lamina reticularis. Blocking of IGF-I expression may contribute to prevent airway remodeling. In other studies, IL-17F has been shown to induce the expression of TGF-β in vein endothelial cells [8]. TGF-β is a profibrotic cytokine and has been implicated in the extracellular matrix changes observed in fibrosis. More recently, direct effect of IL-17F to airway smooth muscle (ASM) cells was demonstrated. IL-17F promotes migration of ASM cells via p38MAPK [50]. Th17 cells contribute to airway remodeling via excessive mucus expression and ASM proliferation [51]. These findings suggest the potential involvement of IL-17F in the process of airway remodeling.
10. Steroid Resistance
Recent studies have demonstrated that IL-17F is involved in steroid resistance in asthma. Th17 cells, but not Th2 cells, mediate steroid resistant airway inflammation and airway hyperreactivity in a mouse model of asthma [52]. In the setting of in vivo polarized Th17 cell transfer, chemokine secretion, cellular influx to the airways, and airway hyperreactivity are not sensitive to dexamethasone treatment. Other studies have reported that IL-17F induced expression of glucocorticoid receptor- (GR-) β mRNA in bronchial epithelial cells from healthy subjects as well as asthmatic patients [53]. GR-β acts as a dominant negative inhibitor of GR-α which is the active isoform of this receptor. Moreover, unlike healthy subjects, IL-6 induced by IL-17A and IL-17F was not inhibited by dexamethasone in bronchial epithelial cells from asthmatic patients. These findings suggest that steroid resistance in subjects with severe asthma may be due to, at least in part, IL-17F and Th17 cells.
11. Expression in the Airway of Asthmatic Patients
The expression of IL-17F is observed in the airway of asthmatic patients. Analyses of its expression in BAL cells from asthmatic subjects challenged with allergen or saline control show that while no detectable expression of IL-17F was seen in the BAL cells from saline-challenged sites, its expression was obviously seen in the BAL cells from allergen-challenged sites of all four study subjects [3]. IL-17F is expressed in both bronchial epithelium and inflammatory infiltrates [54, 55]. Immunocytochemistry showed that IL-17F positive cells in the subepithelial component and epithelium are significantly elevated in severe asthma compared with healthy and mild asthmatic subjects [55]. Additionally, an increased expression of epithelial IL-17F was correlated with disease severity. Moreover, another recent study demonstrated that asthmatic patients have a significantly higher level of serum IL-17F protein as compared to that of healthy subjects [56]. This implies that IL-17F can be used as a clinical biomarker of asthma diagnosis and management. Further validation is needed in the future.
12. Genetic Relevance
We investigated the genetic association of asthma with the common variants of IL-17F, using 867 unrelated Japanese subjects [57]. Five polymorphisms were studied, including the coding-region sequence variant SNP rs763780 (7488T>C), which causes a His-to-Arg substitution at amino acid 161 (H161R). A genotype-based χ 2 association analysis indicated a significant association between the H161R variant and asthma. Importantly, none of the asthmatic subjects were homozygous for H161R. The homozygosity of the H161R variant is associated with the protection against asthma; the odds ratio (OR) for asthma was 0.06 (95% confidence interval, 0.01–0.43, P = 0.0039) among H161R homozygotes compared with wild-type homozygotes. In atopic patients with asthma, prebronchodilator baseline FEV1/forced vital capacity (FVC) values also showed a significant association with the H161R variant [58]. This suggests that the H161R variant of IL-17f is associated with asthma severity. Moreover, in vitro functional studies demonstrated that, compared with wild-type IL-17F, the H161R variant is unable to activate ERK1/2 that is a critical signaling molecule of IL-17F [57] but, interestingly, is able to block the induction of IL-8 by wild-type IL-17F in a dose-dependent manner. These findings suggest that the H161R variant is a natural antagonist for the wild-type IL-17F and may be an attractive therapeutic target in IL-17F-mediated diseases. However, further study is needed to clarify the precise mechanisms by which H161R variant exerts its antagonistic effect. Interestingly, recent studies have demonstrated novel therapeutic options targeting IL-17A, IL-17F, and their signaling pathways. Inhibition of either IL-17RA or IL-17RC expression via siRNA revealed significant reduction of IL-17A/IL-17F-stimulated chemokine production [59]. Similarly the microRNA, miR-23b, suppresses IL-17A-associated autoimmune inflammation by targeting TGF-β-activated kinase 1/MAP3 K7 binding protein (TAB)2, TAB3, and IKK-α [60]. These molecules may provide therapeutic benefit for immune and inflammatory diseases.
13. Conclusions
IL-17F is one of the important cytokines involving in the pathophysiologic events of asthma. In vivo and in vitro studies have implicated that IL-17F shows multiple functions in the pathogenesis of airway allergic inflammation. In particular, CCL20 induced by IL-17F may enhance Th17-mediated airway inflammation via CCR6. Although biological function of IL-17F has become clear, its inducible factors still remain except for IL-33. It is suggested that further investigation of IL-17F is informative in pointing to novel approaches to the diagnosis and treatment of asthma.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (C) 23591455. S. K. Huang was supported, in part, by National Institute of Health (AI-052468), National Science Council/Ministry of Health (EODOH01), Taiwan, and National Health Research Institutes (EOPP10-014 and EOSP07-014), Taiwan.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. Journal of Immunology. 1995;155(12):5483–5486. [PubMed] [Google Scholar]
- 2.Yao Z, Fanslow WC, Seldin MF, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Onuchic LF, Li X-D, et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. Journal of Immunology. 2001;167(8):4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Ullrich SJ, Zhang J, et al. A novel cytokine receptor-ligand pair: identification, molecular characterization, and in vivo immunomodutory activity. Journal of Biological Chemistry. 2000;275(25):19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Chen J, Huang A, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(2):773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. Journal of Immunology. 2002;169(2):642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Ho W-H, Maruoka M, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. Journal of Biological Chemistry. 2001;276(2):1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 8.Starnes T, Robertson MJ, Sledge G, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. Journal of Immunology. 2001;167(8):4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 9.Hymowitz SG, Filvaroff EH, Yin J, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. The EMBO Journal. 2001;20(19):5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine and Growth Factor Reviews. 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 11.Wright JF, Guo Y, Quazi A, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. Journal of Biological Chemistry. 2007;282(18):13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 13.Prause O, Bossios A, Silverpil E, et al. IL-17-producing T lymphocytes in lung tissue and in the bronchoalveolar space after exposure to endotoxin from Escherichia coli in vivo: effects of anti-inflammatory pharmacotherapy. Pulmonary Pharmacology and Therapeutics. 2009;22(3):199–207. doi: 10.1016/j.pupt.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciric B, El-behi M, Cabrera R, Zhang G-X, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. Journal of Immunology. 2009;182(9):5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- 16.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nature Reviews Immunology. 2010;10(7):479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Tello A, Halwani R, Li R, et al. IL-17A and IL-17F expression in B lymphocytes. International Archives of Allergy and Immunology. 2012;157(4):406–416. doi: 10.1159/000329527. [DOI] [PubMed] [Google Scholar]
- 18.Fujita J, Kawaguchi M, Kokubu F, et al. Interleukin-33 induces interleukin-17F in bronchial epithelial cells. Allergy. 2012;67(6):744–750. doi: 10.1111/j.1398-9995.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- 19.Gudbjartsson DF, Bjornsdottir US, Halapi E, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nature Genetics. 2009;41(3):342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 20.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clinical and Experimental Allergy. 2010;40(2):200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi M, Onuchic LF, Huang S-K. Activation of extracellular signal-regulated kinase (ERK)1/2, but not p38 and c-Jun N-terminal kinase, is involved in signaling of a novel cytokine, ML-1. Journal of Biological Chemistry. 2002;277(18):15229–15232. doi: 10.1074/jbc.C100641200. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Kokubu F, Matsukura S, et al. Induction of C-X-C chemokines, growth-related oncogene α expression, and epithelial cell-derived neutrophil-activating protein-78 by ML-1 (interleukin-17F) involves activation of Raf1-Mitogen-activated protein kinase kinase-extracellular signal-regulated kinase 1/2 pathway. Journal of Pharmacology and Experimental Therapeutics. 2003;307(3):1213–1220. doi: 10.1124/jpet.103.056341. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang S-K. IL-17 cytokine family. Journal of Allergy and Clinical Immunology. 2004;114(6):1265–1273. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi M, Adachi M, Huang S-K. Structural and functional analysis of a new cytokine, ML-1 (interleukin-17F) Allergology International. 2003;52(3):117–122. [Google Scholar]
- 25.Kawaguchi M, Kokubu F, Odaka M, et al. Induction of granulocyte-macrophage colony-stimulating factor by a new cytokine, ML-1 (IL-17F), via Raf I-MEK-ERK pathway. Journal of Allergy and Clinical Immunology. 2004;114(2):444–450. doi: 10.1016/j.jaci.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi M, Kokubu F, Huang S-K, et al. The IL-17F signaling pathway is involved in the induction of IFN-γ-inducible protein 10 in bronchial epithelial cells. Journal of Allergy and Clinical Immunology. 2007;119(6):1408–1414. doi: 10.1016/j.jaci.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi M, Fujita J, Kokubu F, et al. IL-17F-induced IL-11 release in bronchial epithelial cells via MSK1-CREB pathway. The American Journal of Physiology: Lung Cellular and Molecular Physiology. 2009;296(5):L804–L810. doi: 10.1152/ajplung.90607.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchi M, Fujita J, Kokubu F, et al. Induction of insulin-like growth factor-I by interleukin-17F in bronchial epithelial cells. Clinical and Experimental Allergy. 2010;40(7):1036–1043. doi: 10.1111/j.1365-2222.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 29.Al-Alwan LA, Chang Y, Baglole CJ, et al. Autocrine-regulated airway smooth muscle cell migration is dependent on IL-17-induced growth-related oncogenes. Journal of Allergy and Clinical Immunology. 2012;130(4):977–985. doi: 10.1016/j.jaci.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Cheung PFY, Wong CK, Lam CWK. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. Journal of Immunology. 2008;180(8):5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 31.Nozato K, Fujita J, Kawaguchi M, et al. IL-17F induces CCL20 in bronchial epithelial cells. Journal of Allergy. 2011;2011:8 pages. doi: 10.1155/2011/587204.587204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toy D, Kugler D, Wolfson M, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. Journal of Immunology. 2006;177(1):36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 33.Kuestner RE, Taft DW, Haran A, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. Journal of Immunology. 2007;179(8):5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XO, Seon HC, Park H, et al. Regulation of inflammatory responses by IL-17F. Journal of Experimental Medicine. 2008;205(5):1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rong Z, Cheng L, Ren Y, et al. Interleukin-17F signaling requires ubiquitination of interleukin-17 receptor via TRAF6. Cellular Signalling. 2007;19(7):1514–1520. doi: 10.1016/j.cellsig.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 37.Traves SL, Donnelly LE. Th17 cells in airway diseases. Current Molecular Medicine. 2008;8(5):416–426. doi: 10.2174/156652408785160998. [DOI] [PubMed] [Google Scholar]
- 38.Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. Journal of Immunology. 2008;180(11):7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Yang J, Gao Y-D, Guo W. Th17 immunity in patients with allergic asthma. International Archives of Allergy and Immunology. 2010;151(4):297–307. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]
- 40.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. Journal of Experimental Medicine. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. Journal of Immunology. 2002;169(1):443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 42.Oda N, Canelos PB, Essayan DM, Plunkett BA, Myers AC, Huang S-K. Interleukin-17F induces pulmonary neutrophilia and amplifies antigen-induced allergic response. The American Journal of Respiratory and Critical Care Medicine. 2005;171(1):12–18. doi: 10.1164/rccm.200406-778OC. [DOI] [PubMed] [Google Scholar]
- 43.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. The American Journal of Respiratory Cell and Molecular Biology. 1994;10(6):587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 44.Renzi PM, Yang JP, Diamantstein T, Martin JG. Effects of depletion of cells bearing the interleukin-2 receptor on immunoglobulin production and allergic airway responses in the rat. The American Journal of Respiratory and Critical Care Medicine. 1996;153(4):1214–1221. doi: 10.1164/ajrccm.153.4.8616544. [DOI] [PubMed] [Google Scholar]
- 45.Tang W, Geba GP, Zheng T, et al. Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. Journal of Clinical Investigation. 1996;98(12):2845–2853. doi: 10.1172/JCI119113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein RH, Poliks CF, Pilch PF, Smith BD, Fine A. Stimulation of collagen formation by insulin and insulin-like growth factor I in cultures of human lung fibroblasts. Endocrinology. 1989;124(2):964–970. doi: 10.1210/endo-124-2-964. [DOI] [PubMed] [Google Scholar]
- 47.Noveral JP, Bhala A, Hintz RL, Grunstein MM, Cohen P. Insulin-like growth factor axis in airway smooth muscle cells. The American Journal of Physiology: Lung Cellular and Molecular Physiology. 1994;267(6):L761–L765. doi: 10.1152/ajplung.1994.267.6.L761. [DOI] [PubMed] [Google Scholar]
- 48.Clemmons DR, Van Wyk JJ. Evidence for a functional role of endogenously produced somatomedinlike peptides in the regulation of DNA synthesis in cultured human fibroblasts and porcine smooth muscle cells. Journal of Clinical Investigation. 1985;75(6):1914–1918. doi: 10.1172/JCI111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoshino M, Nakamura Y, Sim JJ, et al. Inhaled corticosteroid reduced lamina reticularis of the basement membrane by modulation of insulin-like growth factor (IGF)-I expression in bronchial asthma. Clinical and Experimental Allergy. 1998;28(5):568–577. doi: 10.1046/j.1365-2222.1998.00277.x. [DOI] [PubMed] [Google Scholar]
- 50.Chang Y, Al-Alwan L, Risse P-A, et al. TH17 cytokines induce human airway smooth muscle cell migration. Journal of Allergy and Clinical Immunology. 2011;127(4):1046–1053. doi: 10.1016/j.jaci.2010.12.1117. [DOI] [PubMed] [Google Scholar]
- 51.Chang Y, Al-Alwan L, Risse PA, et al. Th17-associated cytokines promote human airway smooth muscle cell proliferation. The FASEB Journal. 2012;26(12):5152–5160. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 52.McKinley L, Alcorn JF, Peterson A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. Journal of Immunology. 2008;181(6):4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vazquez-Tello A, Semlali A, Chakir J, et al. Induction of glucocorticoid receptor-β expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clinical and Experimental Allergy. 2010;40(9):1312–1322. doi: 10.1111/j.1365-2222.2010.03544.x. [DOI] [PubMed] [Google Scholar]
- 54.Kokubu F, Matsukura S, Kawaguchi M, Osakabe Y. Respiratory viruses and bronchial asthma. Arerugi. 2008;57(11):1117–1123. [PubMed] [Google Scholar]
- 55.Al-Ramli W, Préfontaine D, Chouiali F, et al. TH17-associated cytokines (IL-17A and IL-17F) in severe asthma. Journal of Allergy and Clinical Immunology. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Bazzi MD, Sultan MA, Al Tassan N, et al. Interleukin 17A and F and asthma in Saudi Arabia: gene polymorphisms and protein levels. Journal of Investigational Allergology and Clinical Immunology. 2011;21(7):551–555. [PubMed] [Google Scholar]
- 57.Kawaguchi M, Takahashi D, Hizawa N, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. Journal of Allergy and Clinical Immunology. 2006;117(4):795–801. doi: 10.1016/j.jaci.2005.12.1346. [DOI] [PubMed] [Google Scholar]
- 58.Hizawa N, Kawaguchi M, Huang S-K, Nishimura M. Role of interleukin-17F in chronic inflammatory and allergic lung disease. Clinical and Experimental Allergy. 2006;36(9):1109–1114. doi: 10.1111/j.1365-2222.2006.02550.x. [DOI] [PubMed] [Google Scholar]
- 59.Iyoda M, Shibata T, Kawaguchi M, et al. IL-17A and IL-17F stimulate chemokines via MAPK pathways (ERK1/2 and p38 but not JNK) in mouse cultured mesangial cells: synergy with TNF-α and IL-1β . The American Journal of Physiology: Renal Physiology. 2010;298(3):F779–F787. doi: 10.1152/ajprenal.00198.2009. [DOI] [PubMed] [Google Scholar]
- 60.Zhu S, Pan W, Song X, et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nature Medicine. 2012;18(7):1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]


