Abstract
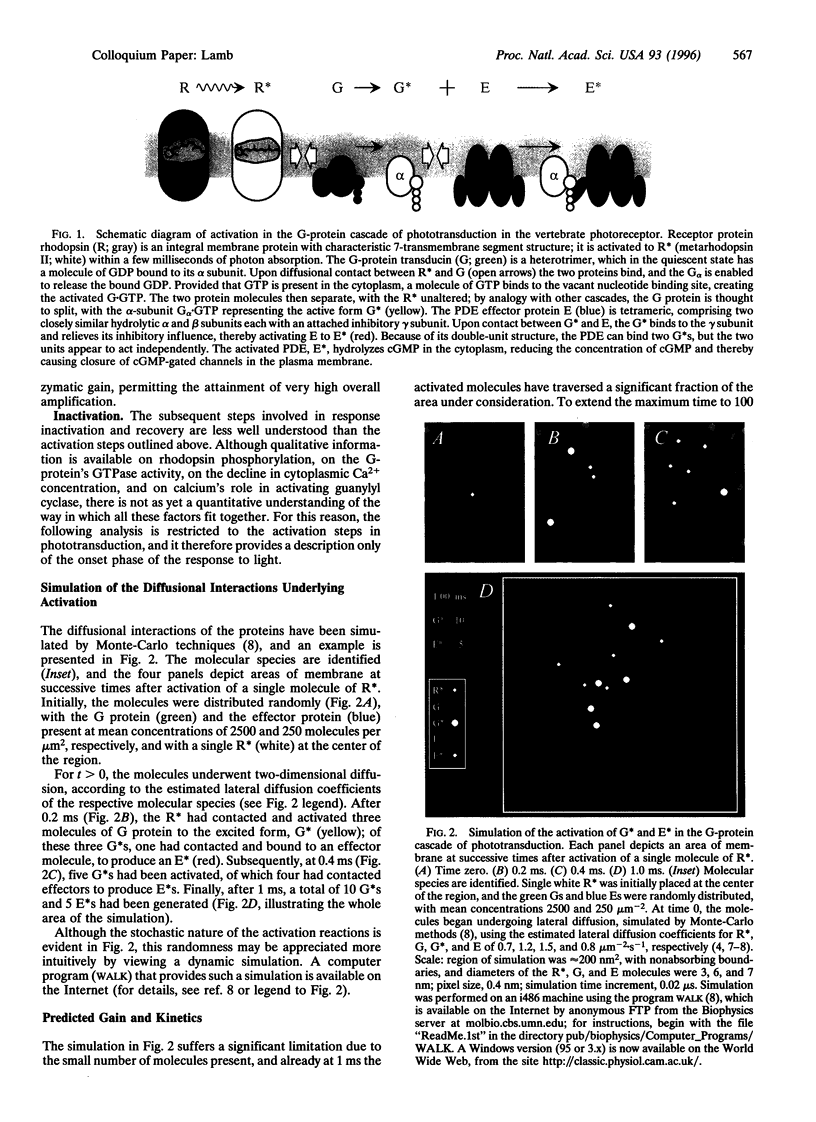
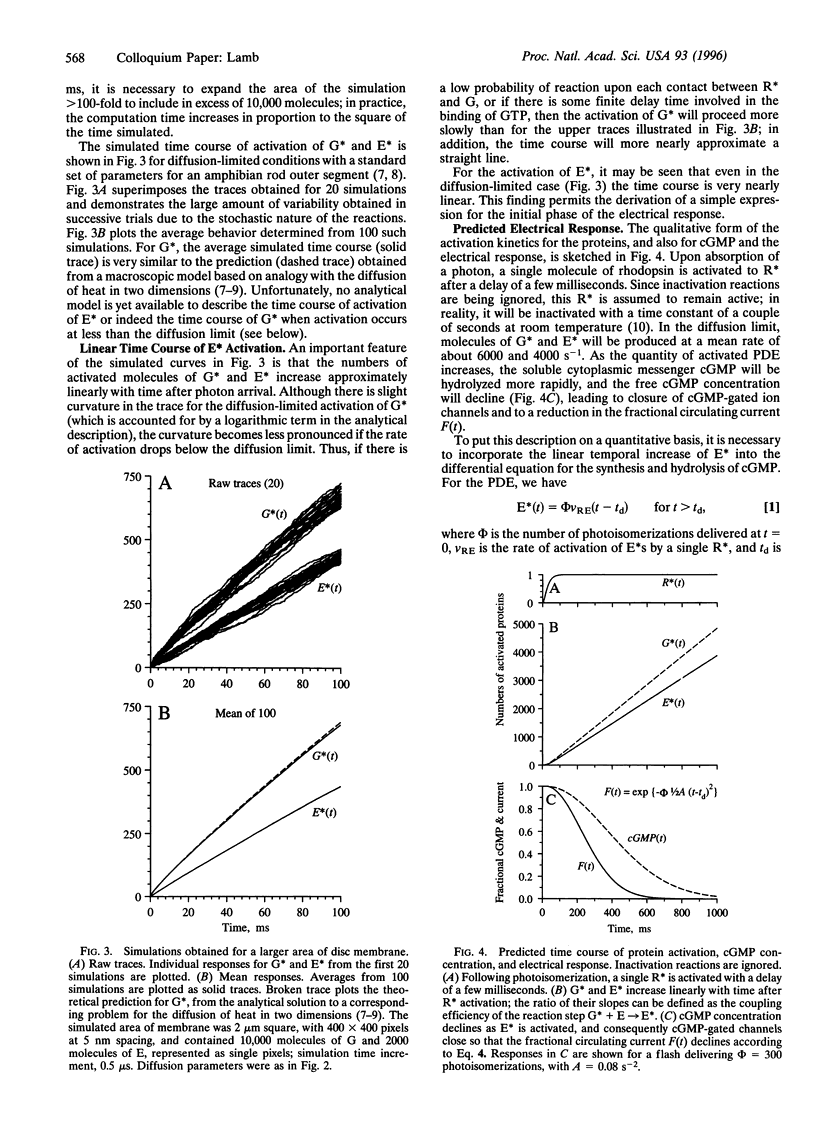
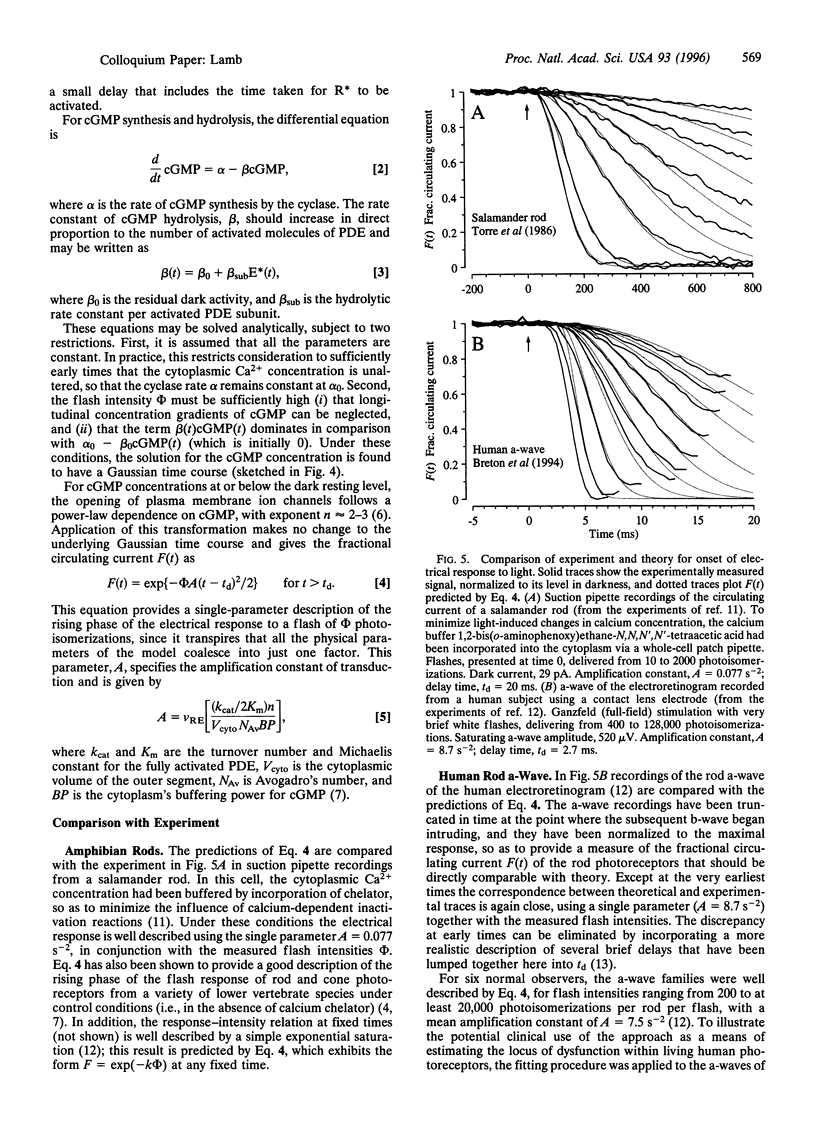
The guanine nucleotide binding protein (G protein) cascade underlying phototransduction is one of the best understood of all signaling pathways. The diffusional interactions of the proteins underlying the cascade have been analyzed, both at a macroscopic level and also in terms of the stochastic nature of the molecular contacts. In response to a single activated rhodopsin (R*) formed as a result of a single photon hit, it can be shown that molecules of the G-protein transducin will be activated approximately linearly with time. This, in turn, will cause the number of activated molecules of the effector protein (the phosphodiesterase) also to increase linearly with time. These kinetics of protein activation provide an accurate description of the time course of the rising phase of the photoreceptor's electrical response over a wide range of flash intensities. Recent estimates indicate that at room temperature each R* triggers activation of the phosphodiesterase at a rate of 1000-2000 subunits.s-1. Now that a quantitative description of the activation steps in transduction has been obtained, perhaps the greatest challenge for the future is to provide a comprehensive description of the shutoff reactions, so that a complete account of the photoreceptor's response to light can be achieved.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. How photons start vision. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):560–565. doi: 10.1073/pnas.93.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton M. E., Schueller A. W., Lamb T. D., Pugh E. N., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):295–309. [PubMed] [Google Scholar]
- Dumke C. L., Arshavsky V. Y., Calvert P. D., Bownds M. D., Pugh E. N., Jr Rod outer segment structure influences the apparent kinetic parameters of cyclic GMP phosphodiesterase. J Gen Physiol. 1994 Jun;103(6):1071–1098. doi: 10.1085/jgp.103.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Yau K. W. Cyclic GMP diffusion coefficient in rod photoreceptor outer segments. Biophys J. 1995 Jan;68(1):373–382. doi: 10.1016/S0006-3495(95)80198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Stochastic simulation of activation in the G-protein cascade of phototransduction. Biophys J. 1994 Oct;67(4):1439–1454. doi: 10.1016/S0006-3495(94)80617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R., Cornwall M. C., Kahlert M., Hofmann K. P., Jin J., Jones G. J., Ripps H. Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis Neurosci. 1992 Jan;8(1):9–18. doi: 10.1017/s0952523800006441. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Stryer L. Visual excitation and recovery. J Biol Chem. 1991 Jun 15;266(17):10711–10714. [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong T. M., Chabre M., Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984 Oct 18;311(5987):659–661. doi: 10.1038/311659a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]





