Abstract
Crocin is a water-soluble carotenoid pigment that is primarily used in various cuisines as a seasoning and coloring agent, as well as in traditional medicines for the treatment of edema, fever, and hepatic disorder. In this study, we demonstrated that crocin markedly induces the expression of heme oxygenase-1 (HO-1) which leads to an anti-inflammatory response. Crocin inhibited inducible nitric oxide synthase (iNOS) expression and nitric oxide production via downregulation of nuclear factor kappa B activity in lipopolysaccharide- (LPS-) stimulated RAW 264.7 macrophages. These effects were abrogated by blocking of HO-1 expression or activity. Crocin also induced Ca2+ mobilization from intracellular pools and phosphorylation of Ca2+/calmodulin-dependent protein kinase 4 (CAMK4). CAMK4 knockdown and kinase-dead mutant inhibited crocin-mediated HO-1 expression, Nrf2 activation, and phosphorylation of Akt, indicating that HO-1 expression is mediated by CAMK4 and that Akt is a downstream mediator of CAMK4 in crocin signaling. Moreover, crocin-mediated suppression of iNOS expression was blocked by CAMK4 inhibition. Overall, these results suggest that crocin suppresses LPS-stimulated expression of iNOS by inducing HO-1 expression via Ca2+/calmodulin-CAMK4-PI3K/Akt-Nrf2 signaling cascades. Our findings provide a novel molecular mechanism for the inhibitory effects of crocin against endotoxin-mediated inflammation.
1. Introduction
Inflammation is a defense response against infection, toxin exposure, or tissue injury. Inflammatory responses involve the infiltration of leukocytes to the site of infection or injury, and macrophages play an important role in regulation of inflammatory and immune responses through phagocytosis and secretion of proinflammatory mediator. In response to LPS, a major constituent of the Gram-negative bacterial cell wall, macrophages induce NF-κB-dependent expression of inducible nitric oxide synthase (iNOS) and subsequently produce nitric oxide (NO). Overproduction of NO resulting from inappropriate activation of macrophages is implicated in the pathologies of many chronic inflammatory diseases such as rheumatoid arthritis, atherosclerosis, multiple sclerosis, and shock [1–3]. Therefore, the inhibition of NO production and suppression of mechanisms responsible for the activation of inflammatory responses are regarded as clinical strategies for the treatment of chronic inflammation.
Heme oxygenases (HOs) catalyze the rate-limiting step in oxidative degradation of cellular heme into carbon monoxide (CO), biliverdin, and free iron [4]. Two HO isoforms, HO-1 and HO-2, have been identified; HO-2 is constitutively expressed, while HO-1 is potently induced in many types of cells by numerous stress stimuli including oxidative stress, hypoxia, heavy metals, and cytokines. HO-1 and its enzymatic by-products provide a host defense mechanism that can protect the body against oxidative injury and also contribute to the anti-inflammatory activity of cells and tissues [5]. Previous studies suggested that, when macrophages are induced to overexpress HO-1 prior to stimulation with LPS, the subsequent proinflammatory response is markedly inhibited [5]. In activated macrophages, HO-1 expression or CO treatment inhibits the production of proinflammatory mediators such as nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1β [5–7]. Moreover, an increasing number of therapeutic agents have been reported to induce HO-1 expression and exert anti-inflammatory effects through HO-1 induction.
HO-1 is primarily regulated at the transcriptional level via signaling pathways involved in survival and stress responses. A redox-sensitive transcription factor NF-E2-related factor 2 (Nrf2) plays a central role in the inducible expression of HO-1. Under normal conditions, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1) and degraded by the ubiquitin-dependent 26S proteasome system [8]. Upon activation, Nrf2 released from Keap1 inhibition translocates to the nucleus, heterodimerizes with Maf, and binds antioxidant response elements (AREs) located in the promoter regions of many detoxifying/antioxidant genes, including HO-1 [9]. HO-1 expression is known to be promoted by phosphatidylinositol 3-kinase (PI3K)/Akt, as well as mitogen-activated protein kinases (MAPKs) including p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK). However, these signaling pathways leading to HO-1 expression vary depending on the type of cells and stimuli.
Crocin, crocetin digentiobiose ester, is a water-soluble carotenoid pigment found in the fruit of Gardenia jasminoides or stigma of Crocus sativus [10, 11]. These plants are primarily used in various cuisines as a seasoning and coloring agent, as well as in traditional medicines for the treatment of edema, fever, and hepatic disorder. A number of pharmacological studies have demonstrated that crocin has a wide range of activities including antioxidant [12–14], anti-inflammatory [15–17], anticancer [18], antiatherosclerotic, and hepatoprotective effects [13, 19–21]. In this study, we examined the molecular mechanism responsible for the anti-inflammatory effects of crocin. The results presented herein provide the first evidence that crocin induces HO-1 expression via Ca2+/calmodulin-dependent protein kinase 4 (CAMK4), which subsequently inhibits the lipopolysaccharide- (LPS-) induced iNOS expression in murine macrophages.
2. Materials and Methods
2.1. Reagents
Crocin (Figure 1(a)), LPS (phenol extracted from Salmonella enteritidis), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), actinomycin D (Act. D), cycloheximide (CHX), 2-(3,6-bis(acetyloxy)-2,7-dichloro-9H-xanthen-9-yl)benzoic acid acetoxymethyl ester (Fluo-3/AM), and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cobalt protoporphyrin (CoPP) and zinc protoporphyrin IX (ZnPP) were bought from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). LY294002, PD98059, SP600125, and SB203580 were obtained from A.G. Scientific (San Diego, CA, USA). Nifedipine, 1,2-bis (o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester (BAPTA/AM), and calmidazolium chloride were acquired from Calbiochem (EMD Millipore, Billerica, MA, USA). KN92, KN93, and KN62 were purchased from Cayman Chemical (Ann Arbor, MI, USA) and Calbiochem.
Figure 1.
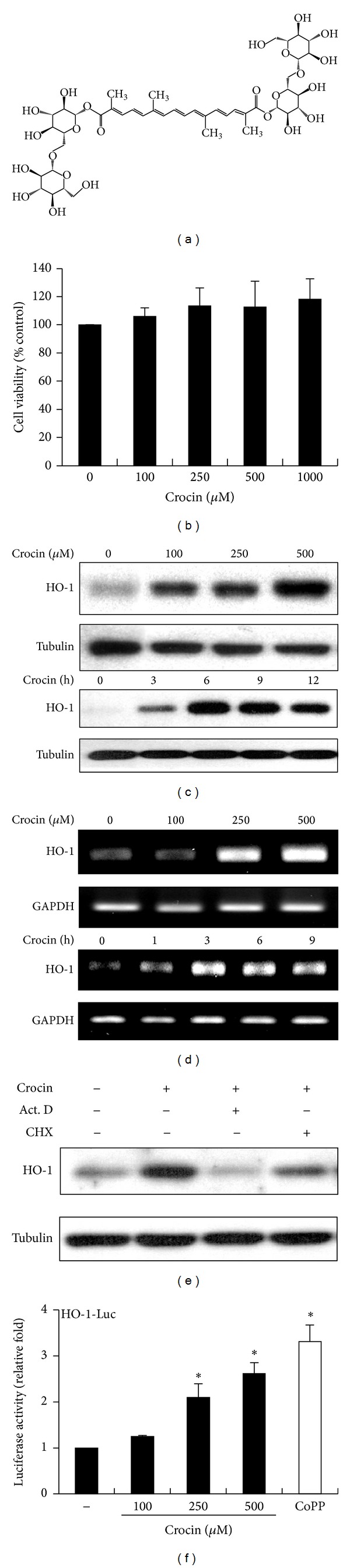
Induction of HO-1 by crocin. (a) Chemical structure of crocin. (b) RAW 264.7 cells were incubated with the indicated concentrations of crocin for 24 h, after which cell viability was measured by MTT assay. (c) RAW 264.7 cells were treated with the indicated concentrations of crocin for 6 h (upper panel) or with 500 μM crocin for various periods (lower panel), and HO-1 protein levels were analyzed by Western blotting. (d) Cells were treated with the indicated concentrations of crocin for 3 h or with 500 μM crocin for various periods and HO-1 mRNA levels were measured by RT-PCR. (e) Cells were treated with crocin (500 μM) for 6 h in the presence of Act. D (1 μg/mL) or CHX (1 μg/mL), after which protein levels of HO-1 were measured by Western blotting. (f) Cells were transfected with HO-1 promoter-luciferase construct and then treated with the indicated concentration of crocin or CoPP (10 μM). Equal amounts of cell extracts were then assayed for dual luciferase activity. *P < 0.05 versus the group treated with PBS (vehicle). GAPDH and tubulin were used as loading controls.
2.2. Macrophage Culture
Murine macrophage RAW264.7 cells were maintained in Dulbecco's modified Eagle's medium (GIBCO, Gaithersburg, MD, USA) supplemented with glutamine (1 mM) and 10% FBS (GIBCO, Gaithersburg, MD, USA) at 37°C under 5% CO2.
2.3. Cell Viability Assay
The cytotoxicity of crocin was assessed by a microculture tetrazolium- (MTT-) based colorimetric assay. Briefly, MTT was added to each well of a 96-well plate containing cells (final concentration 62.5 μg/mL). After incubation for 3 h at 37°C under 5% CO2, the supernatant was removed and the formed formazan crystals in viable cells were solubilized with 150 μL of DMSO. The absorbance of each well was then read at 570 nm using a microplate reader.
2.4. Western Blot Analysis
Cells were washed with phosphate buffered saline (PBS) three times and then scraped off and lysed with lysis buffer (PBS with 1% Triton X-100 and 1% deoxycholate). The protein concentration of lysates was determined using Bradford reagent (Bio-Rad, Hercules, CA, USA), after which equal amounts of protein were separated electrophoretically using 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). The gel was then transferred to 0.45 μm nitrocellulose paper and incubated with anti-iNOS, p65, HO-1, Nrf2, phospho-CAMK4, Akt, ERK1/2, JNK, TBP, HDAC antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), CAMK4, phospho-Akt, phospho-ERK1/2, phospho-JNK antibodies (Cell Signaling Technology, Beverly, MA, USA) or α-tubulin antibody (Bio Genex, Fremont, CA, USA), and secondary antibody and then detected by an enhanced chemiluminescence detection system according to the recommended procedure (GE Healthcare, Piscataway, NJ, USA).
2.5. Reverse Transcription- (RT-) Polymerase Chain Reaction (PCR)
Total cellular RNA was isolated using RNA spin mini RNA isolation kits (GE Healthcare, Piscataway, NJ, USA) according to the manufacturer's instructions. 1 μg of total RNA was reverse-transcribed using Maxime RT PreMix (Intron Biotechnology, Korea) and anchored oligo-dT15-primers. PCR was then performed using a TaKaRa PCR Thermal Cycler Dice (TaKaRa Bio Inc., Japan). The primer sequences were as follows: HO-1-sense (5′-GTTGACGGACCCCAAAAGAT-3′), HO-1-antisense (5′-CCTCATCCTGGAAGGTCCAC-3′), GAPDH-sense (5′-AGGTGGTCTCCTCTGACTTC-3′), and GAPDH-antisense (5′-TACCAGGAAATGAGCTTGAC-3′).
2.6. Measurement of Nitrite Concentration
NO synthesis in cell cultures was measured by a microplate assay method. To measure nitrite, 100 μL aliquots were removed from conditioned medium and incubated with an equal volume of Griess reagent (1% sulfanilamide/0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride/2.5% H3PO4) at room temperature for 10 min. The nitrite concentration was then determined by measuring the absorbance at 540 nm with a Vmax 96-well microplate spectrophotometer (Bio-Rad, Hercules, CA, USA). Sodium nitrite was used as a standard.
2.7. Transient Transfection and Dual Luciferase Assay
RAW 264.7 cells were transfected with antioxidant-response element (ARE) reporter plasmid (Stratagene, La Jolla, CA, USA) or HO-1 promoter reporter plasmid (kindly provided by Dr. Norbert Leitinger at the University of Virginia, School of Medicine) [22] and Renilla luciferase control plasmid pRL-CMV (Promega, Madison, WI, USA) using FuGENE-6 reagent (Roche Applied Science, Penzberg, Germany) according to manufacturer's instruction. Sixteen hours after transfection, the cells were incubated with the indicated concentrations of crocin for 8 h. Luciferase activity was then assayed using a dual-luciferase assay kit (Promega) according to the manufacturer's instructions. Luminescence was measured with a GloMax 96 microplate luminometer (Promega). ARE luciferase activity was normalized to control Renilla luciferase expression. All transfection experiments were performed in duplicate in three independent experiments.
2.8. Interference of HO-1 or CAMK4
The siRNAs for HO-1 and CAMK4 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and cells were transfected with HO-1 siRNA or negative control siRNA using INTERFERin (Polyplus transfection, France). The cells were then incubated for 48 h until protein expression was detected.
2.9. Measurement of Intracellular Ca2+ Concentration
Changes in intracellular Ca2+ concentration were measured using Fluo-3 loaded cells as described by June and Moore [23] with some modifications. For loading, the cells were incubated with 4 μg/mL of Fluo-3/AM in cell-loading media (Dulbecco's PBS, DPBS, with Ca2+ and Mg2+ containing 1% FBS) at 37°C for 30 min and then washed three times with DPBS and resuspended in cell-loading medium (Ca2+-containing medium) or DPBS without Ca2+ and Mg2+ (Ca2+-free medium). After treatment with crocin or other reagents, cells were analyzed using a flow cytometer (Beckman Coulter, Miami, FL, USA) with excitation at 488 nm and emission at 535 nm. A total of 10,000 live cell events gated on forward and side scatter characteristics were acquired. Dead cells were excluded by gating out cells without Fluo-3 AM fluorescence. Data were expressed as a percentage of responding cells.
2.10. Statistical Analysis
All results were expressed as means ± SE. Each experiment was repeated at least three times. Statistical analysis was performed using the SPSS software to determine significant differences. We used one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for comparison of three or more groups. A P < 0.05 was considered statistically significant.
3. Results
3.1. Crocin Induces HO-1 Expression in Murine Macrophages
To examine the effects of crocin on cell viability, murine macrophage RAW 264.7 cells were treated with various concentrations of crocin and an MTT-based cell viability assay was conducted. As shown in Figure 1(b), cell viability was not reduced in response to treatment with up to 1 mM of crocin.
To investigate whether crocin induces HO-1 expression in macrophages, murine macrophage RAW 264.7 cells were incubated with various concentrations of crocin. The HO-1 protein level was significantly increased by crocin in a dose-dependent manner and peaked at 6 h after treatment (Figure 1(c)). Crocin also induced HO-1 in thioglycollate- (TG-) elicited mouse peritoneal macrophages (see Supplementary Figures 1(A) and 1(B) in the Supplementary Material available online at http://dx.doi.org/10.1155/2014/728709). Moreover, the mRNA level of HO-1 increased in response to treatment with crocin in a dose-dependent manner, suggesting that crocin induces the transcription of HO-1 (Figure 1(d)). To confirm that crocin-induced HO-1 expression is mediated by transcription and translation, we used actinomycin D (Act. D) to inhibit DNA-dependent RNA polymerase, and cycloheximide (CHX) to inhibit ribosomal protein synthesis. Cotreatment with crocin and Act. D or CHX significantly reduced HO-1 expression (Figure 1(e)). Furthermore, HO-1 promoter activity also increased in dose-dependent manner in response to crocin (Figure 1(f)). Cobalt protoporphyrin (CoPP), an inducer of HO-1 [24], was used as a positive control. Taken together, these results suggest that crocin primarily upregulates HO-1 expression at the transcriptional level without damaging cells.
3.2. HO-1 Mediates Inhibitory Effects of Crocin on iNOS Expression and NF-κB Activation in LPS-Stimulated Macrophages
To investigate the anti-inflammatory effects of crocin, we examined the effects of crocin on NO synthesis. The amount of LPS-induced NO released into culture supernatant was suppressed by crocin in a dose-dependent manner (Figure 2(a)). To determine whether the decreased NO synthesis was correlated with iNOS expression, we analyzed the amount of iNOS by Western blot analysis. The expression of iNOS was dramatically suppressed by crocin in a dose-dependent manner (Figure 2(b)). These results indicate that crocin inhibits NO release by affecting the iNOS expression level. Crocin also inhibited LPS-induced NO production and iNOS expression in TG-elicited mouse peritoneal macrophages (Supplementary Figures 1(C) and 1(D)). To determine whether crocin exhibits its anti-inflammatory effects through the induction of HO-1, we applied an HO-1 small interfering (si) RNA system to knock down HO-1. As shown in Figure 2(c), decreased HO-1 expression blocked crocin-mediated suppression of LPS-stimulated iNOS expression, whereas transfection with control siRNA had no effect. Moreover, increased HO-1 expression by an inducer of HO-1 (CoPP) suppressed LPS-stimulated iNOS expression (Figure 2(d)). These results suggest that HO-1 induced by crocin suppresses the expression of iNOS.
Figure 2.
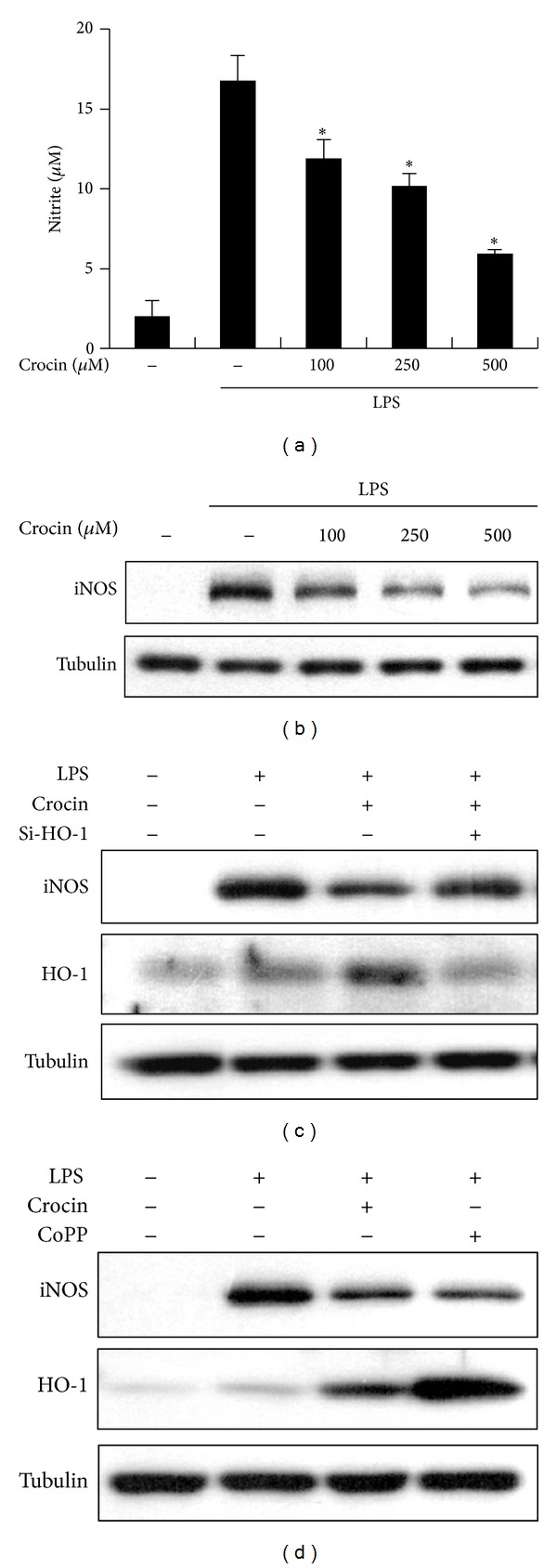
Inhibitory effects of crocin on NO production and iNOS expression in LPS-stimulated macrophages. ((a), (b)) RAW 264.7 cells were incubated with the indicated concentrations of crocin for 3 h and then stimulated with LPS (0.1 μg/mL) for 24 h. Next, the supernatant was harvested and the amount of nitrite released from cells was measured by the Griess method (a). *P < 0.05 versus the group treated with LPS alone (no crocin). Equal amounts of cytosolic extract were analyzed by Western blotting (b). (c) Cells were transfected with HO-1 siRNA or control siRNA. Cells were treated with crocin (500 μM) for 3 h and then were stimulated with LPS (0.1 μg/mL) for 6 h. (d) Cells were incubated with crocin (500 μM) or CoPP (10 μM) for 3 h and then stimulated with LPS (0.1 μg/mL) for 24 h, after which protein levels of iNOS and HO-1 were analyzed by Western blotting. Tubulin was used as a loading control.
Since iNOS promoter which contains two binding sites for transcription factor NF-κB and NF-κB plays a critical role in LPS-induced iNOS expression, we investigated whether crocin suppresses iNOS expression via the regulation of NF-κB activity. Crocin inhibited the translocation of NF-κB p65 from cytosol into nucleus in a dose-dependent manner (Figure 3(a)). Additionally, crocin dose-dependently suppressed LPS-stimulated binding of NF-κB to DNA and IκB-α degradation (Supplementary Figures 2(A) and 2(B)). The involvement of HO-1 in NF-κB activation was tested using a specific HO competitive inhibitor, zinc protoporphyrin IX (ZnPP) [25]. Pretreatment with ZnPP attenuated crocin-mediated suppression of NF-κB translocation (Figure 3(b)). NF-κB translocation was confirmed by immunofluorescence microscopy. Crocin attenuated LPS-mediated nuclear translocation of NF-κB p65 in RAW 264.7 cells, while crocin-mediated suppression of NF-κB translocation was obstructed by HO-1 inhibitor ZnPP (Figure 3(c)). These results suggest that crocin inhibits iNOS expression and NF-κB activation through the induction of HO-1 in macrophages.
Figure 3.
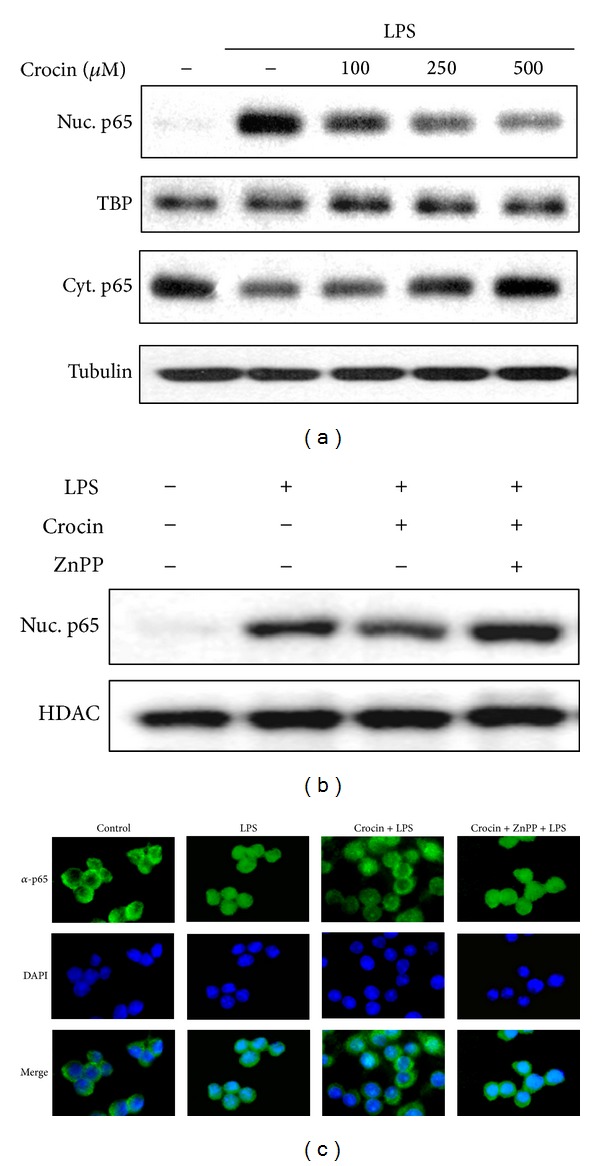
Inhibitory effects of crocin on NF-κB translocation in LPS-stimulated macrophages. (a) Cells were incubated with the indicated concentrations of crocin for 3 h and then stimulated with LPS (0.1 μg/mL) for 30 min, after which nuclear and cytosolic proteins were analyzed by Western blotting. (b) Cells were treated with crocin (500 μM) in the absence or presence of ZnPP (1 μM) for 3 h and then stimulated with LPS (0.1 μg/mL) for 30 min, after which nuclear proteins were analyzed by Western blotting. (c) Cells were incubated with crocin in the absence or presence of ZnPP as described above and then stimulated with LPS (0.1 μg/mL) for 30 min. Fixed cells were subsequently stained with anti-NF-κB, FITC-conjugated anti-rabbit IgG antibody, and DAPI, and images were taken using fluorescence microscope.
3.3. Nrf2 Is Activated by Crocin in Macrophages
Since the promoter region of the ho-1 gene contains binding sites for transcription factor Nrf2 and the expression of HO-1 is known to be regulated by Nrf2, we determined whether crocin activates Nrf2 in RAW 264.7 cells. Nrf2 nuclear translocation, which is critical to its transcriptional activity, increased in response to crocin in a dose-dependent manner, reaching its peak at 3 h (Figure 4(a)). Crocin also induced binding of Nrf2 to the antioxidant response element (ARE) sequence (Supplementary Figure 3). To elucidate the effects of crocin on transactivation activity of Nrf2, RAW 264.7 cells were transiently transfected with luciferase reporter genes driven by ARE to which Nrf2 binds. As shown in Figure 4(b), crocin treatment increased ARE promoter activity in a dose-dependent manner. Taken together, these results suggest that crocin activates Nrf2, which then induces HO-1 expression.
Figure 4.
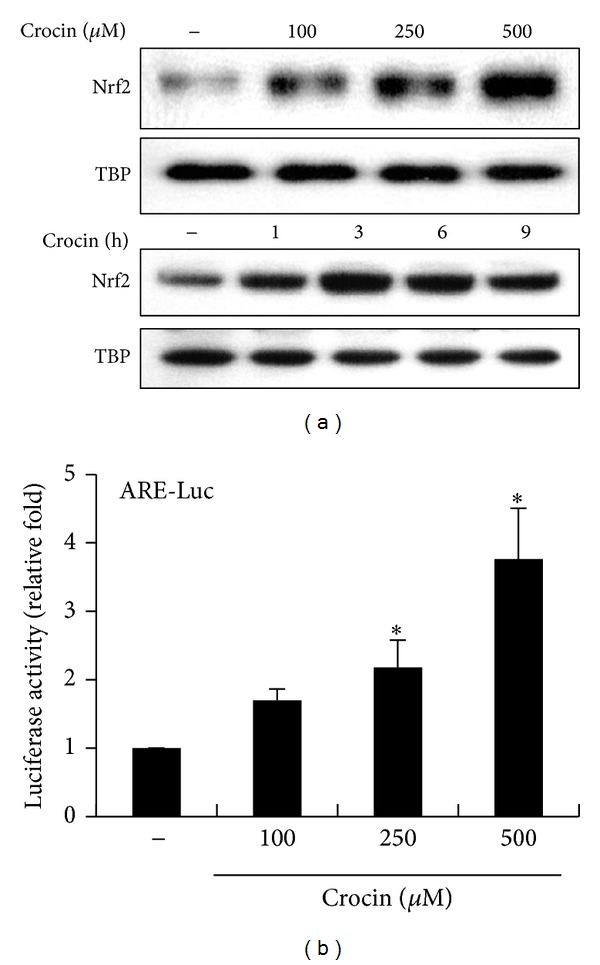
Activation of Nrf2 by crocin. (a) RAW 264.7 cells were incubated with the indicated concentrations of crocin for 3 h (upper panel) or with 500 μM crocin for various periods (lower panel), after which nuclear extracts were harvested and assayed by Western blotting. TATA binding protein (TBP) was used as a loading control. (b) Cells were transfected with ARE-luciferase construct and then treated with the indicated concentration of crocin. Equal amounts of cell extracts were then assayed for dual luciferase activity. *P < 0.05 versus the group treated with PBS (vehicle).
3.4. Crocin Induces HO-1 Expression via Mobilization of Intracellular Free Ca2+
To investigate the signaling pathways associated with crocin-induced HO-1 expression, we examined the effects of several inhibitors related to Ca2+ signaling including BAPTA/AM, the intracellular Ca2+ chelator, calmidazolium chloride, the calmodulin antagonist, and nifedipine, the L-type Ca2+ channel blocker. HO-1 expression and HO-1 promoter activity were inhibited by BAPTA/AM and calmidazolium chloride but not by nifedipine (Figures 5(a) and 5(b)), suggesting that intracellular Ca2+/calmodulin is critical to crocin-mediated HO-1 expression. We next examined whether crocin triggers the release of Ca2+ from intracellular pools. To accomplish this, RAW 264.7 cells were loaded with Fluo-3/AM and the level of intracellular free Ca2+ was measured by FACS in Ca2+-containing or Ca2+-free medium. As shown in Figures 5(c) and 5(d), crocin treatment resulted in an increase in [Ca2+]i in either Ca2+-containing or Ca2+-free medium. Moreover, the crocin-mediated increase in [Ca2+]i was inhibited by BAPTA/AM but not by nifedipine (Figure 5(e)), indicating that crocin induces Ca2+ mobilization from intracellular pools. Taken together, these results suggest that crocin-mediated HO-1 expression is triggered by Ca2+ efflux from intracellular stores rather than Ca2+ influx from extracellular fluid.
Figure 5.
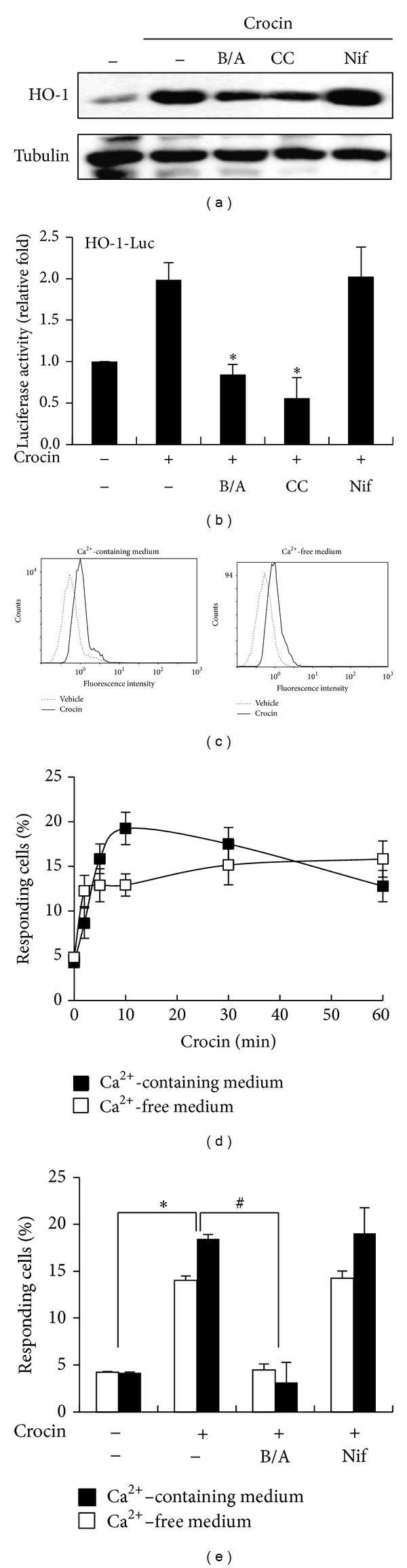
Induction of HO-1 expression via elevation of intracellular Ca2+ levels. (a) RAW 264.7 cells were incubated with BAPTA/AM (B/A, 5 μM), calmidazolium chloride (CC, 5 μM), and nifedipine (Nif, 5 μM) for 1 h and then treated with crocin (500 μM) for 6 h. Equal amounts of cytosolic proteins were subsequently analyzed by Western blotting with HO-1 antibody. Tubulin was used as a loading control. (b) Cells were transfected with HO-1 promoter-luciferase construct and then treated as described above, after which equal amounts of cell extracts were assayed for dual luciferase activity. *P < 0.05 versus the group treated with crocin alone. (c) Intracellular free Ca2+ levels were assessed by flow cytometry. Cells were treated with vehicle or crocin (500 μM) for 10 min in either Ca2+-containing medium (left) or Ca2+-free medium (right). (d) The changes of intracellular Ca2+ levels with time were represented as percentages of responding cells. (e) Cells were treated with crocin (500 μM) in the absence or presence of BAPTA/AM (B/A, 5 μM), or nifedipine (Nif, 5 μM) for 10 min, after which intracellular free Ca2+ levels (represented as percentage of responding cells) were evaluated by flow cytometry. *P < 0.05 versus the group treated with PBS (vehicle); # P < 0.05 versus the group treated with crocin alone in both Ca2+-containing and Ca2+-free medium.
3.5. Ca2+/Calmodulin-Dependent Kinase 4 (CAMK4) Mediates Crocin-Induced HO-1 Expression
Ca2+ is an important second messenger and several signaling molecules are regulated by Ca2+/calmodulin, among which CAMK is a common target. We investigated whether CAMK4 is associated with crocin-induced HO-1 expression using siRNA because no specific inhibitor of CAMK4 was found. As shown in Figure 6(a), crocin-induced HO-1 expression was suppressed by CAMK4 knockdown. Next, we examined the effects of ectopic expression of CAMK4 mutant on HO-1 expression. Transient transfection of RAW 264.7 cells with catalytically inactive CAMK4 mutant (K75E) inhibited crocin-induced activation of HO-1 promoter and HO-1 expression, while constitutively active CAMK4 mutant (c-terminal autoinhibitory domain-deletion construct, dCT) slightly increased these factors (Figures 6(b) and 6(c)). Moreover, crocin induced rapid phosphorylation of CAMK4, which peaked at 5 min and then declined to basal levels within 60 min (Figure 6(d)). These results suggest that activation of CAMK4 is involved in crocin-mediated HO-1 expression.
Figure 6.
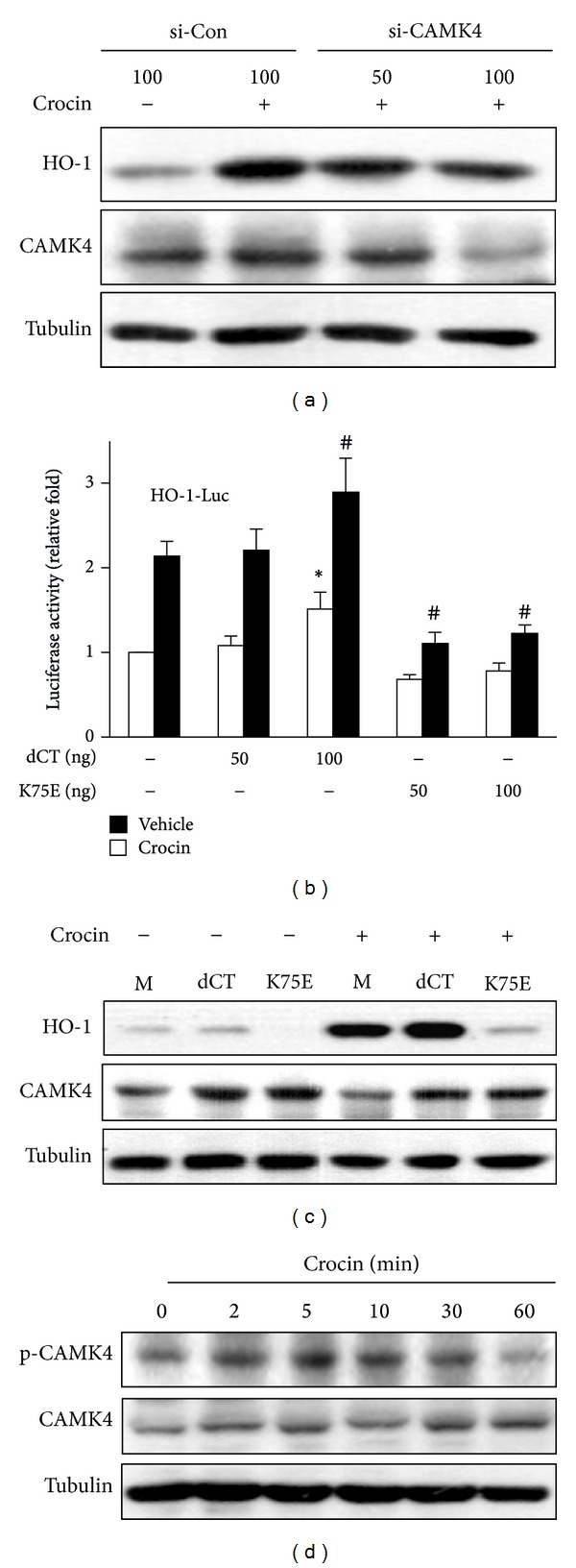
Involvement of CAMK4 in crocin-mediated expression of HO-1. (a) RAW 264.7 cells were transfected with CAMK4 siRNA or control siRNA and then treated with crocin (500 μM) for 6 h. Equal amounts of cytosolic proteins were analyzed by Western blotting. (b) Cells were cotransfected with HO-1 promoter-luciferase construct and pcDNA3.1, CAMK4 constitutive active (dCT), or kinase dead (K75E) construct and then treated with crocin (500 μM). Equal amounts of cell extracts were assayed for dual luciferase activity. *P < 0.05 versus the pcDNA3.1-transfected (vehicle-treated) group; # P < 0.05 versus the pcDNA3.1-transfected (crocin-treated) group. (c) Cells were transfected with pcDNA3.1 (mock, M), dCT or K75E construct and then treated with crocin (500 μM). Equal amounts of cytosolic proteins were analyzed by Western blotting. (d) Cells were treated with crocin (500 μM) for the indicated time and the levels of phosphorylated CAMK4 and total CAMK4 were analyzed by Western blotting. Tubulin was used as a loading control.
3.6. CAMK4 Mediates Crocin-Induced Nrf2 Activation
Since expression of HO-1 is regulated by Nrf2 and crocin induced nuclear translocation of Nrf2 and ARE promoter activity, we examined whether Ca2+ signaling involved crocin-mediated Nrf2 activation using several inhibitors related to Ca2+ signaling. As shown in Figure 7(a), Nrf2 nuclear translocation was inhibited by BAPTA/AM and calmidazolium chloride but not by nifedipine. These findings are consistent with those of HO-1 and indicate that crocin-mediated Nrf2 nuclear translocation is triggered by Ca2+ efflux from intracellular stores. Next, to investigate whether CAMK4 mediates crocin-induced Nrf2 activation, the effects of ectopic expression of CAMK4 mutants were examined. Transient transfection of RAW 264.7 cells with catalytically inactive CAMK4 mutant (K75E) inhibited crocin-induced Nrf2 nuclear translocation (Figure 7(b)). Moreover, K75E mutant inhibited crocin-mediated ARE promoter activity, while constitutively active CAMK4 mutant (dCT) slightly increased ARE promoter activity (Figure 7(c)). These results suggest that CAMK4 mediates crocin-induced Nrf2 activation which contributes to the upregulation of HO-1 by crocin.
Figure 7.
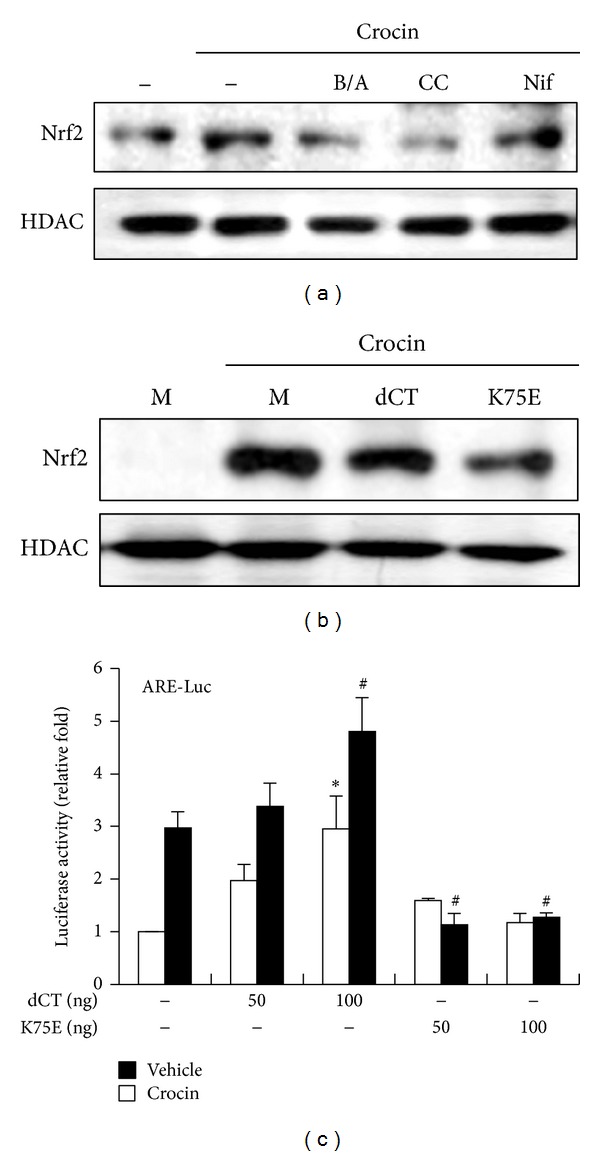
Regulation of Nrf2 activation by CAMK4. (a) RAW 264.7 cells were incubated with BAPTA/AM (B/A, 5 μM), calmidazolium chloride (CC, 5 μM), and nifedipine (Nif, 5 μM) for 1 h and then treated with crocin (500 μM) for 3 h. Equal amounts of nuclear proteins were subsequently analyzed by Western blotting. (b) Cells were transfected with pcDNA3.1 (mock, M), CAMK4 constitutive active (dCT), or kinase dead (K75E) construct and then treated with crocin (500 μM). Equal amounts of nuclear proteins were then analyzed by Western blotting. HDAC was used as a loading control. (c) Cells were cotransfected with ARE-luciferase construct and pcDNA3.1, dCT, or K75E and then treated with crocin (500 μM). Equal amounts of cell extracts were assayed for dual luciferase activity. *P < 0.05 versus the pcDNA3.1-transfected (vehicle-treated) group; # P < 0.05 versus the pcDNA3.1-transfected (crocin-treated) group.
3.7. Akt Acts Downstream of CAMK4 during Crocin-Induced HO-1 Expression
Since PI3K/Akt and three MAPKs (ERK1/2, JNK, and p38 MAPK) have been reported to be involved in HO-1 expression in response to diverse stimuli [1], we investigated whether CAMK4 is associated with these signaling pathways in crocin-induced HO-1 expression. To accomplish this, we examined which signaling pathway regulates crocin-mediated expression of HO-1 using the pharmaceutical protein kinase inhibitors, LY294002 (PI3K inhibitor), SP600125 (JNK inhibitor), PD98059 (ERK1/2 inhibitor), and SB203580 (p38 MAPK inhibitor). As shown in Figure 8(a), crocin-induced HO-1 expression and Nrf2 nuclear translocation were significantly inhibited by LY294002, SP600125, and PD98059 but not by SB203580. Moreover, crocin increased the phosphorylation of Akt (Figure 8(b)), ERK, and JNK, although ERK and JNK were slightly phosphorylated by crocin (Supplementary Figure 4(A)). These results indicate that PI3K/Akt, ERK, and JNK signalings occur upstream of crocin-mediated HO-1 expression. Next, to determine the relationship of CAMK4 and these kinases during crocin-induced HO-1 expression, the effects of CAMK4 inhibition on the activities of these kinases were examined. Western blot analysis showed that crocin-induced Akt activation was suppressed by CAMK4 knockdown (Figure 8(c)) and catalytically inactive CAMK4 mutant (Figure 8(d)), but ERK and JNK activation were not influenced (Supplementary Figures 4(B) and 4(C)). Moreover, increased HO-1 promoter activity by constitutively active CAMK4 mutant was significantly reduced following treatment with LY294002 (Figure 8(e)). These results suggest that CAMK4 acts as an upstream regulator of PI3K/Akt in crocin-mediated HO-1 expression.
Figure 8.
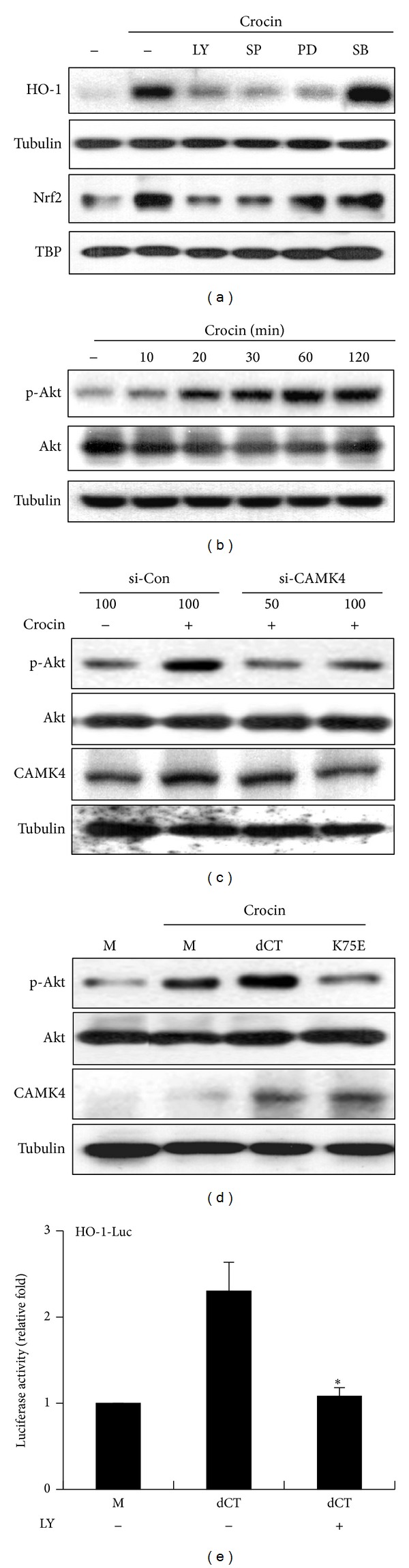
Akt acts as a downstream regulator of CAMK4 in crocin-mediated HO-1 expression. (a) RAW 264.7 cells were incubated with LY294002 (20 μM), SP600125 (20 μM), PD98059 (20 μM), and SB203580 (20 μM) for 1 h and then treated with crocin (500 μM) for 6 h. Equal amounts of cytosolic and nuclear extract were subsequently analyzed by Western blotting with HO-1 and Nrf2 antibody, respectively. Tubulin and TBP were used as loading controls. (b) Cells were incubated with crocin (500 μM) for the indicated times. (c) Cells were transfected with CAMK4 siRNA or control siRNA and then treated with crocin (500 μM) for 1 h. (d) Cells were transfected with pcDNA3.1 (mock, M), CAMK4 constitutive active (dCT), or kinase dead (K75E) construct and then treated with crocin (500 μM) for 1 h, after which equal amounts of cytosolic extract were analyzed by Western blotting. (e) Cells were cotransfected with HO-1-luciferase construct and pcDNA3.1, or dCT and then treated with LY294002 (20 μM). Equal amounts of cell extracts were then assayed for dual luciferase activity. *P < 0.05 versus the dCT-transfected (no LY294002) group.
3.8. CAMK4 Is Required for Crocin-Mediated Inhibition of iNOS Expression
To determine whether CAMK4 contributes to the inhibitory effects of crocin on iNOS expression, cells were treated with CAMK4 siRNA and iNOS expression was examined. As shown in Figure 9, CAMK4 knockdown rescued crocin-mediated inhibition of iNOS expression in LPS-stimulated macrophages. These results suggest that CAMK4 plays a pivotal role in crocin-mediated suppression of iNOS expression in LPS-stimulated macrophages.
Figure 9.
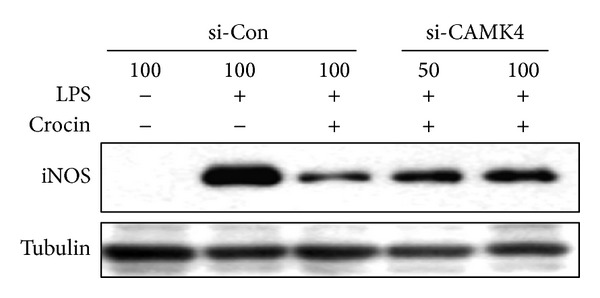
CAMK4 is necessary for crocin-mediated inhibition of iNOS expression in LPS-stimulated macrophages. RAW 264.7 cells were transfected with CAMK4 siRNA or control siRNA and then treated with crocin (500 μM). After 3 h, cells were incubated with LPS (0.1 μg/mL) for 24 h. Equal amounts of cytosolic extract were analyzed by Western blotting. Tubulin was used as a loading control.
4. Discussion
HO-1 is believed to exhibit anti-inflammatory activities by inhibiting production of proinflammatory mediators in a variety of cells [26–28]. However, the precise mechanism by which HO-1 exerts its anti-inflammatory effects has yet to be fully elucidated. The results of the present study demonstrate that crocin inhibited LPS-stimulated iNOS expression by inducing HO-1 in macrophages providing the first evidence of the pivotal roles of CAMK4 in crocin-induced expression of HO-1.
We found that crocin markedly induced HO-1 expression at the transcriptional level in the murine macrophage cell line, RAW 264.7, as well as in mouse peritoneal macrophages. Because HO-1 is known to be induced by chemical-mediated oxidative stress, we examined whether crocin increased HO-1 expression by inducing cell damage. A cell viability assay (MTT assay) revealed that crocin does not affect cell viability, suggesting that HO-1 is specifically induced by crocin, not by cell damage. Next, we examined whether HO-1 expression is correlated with the inhibition of LPS-induced iNOS expression. Crocin significantly inhibited iNOS expression and NO production in LPS-stimulated macrophages, while the knockdown of HO-1 expression by siRNA markedly reversed the inhibitory effects of crocin on iNOS expression in LPS-induced macrophages. These data indicate that crocin inhibits iNOS expression via the modulation of HO-1 expression and are consistent with other reports which demonstrated the induction of HO-1 expression plays a significant role in mediating the anti-inflammatory effects of various natural compounds in LPS-stimulated macrophages. Although the contribution of HO-1 products (i.e., CO, biliverdin, and iron) was not examined in this study, several studies point to HO-1-derived CO and biliverdin as a potential metabolite to combat inflammation [29–32]. In particular, recent reports demonstrated that biliverdin administration protects against endotoxin-induced acute lung injury in rats [32], and CO and biliverdin ameliorate murine collagen induced arthritis [29]. Taken together, these results suggest that crocin could be useful as a therapeutic agent for many kinds of inflammatory diseases.
Since dysregulation of NF-κB function is associated with inflammation, the development of drugs that control NF-κB is one of promising strategies for treatment of various inflammatory diseases [33–35]. The results of the present study showed that crocin significantly inhibited the translocation of NF-κB p65 subunit into the nucleus and its binding activity to κB-binding motifs on DNA (Supplementary Figure 2). Crocin also suppressed the degradation of IκB-α in LPS-stimulated macrophages (Supplementary Figure 2). Additionally, a specific HO competitive inhibitor, ZnPP, blocked crocin-mediated suppression of LPS-induced NF-κB translocation. These results indicate that NF-κB is inhibited by crocin-induced HO-1, which consequently attenuates the expression of iNOS. Because NF-κB regulates many genes involved in inflammation, crocin might inhibit the expression of inflammatory mediators such as TNF-α, IL-6, and IL-1β, although the effects of crocin on cytokine production have to be confirmed in macrophages.
Despite its role as a potent anti-inflammatory mediator, the intracellular signaling pathway leading to the induction of HO-1 expression is not well understood. Our study showed that crocin mobilized Ca2+ from intracellular stores and that crocin-induced expression of HO-1 was dependent on intracellular Ca2+/calmodulin. This role of Ca2+ signaling in regulation of HO-1 expression has also been observed in several types of cells treated with other compounds such as tertiary-butylhydroquinone, nicotine, and curcumin, suggesting that Ca2+-linked signaling could be a common regulatory mechanism of HO-1 expression [36–39]. One of the common targets of Ca2+-mediated signaling is CAMK. Kim et al. [36] reported that bisdemethoxycurcumin, an analog of curcumin, mediates induction of HO-1 expression via a Ca2+/CAMK2/ERK cascade. However, in the present study, crocin-induced HO-1 expression was not suppressed by CAMK2 inhibitors such as KN62 and KN93 (Supplementary Figure 5). Instead, it was markedly inhibited by CAMK4 siRNA or kinase-dead mutant. Although KN62 and KN93 are known to inhibit CAMK4 activity as well as CAMK2, HO-1 expression was not inhibited by these inhibitors. This might have been due to differences in the sensitivity of pharmacological inhibitors to each CAMK or the unknown side effects of these inhibitors. Because we did not examine the effects of CAMK2 siRNA or mutant, we cannot exclude the possibility that CAMK2 is involved in crocin-induced HO-1 expression. However, the results derived from more specific inhibition of CAMK4 with siRNA and mutant suggest that the Ca2+/calmodulin-CAMK4-linked pathway regulates crocin-mediated expression of HO-1.
Nrf2, a bZIP transcription factor, is an essential ARE-binding factor involved in expression of the ho-1 gene. In this study, Nrf2 activation by crocin was dependent on Ca2+/calmodulin-CAMK4. Previous studies demonstrated that PI3K and MAPKs such as ERK, JNK, and p38 participate in the induction of Nrf2/ARE-mediated HO-1 expression in response to various stimuli [40, 41]. However, these signaling pathways depend on the type of cells and stimuli in terms of their contribution to HO-1 expression. Crocin-mediated Nrf2 activation and HO-1 expression were regulated by PI3k/Akt, ERK, and JNK. Moreover, use of siRNA or kinase-dead mutant for CAMK4 showed that CAMK4 acts upstream of Akt and that activation of ERK and JNK is independent of CAMK4. These data demonstrate that, although necessary for the optimal expression of HO-1 by crocin, CAMK4-Akt and MAPKs (ERK and JNK) are activated independently.
In summary, the results of this study demonstrate that crocin induces HO-1 expression though a rapid elevation of [Ca2+]i and subsequent activation of CAMK4, Akt, and Nrf2 in macrophages. Furthermore, the results presented herein also demonstrate that crocin-mediated HO-1 inhibits LPS-induced iNOS expression and that knockdown of CAMK4 blocks this suppressive effect on iNOS expression. These findings suggest that crocin exhibits anti-inflammatory effects by upregulation of HO-1 expression via CAMK4-PI3K/Akt-Nrf2 signaling in LPS-stimulated macrophages. Our findings could help elucidate the molecular mechanism of anti-inflammatory action of crocin and suggest that crocin may have therapeutic potential for treatment of inflammatory diseases.
Supplementary Material
Supplementary Fig. 1: Effects of crocin on HO-1 induction, NO production and iNOS expression in murine peritoneal macrophages.
Supplementary Fig. 2: Inhibitory effects of crocin on DNA binding activity of NF-κB and IκB-α degradation in LPS-stimulated macrophages.
Supplementary Fig. 3: Effects of crocin on DNA binding activity of Nrf2.
Supplementary Fig. 4: Effects of crocin and CAMK4 on activity of ERK and JNK.
Supplementary Fig. 5: Effects of CAMK inhibitors on crocin-mediated HO-1 expression.
Acknowledgments
The authors thank Dr. Jiuyong Xie (University of Manitoba, Canada) for providing CAMK4-dCT and CAMK4-dCTK75E constructs and Dr. Jaehun Cheong (Pusan National University, Korea) for providing CAMK4-dCT. This study was financially supported by the “2012 Post-Doc. Development Program” of Pusan National University and the Research Fund Program of Research Institute for Basic Sciences, Pusan National University, Korea, 2013, Project No. RIBS-PNU-2013-201.
Conflict of Interests
The authors declare that they have no conflict of interests.
Authors' Contribution
Ji-Hee Kim and Ga-Young Park equally contributed to this work.
References
- 1.Hua LL, Liu JSH, Brosnan CF, Lee SC. Selective inhibition of human glial inducible nitric oxide synthase by interferon-β: implications for multiple sclerosis. Annals of Neurology. 1998;43(3):384–387. doi: 10.1002/ana.410430317. [DOI] [PubMed] [Google Scholar]
- 2.Levy B, Valtier M, de Chillou C, Bollaert P-E, Cane D, Mallie JP. Beneficial effects of L-canavanine, a selective inhibitor of inducible nitric oxide synthase, on lactate metabolism and muscle high energy phosphates during endotoxic shock in rats. Shock. 1999;11(2):98–103. doi: 10.1097/00024382-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Manzi S, Wasko MCM. Inflammation-mediated rheumatic diseases and atherosclerosis. Annals of the Rheumatic Diseases. 2000;59(5):321–325. doi: 10.1136/ard.59.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiological Reviews. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 5.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nature Medicine. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 6.Oh GS, Pae HO, Lee BS, et al. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radical Biology and Medicine. 2006;41(1):106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Suh GY, Jin Y, Yi AK, Wang XM, Choi AMK. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. The American Journal of Respiratory Cell and Molecular Biology. 2006;35(2):220–226. doi: 10.1165/rcmb.2005-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxidants and Redox Signaling. 2005;7(3-4):385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K, Mimura J, Yamamoto M. Discovery of the negative regulator of Nrf2, keap1: a historical overview. Antioxidants and Redox Signaling. 2010;13(11):1665–1678. doi: 10.1089/ars.2010.3222. [DOI] [PubMed] [Google Scholar]
- 10.Bolhassani A, Khavari A, Bathaie SZ. Saffron and natural carotenoids: biochemical activities and anti-tumor effects. Biochimica Et Biophysica Acta. 2014;1845(1):20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Chen YF, Li F, Zhang HY. Fructus gardenia (gardenia jasminoides J. ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. Journal of Asian Natural Products Research. 2013;15(1):94–110. doi: 10.1080/10286020.2012.723203. [DOI] [PubMed] [Google Scholar]
- 12.Papandreou MA, Kanakis CD, Polissiou MG, et al. Inhibitory activity on amyloid-β aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. Journal of Agricultural and Food Chemistry. 2006;54(23):8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 13.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytotherapy Research. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 14.Goyal SN, Arora S, Sharma AK, et al. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17(3-4):227–232. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Nam KN, Park YM, Jung HJ, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. European Journal of Pharmacology. 2010;648(1–3):110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Deslauriers AM, Afkhami-Goli A, Paul AM, et al. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. Journal of Immunology. 2011;187(9):4788–4799. doi: 10.4049/jimmunol.1004111. [DOI] [PubMed] [Google Scholar]
- 17.Hemshekhar M, Sebastin Santhosh M, Sunitha K, et al. A dietary colorant crocin mitigates arthritis and associated secondary complications by modulating cartilage deteriorating enzymes, inflammatory mediators and antioxidant status. Biochimie. 2012;94(12):2723–2733. doi: 10.1016/j.biochi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Escribano J, Alonso GL, Coca-Prados M, Fernández JA. Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Letters. 1996;100(1-2):23–30. doi: 10.1016/0304-3835(95)04067-6. [DOI] [PubMed] [Google Scholar]
- 19.He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sciences. 2005;77(8):907–921. doi: 10.1016/j.lfs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biological and Pharmaceutical Bulletin. 2005;28(11):2106–2110. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 21.Lin JK, Wang CJ. Protection of crocin dyes on the acute hepatic damage induced by aflatoxin B1 and dimethylnitrosamine in rats. Carcinogenesis. 1986;7(4):595–599. doi: 10.1093/carcin/7.4.595. [DOI] [PubMed] [Google Scholar]
- 22.Krönke G, Bochkov VN, Huber J, et al. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. Journal of Biological Chemistry. 2003;278(51):51006–51014. doi: 10.1074/jbc.M304103200. [DOI] [PubMed] [Google Scholar]
- 23.June CH, Moore JS. Unit 5. 5 measurement of intracellular ions by flow cytometry. In: Coligan JE, editor. Current Protocols in Immunology. chapter 5. 2004. [DOI] [PubMed] [Google Scholar]
- 24.Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. The FASEB Journal. 2006;20(14):2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 25.Hirai K, Sasahira T, Ohmori H, Fujii K, Kuniyasu H. Inhibition of heme oxygenase-1 by zinc protoporphyrin IX reduces tumor growth of LL/2 lung cancer in C57BL mice. International Journal of Cancer. 2007;120(3):500–505. doi: 10.1002/ijc.22287. [DOI] [PubMed] [Google Scholar]
- 26.Takagi T, Naito Y, Uchiyama K, Yoshikawa T. The role of heme oxygenase and carbon monoxide in inflammatory bowel disease. Redox Report: Communications in Free Radical Research. 2010;15(5):193–201. doi: 10.1179/174329210X12650506623889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu ML, Ho YC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. The American Journal of Cardiovascular Disease. 2011;1(2):150–158. [PMC free article] [PubMed] [Google Scholar]
- 28.Ryter SW, Choi AMK. Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Current drug targets. 2010;11(12):1485–1494. doi: 10.2174/1389450111009011485. [DOI] [PubMed] [Google Scholar]
- 29.Bonelli M, Savitskaya A, Steiner CW, et al. Heme oxygenase-1 end-products carbon monoxide and biliverdin ameliorate murine collagen induced arthritis. Clinical and Experimental Rheumatology. 2012;30(1):73–78. [PubMed] [Google Scholar]
- 30.Fagone P, Mangano K, Coco M, et al. Therapeutic potential of carbon monoxide in multiple sclerosis. Clinical and Experimental Immunology. 2012;167(2):179–187. doi: 10.1111/j.1365-2249.2011.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Overhaus M, Moore BA, Barbato JE, Behrendt FF, Doering JG, Bauer AJ. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. The American Journal of Physiology—Gastrointestinal and Liver Physiology. 2006;290(4):G695–G703. doi: 10.1152/ajpgi.00152.2005. [DOI] [PubMed] [Google Scholar]
- 32.Sarady-Andrews JK, Liu F, Gallo D, et al. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. The American Journal of Physiology—Lung Cellular and Molecular Physiology. 2005;289(6):L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 33.Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-κB pathway in asthma and chronic obstructive pulmonary disease. Pharmacology and Therapeutics. 2009;121(1):1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roman-Blas JA, Jimenez SA. Targeting NF-κB: a promising molecular therapy in inflammatory arthritis. International Reviews of Immunology. 2008;27(5):351–374. doi: 10.1080/08830180802295740. [DOI] [PubMed] [Google Scholar]
- 35.Yan J, Greer JM. NF-κB, a potential therapeutic target for the treatment of multiple sclerosis. CNS and Neurological Disorders—Drug Targets. 2008;7(6):536–557. doi: 10.2174/187152708787122941. [DOI] [PubMed] [Google Scholar]
- 36.Kim AN, Jeon WK, Lee JJ, Kim BC. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radical Biology and Medicine. 2010;49(3):323–331. doi: 10.1016/j.freeradbiomed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Cheung KL, Yu S, Pan Z, Ma J, Wu TY, Kong ANT. TBHQ-induced HO-1 expression is mediated by calcium through regulation of Nrf2 binding to enhancer and polymerase II to promoter region of HO-1. Chemical Research in Toxicology. 2011;24(5):670–676. doi: 10.1021/tx1004369. [DOI] [PubMed] [Google Scholar]
- 38.Tsoyi K, Jang HJ, Kim JW, et al. Stimulation of Alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxidants and Redox Signaling. 2011;14(11):2057–2070. doi: 10.1089/ars.2010.3555. [DOI] [PubMed] [Google Scholar]
- 39.Gissel C, Doutheil J, Paschen W. Activation of heme oxygenase-1 expression by disturbance of endoplasmic reticulum calcium homeostasis in rat neuronal cell culture. Neuroscience Letters. 1997;231(2):75–78. doi: 10.1016/s0304-3940(97)00528-4. [DOI] [PubMed] [Google Scholar]
- 40.Nemmiche S, Chabane-Sari D, Kadri M, Guiraud P. Cadmium-induced apoptosis in the BJAB human B cell line: involvement of PKC/ERK1/2/JNK signaling pathways in HO-1 expression. Toxicology. 2012;300(3):103–111. doi: 10.1016/j.tox.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Cui W, Li G, et al. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCalpha and PI3K/AKT signaling pathways. Journal of Agricultural and Food Chemistry. 2012;60(33):8171–8182. doi: 10.1021/jf301511m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Effects of crocin on HO-1 induction, NO production and iNOS expression in murine peritoneal macrophages.
Supplementary Fig. 2: Inhibitory effects of crocin on DNA binding activity of NF-κB and IκB-α degradation in LPS-stimulated macrophages.
Supplementary Fig. 3: Effects of crocin on DNA binding activity of Nrf2.
Supplementary Fig. 4: Effects of crocin and CAMK4 on activity of ERK and JNK.
Supplementary Fig. 5: Effects of CAMK inhibitors on crocin-mediated HO-1 expression.


