Abstract
Background. The hanging drop (HD) technique presumably relies on the presence of subatmospheric epidural pressure. It is not clear whether this negative pressure is intrinsic or an artifact and how it is affected by body position. There are few data to indicate how often HD is currently being used. Methods. We identified studies that measured subatmospheric pressures and looked at the effect of the sitting position. We also looked at the technique used for cervical and thoracic epidural anesthesia in the last 10 years. Results. Intrinsic subatmospheric pressures were measured in the thoracic and cervical spine. Three trials studied the effect of body position, indicating a higher incidence of subatmospheric pressures when sitting. The results show lower epidural pressure (−10.7 mmHg) with the sitting position. 28.8% of trials of cervical and thoracic epidural anesthesia that documented the technique used, utilized the HD technique. When adjusting for possible bias, the rate of HD use can be as low as 11.7%. Conclusions. Intrinsic negative pressure might be present in the cervical and thoracic epidural space. This effect is more pronounced when sitting. This position might be preferable when using HD. Future studies are needed to compare it with the loss of resistance technique.
1. Introduction
Epidural anesthesia is popular in the treatment of acute and chronic pain. Most commonly the “loss of resistance” (LOR) technique is used. An alternative method is the “hanging drop” (HD) technique. It relies upon the aspiration of a small volume of fluid from the hub of the needle as the pressure at the tip decreases below atmospheric level upon entry into the epidural space (Figure 1 and Video, Supplemental Digital Content 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2014/146750, demonstrating the HD technique). Even though the technique has been used for 80 years there are still questions that remain unanswered. It is not clear whether the negative pressure required for entry into the epidural space is intrinsic or an artifact and how it is affected by body position. In addition, there are very few data to indicate how often this technique is currently used. The primary objective of this systematic review was to identify studies that measured subatmospheric pressures in the epidural space and to determine how these compared in the sitting versus the prone or lateral decubitus position. We also tried to determine how often the HD technique has been used in clinical trials in the last 10 years.
Figure 1.
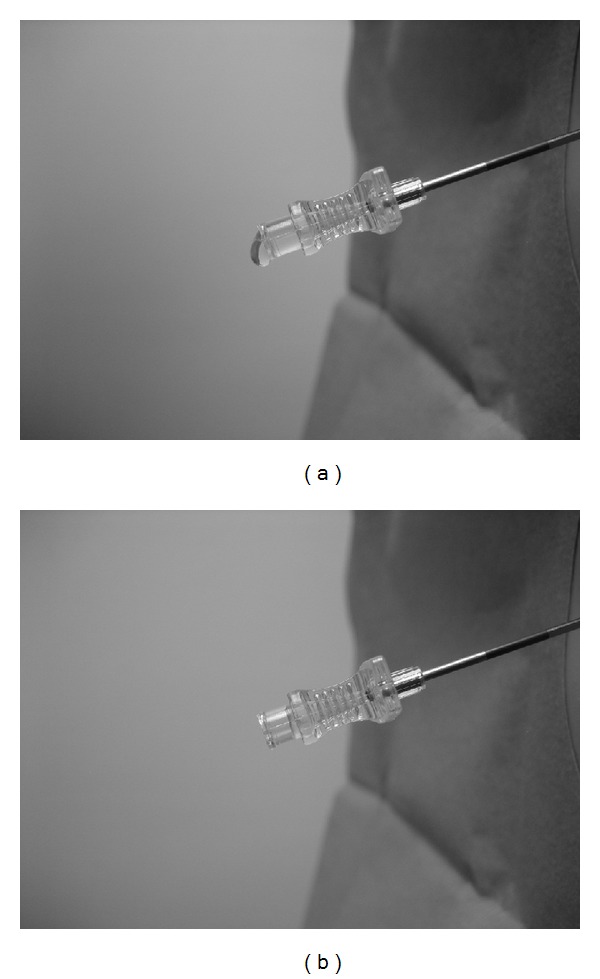
(a) A small volume of fluid is injected into the hub of the needle. (b) As the needle is advanced into the epidural space, the pressure at the tip decreases below atmospheric and the fluid is aspirated from the hub.
2. Methods
We performed a search of the databases MEDLINE, CINAHL, Turning Research into Practice (TRIP), and the Cochrane Library using the terms: Epidural Space/physiology [Mesh] or hanging drop and epidural or subatmospheric epidural pressure or negative epidural pressure. The search was limited to publications in English and humans. Since epidural anesthesia is usually performed in awake, spontaneously breathing patients, studies performed under general anesthesia and positive pressure ventilation were excluded. Case reports, reviews, editorials, practice guidelines, comments, and letters to the editor were also excluded. The reference sections of the articles that fulfilled the inclusion criteria were examined for any relevant sources not identified in the initial search. All publications that fulfilled the eligibility criteria were screened to identify trials demonstrating the presence of intrinsic subatmospheric epidural pressure and studying the effect of body position.
To identify how often the HD technique is used we searched MEDLINE using the terms: thoracic epidural or cervical epidural and anesthesia. The search was limited to clinical trials in human adults in the last 10 years and English language.
2.1. Data Extraction
The reviewers independently reviewed the full manuscripts of all included trials. The data extracted included the number of patients, epidural pressures, and body position. The timing of pressure measurements was also recorded. Only those pressures that were measured with the needle (or catheter) stationary in the epidural space were considered to be intrinsic. This was done to exclude any negative pressure artifactsrelated to the interaction of the advancing needle with the ligamentum flavum and the dura.When assessing the intrinsic epidural pressures, the measurements were identified as positive (above atmospheric), atmospheric, or negative (below atmospheric) without considering the pressure magnitude. When studying the effect of body position, the mean and standard deviation of the measured epidural pressures, as well as the incidence of subatmospheric pressure, were recorded. For the second search, each article was screened and the results were recorded as HD, LOR, both (LOR and HD), or unknown if the technique of epidural cannulation could not be identified from the text.
2.2. Risk of Bias Assessment
Risk of bias assessment was performed for those publications that demonstrated the presence of intrinsic subatmospheric epidural pressure. For randomized controlled studies, we used the 12 criteria established by the Cochrane Back Review Group (CBRG) [1]. These are presented in Table 1. Studies are rated as having a “high risk” of bias if less than 50% of CBRG criteria have been met. For all other studies, we used a modification of the Methodological Index for Non-Randomized Studies (MINORS) [2]. This tool contains 8 items with 4 additional items for comparative studies. Items can be scored as “adequate,” “inadequate,” or “unclear” if there is insufficient information (Table 2). To reduce the chance of publication bias, multiple clinical trial registries were screened for evidence of missing information (Table 3).
Table 1.
Risk of bias assessment using the CBRG criteria.
| Gil et al. (2008) [3] | Moon et al. (2010) [4] | Usubiaga et al. (1967) [5] | |
|---|---|---|---|
| Was the method of randomization adequate? | Yes | Yes | No |
| Was the treatment allocation concealed? | No | No | No |
| Was the patient blinded to the intervention? | No | No | No |
| Was the care provider blinded to the intervention? | No | No | No |
| Was the outcome assessor blinded to the intervention? | No | Yes | No |
| Was the drop-out rate described and acceptable? | Yes | Yes | Yes |
| Were all randomized participants analyzed in the group to which they were allocated? | Yes | Yes | Yes |
| Are reports of the study free of suggestion of selective outcome reporting? | Yes | Yes | Yes |
| Were the groups similar at baseline regarding the most important prognostic indicators? | No | Unsure | No |
| Were cointerventions avoided or similar? | Yes | Yes | Unsure |
| Was the compliance acceptable in all groups? | Yes | Yes | Yes |
| Was the timing of the outcome assessment similar in all groups? | Yes | Yes | Yes |
Table 2.
Risk of bias assessment using MINORS.
| Visser et al. (2006) [18] | Okutomi et al. (1993) [6] | |
|---|---|---|
| A clearly stated aim | A | A |
| Inclusion of consecutive patients | A | U |
| Prospective collection of data | A | A |
| Endpoints appropriate to the aim of the study | A | A |
| Unbiased assessment of the study endpoint | I | I |
| Follow-up period appropriate to the aim of the study | ∗ | ∗ |
| Loss to follow-up less than 5% | ∗ | ∗ |
| Prospective calculation of the study size | A | I |
| Additional criteria for comparative studies | ||
| Adequate control group | A | |
| Contemporary groups | A | |
| Baseline equivalence of groups | A | |
| Adequate statistical analyses | A |
*No follow-up indicated. A: adequate; I: inadequate; U: unclear.
Table 3.
Clinical trial registries that were screened for evidence of missing information.
| U.S. National Institutes of Health | http://www.clinicaltrials.gov/ |
| International Clinical Trials Registry Platform (ICTRP) of the World Health Organization | http://www.who.int/ictrp/en/ |
| Australian New Zealand Clinical Trials Registry (ANZCTR) | http://www.anzctr.org.au/ |
| Clinical Trials Registry-India | http://ctri.nic.in/ |
| European Union Clinical Trials Register (EU-CTR) | https://www.clinicaltrialsregister.eu/ |
| German Clinical Trials Register (DRKS) | http://www.drks.de/ |
| Netherlands Trial Register | http://www.trialregister.nl/ |
| International Standard Randomized Controlled Trial Number Register | http://www.isrctn.org/ |
2.3. Statistical Analysis
Meta-analysis was used to calculate the pooled effects of the sitting versus the horizontal position (prone or lateral decubitus). Analyses were performed using RevMan 5.2 software (Cochrane Collaboration, Oxford, UK). When looking at epidural pressures, the results were expressed as mean differences with 95% confidence intervals using the random effects model. Odds ratios with 95% confidence intervals were calculated using the Mantel-Haenszel random effects model when assessing the incidence of subatmospheric pressures. Statistical heterogeneity of the included studies was determined using the I 2 statistic which describes the degree of total variation between studies that cannot be explained by chance alone. Values <25% indicate low levels, whereas values >50% are consistent with substantive heterogeneity.
3. Results
A total of 17 article publications were identified that matched our criteria [3–19]. The initial search identified 296 records in MEDLINE, 22 in CINAHL, 127 in the Cochrane library, and 360 in TRIP (Figure 2). The titles were examined and those that were not considered relevant to our search were discarded. After reviewing the abstracts and full-text articles, a total of 15 studies were identified that matched the inclusion criteria with a total of 802 patients. A search of the references identified 2 additional articles with a total of 28 patients [7, 17]. 13 studies reported on pressures in the lumbar spine, 5 in the thoracic spine, and 3 in the cervical spine. A brief summary of the main findings is listed in Table 4. In the lumbar spine, subatmospheric pressures were only measured at the time of needle entry into the epidural space. In those cases where the needle was stationary or the pressures were measured using an epidural catheter, the pressures were always positive. This was true independent of patient position. In the thoracic spine, subatmospheric pressures were measured in all 5 studies. Only three studies measured intrinsic epidural pressures after needle stabilization [3, 6, 18]. In all three, epidural pressures were measured with the patient in the lateral decubitus position and even though there were instances of negative epidural pressures measured at least 90 seconds after needle entry, the mean epidural pressures were positive (1 mmHg at T3–5, 5.1 mmHg at T5-6, and 3.7 mmHg at T7-8) [3, 6, 18]. Consistent intrinsic negative pressure measurements were only found in the one study in which the sitting position was also chosen [3]. In all 3 studies that focused on the cervical spine, negative pressures were measured [4, 5, 17]. The recordings were made after needle stabilization only in one study [4]. Moon et al. consistently measured positive pressures with the patients in the prone position, whereas 10 out of 15 measurements were subatmospheric when sitting [4].
Figure 2.
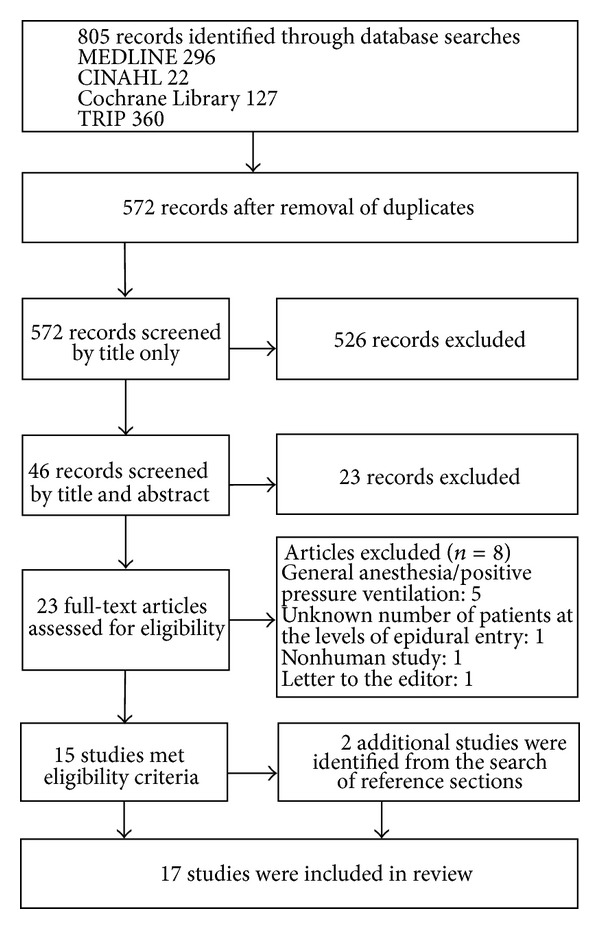
Flow diagram of the literature search results on epidural pressures.
Table 4.
Summary of studies on epidural pressure.
| Author (year) | Level | n | Epidural pressures | Comments |
|---|---|---|---|---|
| Galbert and Marx (1974) [7] | Lumbar | 12 | All positive | Pressures transduced from epidural catheter |
|
| ||||
| Gil et al. (2008) [3] | Thoracic | 28 | Consistent negative epidural pressures in the sitting position only at T5-6 | Pressures measured 120 s after entry into the epidural space |
|
| ||||
| Johnston et al. (1989) [8] | Lumbar | 14 | All positive | Pressures transduced from epidural catheter |
|
| ||||
| Messih (1981) [9] | Lumbar | 21 | All positive upon needle entry and after catheter insertion | Pressures measured in parturients |
|
| ||||
| Moon et al. (2010) [4] | Cervical | 30 | All positive in the prone position, 10/15 negative in the sitting position | Pressures measured 120 s after entry into the epidural space |
|
| ||||
| Okutomi et al. (1993) [6] | Thoracic | 13 | Initial negative pressure right after puncture, positive in 12/13 patients after 90 s. | Lateral decubitus position at T7-8 |
|
| ||||
| Rocco et al. (1997) [10] | Lumbar | 25 | All positive after needle was stabilized in the epidural space | negative in 4/4 patients where pressure was measured upon entry |
|
| ||||
| Rodiera et al. (1995) [11] | Lumbar | 20 | All positive | Pressures measured >5 s. after entry into the epidural space |
|
| ||||
| Shah (1981) [12] | Lumbar | 43 | All positive | Pressures transduced from epidural catheter |
|
| ||||
| Shah (1984) [13] | Lumbar | 40 | All positive | Pressures transduced from epidural catheter |
|
| ||||
| Takahashi et al. (1995) [14] | Lumbar | 10 | All positive | Measurements performed with a catheter transducer |
|
| ||||
| Takahashi et al. (1995) [15] | Lumbar | 19 | All positive | Measurements performed with a catheter transducer |
|
| ||||
| Thomas et al. (1992) [16] | Lumbar | 39 | All positive | Pressures measured 180 s after entry into the epidural space |
|
| ||||
| Usubiaga et al. (1967) [17] | Lumbar | 16 | Consistent negative pressures measured at the time of entry into the epidural space, all positive pressure measurements after needle stabilization | Lateral decubitus position |
| Thoracic | ||||
| Cervical | ||||
|
| ||||
| Usubiaga et al. (1967) [5] | Cervical | 405 | Consistent negative thoracic/cervical pressures in the sitting position | Pressures measured at the time of entry into the epidural space |
| Thoracic | Negative lumbar pressures in 42/48 patients in the sitting position | |||
| Lumbar | Negative lumbar pressures in 202/228 patients in the lateral decubitus position | |||
|
| ||||
| Visser et al. (2006) [18] | Thoracic | 40 | Negative pressures in 8/17 patients at T3–T5 and 2/20 at T7–10 (lateral decubitus) | Pressures measured 120 s after entry into the epidural space |
|
| ||||
| Zarzur (1984) [19] | Lumbar | 30 | Negative pressures in 24/30 patients upon entry into the epidural space | |
Results are listed as either below (negative), or above (positive) atmospheric pressure.
When looking at those publications where negative pressures were measured, 3 studies compared the sitting position to the lateral decubitus or prone position. All three (one cervical, one thoracic, and one with cervical, thoracic, and lumbar measurements) studies showed a greater incidence of measured subatmospheric pressures when sitting [3–5]. Table 1 summarizes the risk of bias assessment of these trials. Our search of trial registries did not reveal any unpublished trials. There was no evidence of missing data upon close examination of the articles and in one case upon examination of the study protocol [4]. The data from the largest study by Usubiaga et al. [5] were only presented as range of measured epidural pressures and incidence of subatmospheric pressure which made it impossible to calculate the mean and standard deviation. The results of the two remaining studies were combined in a forest plot of epidural pressures (Figure 3). The combined effect of the meta-analysis was −10.7 mm Hg mean difference (95% confidence interval −12.9 to −8.5) suggesting a lower epidural pressure in the sitting position that was statistically significant (P < 0.0001). When looking at the incidence of subatmospheric pressure, all 3 studies were included in the meta-analysis (Figure 4). The pooled effect showed no difference between the two positions. There was high heterogeneity (I 2 = 87). A funnel plot was not done due to the small number of studies.
Figure 3.
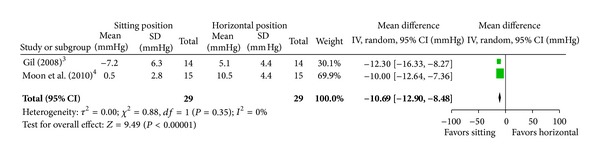
Pooled data evaluating the difference in epidural pressure (mm Hg) between the sitting and horizontal position. Expressed as mean difference with 95% confidence intervals.
Figure 4.
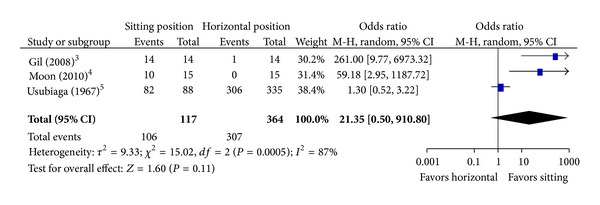
Effect of the sitting position on the incidence of subatmospheric pressure. Expressed as pooled odds ratio with 95% confidence intervals. The 95% confidence interval crosses “1,” suggesting that there is no difference between the sitting and horizontal position.
The second MEDLINE search using the terms: “thoracic epidural or cervical epidural and anesthesia” revealed 209 articles. After discarding articles that were not relevant for reasons such as levels other than thoracic or cervical, no epidural technique used, or retracted publications, either the abstract or full text of the remaining articles was used to identify the technique of epidural cannulation. 78 studies did not indicate what technique was used, in 47 studies only the LOR technique was used, in 14 studies only HD was used, and 5 studies used both techniques. This gives a rate of 28.8% (19/66) for the HD technique in those published controlled studies in the last 10 years where the technique could be identified from the article. Those studies that utilized the HD technique are listed in Table 5 [20–38]. To avoid any bias due to multiple publications from the same source, the articles were screened, and in all cases where several articles came from the same institution or the same authors, they were counted as one. The revised numbers were LOR: 43, HD: 11, and both: 5, giving an overall rate of 27.1% (16/59) for HD in the last 10 years. The total number of patients receiving epidural anesthesia in these trials was 3319 (LOR-1855, HD-485, both-979). To avoid any bias due to the omission of the 78 studies that did not mention the technique of epidural cannulation, it was assumed that they all used LOR, thus allowing us to calculate the lowest possible rate of HD use, namely, 11.7% (16/137). The majority of studies focused on the thoracic spine, while only two were performed in the cervical spine (1 LOR and 1 with both LOR and HD). All studies that used the HD technique came from continental Europe and Asia.
Table 5.
Summary of studies where the hanging drop technique was used.
| Author | Year | Level | n | Patients receiving epidural anesthesia | Country of origin | Technique |
|---|---|---|---|---|---|---|
| Bauer et al. [20] | 2007 | Thoracic | 68 | 34 | France | HD |
| Berendes et al. [21] | 2003 | Thoracic | 73 | 36 | Germany | HD |
| Gupta et al. [22] | 2006 | Thoracic | 60 | 30 | Sweden | HD/LOR |
| Han et al. [23] | 2003 | Cervical | 816 | 816 | Korea | HD/LOR |
| Hansdottir et al. [24] | 2006 | Thoracic | 113 | 58 | Sweden | HD/LOR |
| Heijmans et al. [25] | 2007 | Thoracic | 60 | 15 | Netherlands | HD |
| Kessler et al. [26] | 2005 | Thoracic | 90 | 60 | Germany | HD |
| Kunstyr et al. [27] | 2008 | Thoracic | 32 | 16 | Czech Republic | HD |
| Kurtoğlu et al. [28] | 2009 | Thoracic | 76 | 34 | Turkey | HD/LOR |
| Lagunilla et al. [29] | 2006 | Thoracic | 52 | 52 | Spain | HD |
| Lundstrøm et al. [30] | 2005 | Thoracic | 50 | 25 | Denmark | HD |
| Mehta et al. [31] | 2008 | Thoracic | 36 | 18 | India | HD |
| Mehta et al. [32] | 2010 | Thoracic | 62 | 31 | India | HD |
| Nishi et al. [33] | 2006 | Thoracic | 41 | 41 | Japan | HD/LOR |
| Nygård et al. [34] | 2004 | Thoracic | 163 | 79 | Denmark | HD |
| Porizka et al. [35] | 2011 | Thoracic | 47 | 32 | Czech Republic | HD |
| Schmidt et al. [36] | 2005 | Thoracic | 37 | 37 | Germany | HD |
| Sharma et al. [37] | 2010 | Thoracic | 60 | 30 | India | HD |
| Visser et al. [38] | 2006 | Thoracic | 20 | 20 | Netherlands | HD |
HD: hanging drop; LOR: loss of resistance.
4. Discussion
Our review of the literature suggests that intrinsic negative pressures might be present in the cervical and thoracic epidural space. Subatmospheric pressures are more pronounced when the sitting position is chosen. We also found that the HD technique was used in 28.8% of those controlled trials of cervical and thoracic epidural anesthesia in the last 10 years that documented the method of cannulation.
4.1. Etiology of the Negative Epidural Pressure
In 1933, Guttierez described the sign of the “hanging drop,” whereby a drop of saline hanging in the hub of a needle was “aspirated” when the needle entered the epidural space [39]. This phenomenon presumably occurs due to the presence of subatmospheric pressure at the needle tip. In 1926, Ernst Janzen was the first to describe the presence of subatmospheric (negative) pressure in the epidural space [40]. There has been controversy whether there is intrinsic negative epidural pressure or if it is an artifact produced by the needle entering the epidural space [40–43]. Our literature search suggests that there is no evidence of intrinsic subatmospheric pressure in the lumbar epidural space that can be measured after needle stabilization. The negative pressures recorded at the time of lumbar epidural entry have been described as artifacts caused by the initial bulging of the ligamentum flavum, followed by its rapid return to the resting position once the needle has perforated the ligament or due to tenting of the dura by the advancing needle [19, 40, 44]. Our literature search suggests that intrinsic subatmospheric epidural pressure might be present in the human thoracic and cervical spine. This was the case in all 4 studies (3 thoracic and 1 cervical) that measured epidural pressures after needle stabilization. In the studies identified by our systematic search, we only found evidence of consistent intrinsic negative pressure in the sitting position in the thoracic spine, based on the observations in one report [3]. A possible explanation for this phenomenon is that blood in the epidural venous plexus may be distributed to the lower part of the body due to gravity and the volume of the epidural plexus may decrease, producing a lower epidural pressure. CSF in the dural sac may play a similar role [3]. Intrinsic subatmospheric pressure is not present consistently in the sitting position in the cervical spine and this might be caused by neck flexion, which is necessary to widen the interlaminar space during needle placement. This can prevent venous run-off and cause venous engorgement resulting in a decrease in the epidural volume and increase in pressure [4, 17]. Neck flexion can also cause compression of the jugular veins resulting in increased cerebrospinal fluid pressure, which in turn raises the epidural pressure [45].
Why would the existence of intrinsic subatmospheric pressure be important? It is not a requirement for the successful use of the hanging drop technique due to the occurrence of negative pressure artifacts [46–49]. The existence of intrinsic subatmospheric pressure may be important for the identification of the epidural space in those areas that have ligamentum flavum gaps or where dural tenting is limited by very small amounts of CSF and the presence of the spinal cord, which could potentially make negative pressure artifacts less reliable.
4.2. Comparison to Animal Studies
Negative epidural pressures have also been measured in dogs, horses, and cows [50–54]. Some of the animal data were recorded from chronically implanted (7–14 days) epidural catheters [50]. It is believed that such a time period would allow healing and resolution of any changes related to the needle trauma and produce measurements representing the intrinsic epidural pressure [50]. In all these studies, the pressures were either measured in standing animals (cows, horses) [51–54] or animals placed on their sternum (dogs) [50]. Such positioning produces negative intraabdominal pressure, probably related to the gravity of the organs in the abdominal cavity. The negative pressures are then transmitted to the epidural space through the intervertebral foramina [52]. Changing the body position in cattle from standing to lateral recumbent changes the mean lumbar epidural pressures from negative to positive [54]. This might explain why there is a positive “hanging drop” sign in 88% of dogs positioned on their sternum as opposed to 0% in those that are in the lateral recumbent position [55]. In human studies, only Shah et al. looked at the effect of the prone position in 5 pregnant patients on their hands and knees and showed that the pressures were lower, yet still positive in comparison to the supine and lateral positions (+2.2 cm H2O prone, +14.8 cm H2O lateral, and +22.6 cm H2O supine) [13]. The above-mentioned animal studies did not look at thoracic or cervical epidural pressures. Only Nystrom et al. looked at thoracic pressures in pigs [56]. The epidural pressures were consistently positive; however, they were measured in the lateral recumbent position and the animals were mechanically ventilated.
4.3. Effect of the Sitting Position
The three studies that were analyzed demonstrated a higher incidence of negative epidural pressures with the sitting position. When the data was pooled, there was a statistically significant decrease in epidural pressure when sitting. This evidence is weak as it is only based on 2 randomized controlled trials with a total of 29 patients. Looking at the incidence of subatmospheric pressures allowed us to include a larger trial with 405 patients in the meta-analysis. Although the pooled results suggest that the incidence of subatmospheric pressures might not be increased by the sitting position, it is possible that methodological flaws in the study by Usubiaga et al. [5], including lack of randomization and inconsistencies in the methods of epidural pressure measurement, could have contributed to these findings. Further studies are needed to examine the effect of body position on epidural pressure and how this might affect the reliability of epidural space cannulation with the HD technique.
4.4. Use of the HD Technique
We found that the HD technique was used in 28.8% of those controlled trials of cervical and thoracic epidural anesthesia in the last 10 years that documented the method of cannulation. When accounting for multiple publications by the same institution and assuming that the studies that did not document the technique all used LOR, the rate decreases to 11.7%. This number is speculative but it represents the lowest possible rate for HD use from the included trials. However, even after such corrections, the results are still somewhat surprising when compared to the limited available information on the use of the HD technique, coming mainly from surveys of anesthesiologists. Wantman et al. carried out a survey of 1285 obstetric anesthesiologists in the United Kingdom regarding their preferred method of identifying the epidural space. When performing thoracic epidurals, 98% of the respondents chose the LOR method. 2% preferred alternative methods, including HD [57]. This study did not mention how often obstetric anesthesiologists perform thoracic epidurals and how their rate of HD use compares to the overall rate for anesthesiologists in the UK. In a survey of 617 Spanish anesthesiologists by Figueredo et al., 0.8% of respondents identified HD as the technique they most commonly use [58]. The overall rate of HD use was not mentioned. However, since this study identified only the most commonly used technique for epidural space cannulation, it did not account for the fact that some anesthesiologists, including the authors of this article, use both the LOR and HD techniques.
The articles that we identified in our search came from Europe and Asia. The greatest use of the hanging drop technique in the US might be in the field of chronic pain management. A national survey from 2002 demonstrated that this technique was used in 62% of academic centers, and 30% of private practices for cervical epidural steroid injections [59]. To ensure accuracy of placement and decrease patient discomfort, the routine use of fluoroscopy is recommended [60]. Most commonly, the prone position is chosen, so that both anteroposterior and lateral fluoroscopic images of the needle can be obtained [59–64]. In this position, intrinsic cervical epidural pressures are usually positive [4]. This could mean that a successful HD technique will have to rely on negative pressure artifacts, which might not occur due to ligamentum flavum gaps and the possibility of decreased dural tenting related to the presence of the spinal cord. Abram and Hogan suggested avoiding the HD technique with cervical epidural steroid injections based on 2 malpractice cases in which this method failed to reliably identify the epidural space [65].
Despite the long coexistence of the HD and LOR techniques, just two studies have directly compared them and only in the lumbar spine [46, 47]. Even though both techniques were equally successful in identifying the epidural space and there were no dural punctures, Hoffmann et al. showed that the tip of the epidural needle was 2.8 mm closer to the subarachnoid space with HD than with the LOR [46]. Both studies were performed in nonobstetric patients. Many lumbar epidurals are performed in parturients who demonstrate increasing positive epidural pressures as labor progresses [7]. Janzen observed that increasing the abdominal pressure decreased the amplitude of the subatmospheric pressure during needle placement [40]. When performing lumbar and low (below the T8 level) thoracic epidural puncture, Bonica et al. observed a positive hanging drop sign in only 80% of cases [48]. In a study of 1002 single-shot lumbar epidural blocks by Sheehan et al., a positive sign occurred in 91% of cases [49]. It is therefore unlikely that using the HD technique in the lumbar spine will have any advantage over the LOR technique, and its use may be contraindicated because of the higher potential for failure. Future studies are needed to compare the safety and effectiveness of the LOR and HD techniques.
5. Limitations
We only pooled the data from the randomized trials. Pooling of both randomized and nonrandomized studies with the aim of performing meta-analysis of epidural pressures would have been difficult due to the fact that the studies performed measurements at different levels in the spine which can account for significant variations. Due to the limited number of publications that identified intrinsic subatmospheric epidural pressures, we looked at data from animal studies. The meta-analysis of the effect of the sitting position includes only three studies with the largest one demonstrating significant methodological flaws and a high risk of bias. The limited number of studies did not allow us to use methods such as funnel plots or formal testing to look for publication bias. Instead, we examined multiple registries of clinical trials for missing data. Future studies are needed to confirm our findings.
With respect to the use of the hanging drop technique, we are aware of the limitations of our findings due to the fact that 78 out of 144 articles that were screened did not mention the technique used. There might also be bias towards academic institutions, and thus not reflecting the overall use of the HD technique. We only chose the cervical and thoracic spine because we found no documented advantage of the HD technique over the LOR in the lumbar spine. In addition, we found very little evidence to suggest that the HD technique is routinely used in the lumbar spine. In the study by Hoffman et al., the cannulation was performed for the placement of intrathecal catheters for neurosurgical procedures [46]. The study by Gülen et al. used HD together with LOR in the control groups for the trial of the Episure spring-loaded syringe [47].
In conclusion, our review suggests that intrinsic negative pressure might be present in the cervical and thoracic epidural space. This effect is more pronounced when sitting, which is why this position might be preferable when using the HD technique. We found no information suggesting the presence of intrinsic negative pressure in the lumbar spine. There is also no evidence of any advantage of the HD technique over the LOR technique in the lumbar spine. If performing cervical epidural steroid injection with fluoroscopy in the prone position, the LOR technique should be used. Only studies directly comparing the LOR and HD techniques can determine which technique is better suited for the identification of the epidural space.
Supplementary Material
Demonstration of the hanging drop technique. As the needle enters the epidural space the pressure at the tip decreases below atmospheric level. This results in aspiration of the fluid from the hub.
Acknowledgment
The authors would like to thank Dr. Guy Weinberg, Professor, Department of Anesthesiology, University of Illinois, College of Medicine, Jesse Brown VA Medical Center, Chicago, for his advice and assistance with the preparation of this paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Both authors helped design the study, conduct the study, analyze the data, and write the paper.
References
- 1.Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 Updated method guidelines for systematic reviews in the cochrane back review group. Spine. 2009;34(18):1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 2.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ Journal of Surgery. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 3.Gil NS, Lee J-H, Yoon SZ, Jeon Y, Lim YJ, Bahk JH. Comparison of thoracic epidural pressure in the sitting and lateral decubitus positions. Anesthesiology. 2008;109(1):67–71. doi: 10.1097/ALN.0b013e31817b80df. [DOI] [PubMed] [Google Scholar]
- 4.Moon JY, Lee P-B, Nahm FS, Kim Y-C, Choi J-B. Cervical epidural pressure measurement: comparison in the prone and sitting positions. Anesthesiology. 2010;113(3):666–671. doi: 10.1097/ALN.0b013e3181e898e8. [DOI] [PubMed] [Google Scholar]
- 5.Usubiaga JE, Wikinski JA, Usubiaga LE. Epidural pressure and its relation to spread of anesthetic solutions in epidural space. Anesthesia and Analgesia. 1967;46(4):440–446. [PubMed] [Google Scholar]
- 6.Okutomi T, Watanabe S, Goto F. Time course in thoracic epidural pressure measurement. Canadian Journal of Anaesthesia. 1993;40(11):1044–1048. doi: 10.1007/BF03009475. [DOI] [PubMed] [Google Scholar]
- 7.Galbert MW, Marx GF. Extradural pressures in the parturient patient. Anesthesiology. 1974;40(5):499–502. doi: 10.1097/00000542-197405000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Johnston GM, Rodgers RC, Tunstall ME. Alteration of maternal posture and its immediate effect on epidural pressure. Anaesthesia. 1989;44(9):750–752. doi: 10.1111/j.1365-2044.1989.tb09262.x. [DOI] [PubMed] [Google Scholar]
- 9.Messih MNA. Epidural space pressures during pregnancy. Anaesthesia. 1981;36(8):775–782. doi: 10.1111/j.1365-2044.1981.tb08815.x. [DOI] [PubMed] [Google Scholar]
- 10.Rocco AG, Philip JH, Boas RA, Scott D. Epidural space as a Starling resistor and elevation of inflow resistance in a diseased epidural space. Regional Anesthesia. 1997;22(2):167–177. doi: 10.1016/s1098-7339(06)80037-4. [DOI] [PubMed] [Google Scholar]
- 11.Rodiera J, Calabuig R, Aliaga L, et al. Mathematical analysis of epidural space location. International Journal of Clinical Monitoring and Computing. 1995;12(4):213–217. doi: 10.1007/BF01207201. [DOI] [PubMed] [Google Scholar]
- 12.Shah JL. Influence of cerebrospinal fluid on epidural pressure. Anaesthesia. 1981;36(6):627–631. doi: 10.1111/j.1365-2044.1981.tb10329.x. [DOI] [PubMed] [Google Scholar]
- 13.Shah JL. Effect of posture on extradural pressure. British Journal of Anaesthesia. 1984;56(12):1373–1377. doi: 10.1093/bja/56.12.1373. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Miyazaki T, Takino T, Matsui T, Tomita K. Epidural pressure measurements: relationship between epidural pressure and posture in patients with lumbar spinal stenosis. Spine. 1995;20(6):650–653. [PubMed] [Google Scholar]
- 15.Takahashi K, Kagechika K, Takino T, Matsui T, Miyazaki T, Shima I. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine. 1995;20(24):2746–2749. doi: 10.1097/00007632-199512150-00017. [DOI] [PubMed] [Google Scholar]
- 16.Thomas PS, Gerson JI, Strong G. Analysis of human epidural pressures. Regional Anesthesia. 1992;17(4):212–215. [PubMed] [Google Scholar]
- 17.Usubiaga JE, Moya F, Usubiaga LE. Effect of thoracic and abdominal pressure changes on the epidural space pressure. British Journal of Anaesthesia. 1967;39(8):612–618. doi: 10.1093/bja/39.8.612. [DOI] [PubMed] [Google Scholar]
- 18.Visser WA, Gielen MJM, Giele JLP, Scheffer GJ. A comparison of epidural pressures and incidence of true subatmospheric epidural pressure between the mid-thoracic and low-thoracic epidural space. Anesthesia and Analgesia. 2006;103(5):1318–1321. doi: 10.1213/01.ane.0000244325.46807.b6. [DOI] [PubMed] [Google Scholar]
- 19.Zarzur E. Genesis of the “true” negative pressure in the lumbar epidural space. A new hypothesis. Anaesthesia. 1984;39(11):1101–1104. doi: 10.1111/j.1365-2044.1984.tb08931.x. [DOI] [PubMed] [Google Scholar]
- 20.Bauer C, Hentz J-G, Ducrocq X, et al. Lung function after lobectomy: a randomized, double-blinded trial comparing thoracic epidural ropivacaine/sufentanil and intravenous morphine for patient-controlled analgesia. Anesthesia and Analgesia. 2007;105:238–244. doi: 10.1213/01.ane.0000266441.58308.42. [DOI] [PubMed] [Google Scholar]
- 21.Berendes E, Schmidt C, van Aken H, et al. Reversible cardiac sympathectomy by high thoracic epidural anesthesia improves regional left ventricular function in patients undergoing coronary artery bypass grafting: a randomized trial. Archives of Surgery. 2003;138(12):1283–1290. doi: 10.1001/archsurg.138.12.1283. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Fant F, Axelsson K, et al. Postoperative analgesia after radical retropubic prostatectomy: a double-blind comparison between low thoracic epidural and patient-controlled intravenous analgesia. Anesthesiology. 2006;105(4):784–793. doi: 10.1097/00000542-200610000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Han K-R, Kim C, Park S-K, Kim J-S. Distance to the adult cervical epidural space. Regional Anesthesia and Pain Medicine. 2003;28(2):95–97. doi: 10.1053/rapm.2003.50025. [DOI] [PubMed] [Google Scholar]
- 24.Hansdottir V, Philip J, Olsen MF, Eduard C, Houltz E, Ricksten S-E. Thoracic epidural versus intravenous patient-controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient-perceived quality of recovery. Anesthesiology. 2006;104(1):142–151. doi: 10.1097/00000542-200601000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Heijmans J, Fransen E, Buurman W, Maessen J, Roekaerts P. Comparison of the modulatory effects of four different fast-track anesthetic techniques on the inflammatory response to cardiac surgery with cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia. 2007;21(4):512–518. doi: 10.1053/j.jvca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Kessler P, Aybek T, Neidhart G, et al. Comparison of three anesthetic techniques for off-pump coronary artery bypass grafting: general anesthesia, combined general and high thoracic epidural anesthesia, or high thoracic epidural anesthesia alone. Journal of Cardiothoracic and Vascular Anesthesia. 2005;19(1):32–39. doi: 10.1053/j.jvca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Kunstyr J, Klein A, Lindner J, et al. Use of high-thoracic epidural analgesia in pulmonary endarterectomy: a randomized feasibility study. The Heart Surgery Forum. 2008;11(4):E202–E208. doi: 10.1532/HSF98.20081036. [DOI] [PubMed] [Google Scholar]
- 28.Kurtoğlu M, Ateş S, Bakkaloğlu B. Epidural anesthesia versus general anesthesia in patients undergoing minimally invasive direct coronary artery bypass surgery. Anadolu Kardiyoloji Dergisi. 2009;9:54–58. [PubMed] [Google Scholar]
- 29.Lagunilla J, García-Bengochea JB, Fernández ÁL, et al. High thoracic epidural blockade increases myocardial oxygen availability in coronary surgery patients. Acta Anaesthesiologica Scandinavica. 2006;50(7):780–786. doi: 10.1111/j.1399-6576.2006.01059.x. [DOI] [PubMed] [Google Scholar]
- 30.Lundstrøm LH, Nygård E, Hviid LB, et al. The effect of thoracic epidural analgesia on the occurence of late postoperative hypoxemia in patients undergoing elective coronary bypass surgery: a randomized controlled trial. Chest. 2005;128(3):1564–1570. doi: 10.1378/chest.128.3.1564. [DOI] [PubMed] [Google Scholar]
- 31.Mehta Y, Arora D, Sharma KK, Mishra Y, Wasir H, Trehan N. Comparison of continuous thoracic epidural and paravertebral block for postoperative analgesia after robotic-assisted coronary artery bypass surgery. Annals of cardiac anaesthesia. 2008;11(2):91–96. doi: 10.4103/0971-9784.41576. [DOI] [PubMed] [Google Scholar]
- 32.Mehta Y, Vats M, Sharma M, Arora R, Trehan N. Thoracic epidural analgesia for off-pump coronary artery bypass surgery in patients with chronic obstructive pulmonary disease. Annals of Cardiac Anaesthesia. 2010;13(3):224–230. doi: 10.4103/0971-9784.69062. [DOI] [PubMed] [Google Scholar]
- 33.Nishi M, Usukaura A, Kidani Y, Tsubokawa T, Yamamoto K. Which is a better position for insertion of a high thoracic epidural catheter: sitting or lateral decubitus? Journal of Cardiothoracic and Vascular Anesthesia. 2006;20(5):656–658. doi: 10.1053/j.jvca.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Nygård E, Sørensen LH, Hviid LB. Effects of amiodarone and thoracic epidural analgesia on atrial fibrillation after coronary artery bypass grafting. Journal of Cardiothoracic and Vascular Anesthesia. 2004;18:709–714. doi: 10.1053/j.jvca.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Porizka M, Stritesky M, Semrad M, Dobias M, Dohnalova A. Postoperative outcome in awake, on-pump, cardiac surgery patients. Journal of Anesthesia. 2011;25(4):500–508. doi: 10.1007/s00540-011-1159-7. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt C, Hinder F, van Aken H, et al. The effect of high thoracic epidural anesthesia on systolic and diastolic left ventricular function in patients with coronary artery disease. Anesthesia and Analgesia. 2005;100(6):1561–1569. doi: 10.1213/01.ANE.0000154963.29271.36. [DOI] [PubMed] [Google Scholar]
- 37.Sharma M, Mehta Y, Sawhney R, Vats M, Trehan N. Thoracic epidural analgesia in obese patients with body mass index of more than 30 kg/m2 for off pump coronary artery bypass surgery. Annals of Cardiac Anaesthesia. 2010;13(1):28–33. doi: 10.4103/0971-9784.58831. [DOI] [PubMed] [Google Scholar]
- 38.Visser WA, Gielen MJM, Giele JLP. Continuous positive airway pressure breathing increases the spread of sensory blockade after low-thoracic epidural injection of lidocaine. Anesthesia and Analgesia. 2006;102(1):268–271. doi: 10.1213/01.ane.0000184813.18470.52. [DOI] [PubMed] [Google Scholar]
- 39.Aldrete JA, Auad OA, Gutierrez VP, Wright AJ. Alberto Gutierrez and the hanging drop. Regional Anesthesia and Pain Medicine. 2005;30(4):397–404. doi: 10.1016/j.rapm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Janzen E. Der negative Vorschlag bei Lnmbalpunktion. Deutsche Zeitschrift für Nervenheilkunde. 1926;94(1):280–292. [Google Scholar]
- 41.Bryce-Smith R. Pressures in the extra-dural space. Anaesthesia. 1950;5(4):213–216. doi: 10.1111/j.1365-2044.1950.tb12686.x. [DOI] [PubMed] [Google Scholar]
- 42.Andrade P. A new interpretation of the origin of extradural space negative pressure. British Journal of Anaesthesia. 1983;55(1):85–88. doi: 10.1093/bja/55.1.85. [DOI] [PubMed] [Google Scholar]
- 43.McKinney MS. Hanging drop and extradural pressures. Anaesthesia. 1987;42(10):1123–1124. doi: 10.1111/j.1365-2044.1987.tb05200.x. [DOI] [PubMed] [Google Scholar]
- 44.Telford RJ, Hollway TE. Observations on deliberate dural puncture with a Tuohy needle: pressure measurements. Anaesthesia. 1991;46(9):725–727. doi: 10.1111/j.1365-2044.1991.tb09765.x. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama T, Ushida T, Yamasaki F, Inoue S, Sluka KA. Epidural puncture can be confirmed by the Queckenstedt-test procedure in patients with cervical spinal canal stenosis. Acta Anaesthesiologica Scandinavica. 2008;52(2):256–261. doi: 10.1111/j.1399-6576.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann VLH, Vercauteren MP, Vreugde J-P, Hans GH, Coppejans HC, Adriaensen HA. Posterior epidural space depth: safety of the loss of resistance and hanging drop techniques. British Journal of Anaesthesia. 1999;83(5):807–809. doi: 10.1093/bja/83.5.807. [DOI] [PubMed] [Google Scholar]
- 47.Gülen G, Akkaya T, Ozkan D. Comparison of spring-loaded, loss of resistance and hanging drop techniques in lumbar epidural blocks. Agri. 2012;24:37–41. doi: 10.5505/agri.2012.98705. [DOI] [PubMed] [Google Scholar]
- 48.Bonica JJ, Backup PH, Anderson CE, HADFIELD D, Crepps WF, Monk BF. Peridural block: analysis of 3,637 cases and a review. Anesthesiology. 1957;18(5):723–784. doi: 10.1097/00000542-195709000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan JM, Wright IV, Shepard BM. An evaluation of the Cheng needle in the administration of 1002 single-dose lumbar epidural anesthetics. Anesthesia and Analgesia. 1962;41:552–556. [PubMed] [Google Scholar]
- 50.Bengis RG, Goyton AC. Some pressure and fluid dynamic characteristics of the canine epidural space. American Journal of Physiology. 1977;232(3):h255–h259. doi: 10.1152/ajpheart.1977.232.3.H255. [DOI] [PubMed] [Google Scholar]
- 51.Iff I, Mosing M, Moens Y. Pressure profile in the caudal extradural space of standing horses before and after extradural drug administration. Veterinary Journal. 2009;180(1):112–115. doi: 10.1016/j.tvjl.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Lee IH, Yamagishi N, Yamada H. Lumbar epidural pressure in cattle. Veterinary Record. 2001;149(17):525–526. doi: 10.1136/vr.149.17.525. [DOI] [PubMed] [Google Scholar]
- 53.Lee I, Yamagishi N, Oboshi K, Yamada H, Ohtani M. Multivariate regression analysis of epidural pressure in cattle. American Journal of Veterinary Research. 2002;63(7):954–957. doi: 10.2460/ajvr.2002.63.954. [DOI] [PubMed] [Google Scholar]
- 54.Lee IH, Yamagishi N, Oboshi K, Yamada H. Effect of postural change on lumbar epidural pressure in cattle. Veterinary Journal. 2002;164(3):292–294. doi: 10.1053/tvjl.2002.0709. [DOI] [PubMed] [Google Scholar]
- 55.Naganobu K, Hagio M. The effect of body position on the “hanging drop” method for identifying the extradural space in anaesthetized dogs. Veterinary Anaesthesia and Analgesia. 2007;34(1):59–62. doi: 10.1111/j.1467-2995.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 56.Nystrom EUM, Blomberg SG, Buffington CW. Transmural pressure of epidural veins in the thoracic and lumbar spine of pigs. Anesthesiology. 1998;89(2):449–555. doi: 10.1097/00000542-199808000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Wantman A, Hancox N, Howell PR. Techniques for identifying the epidural space: a survey of practice amongst anaesthetists in the UK. Anaesthesia. 2006;61(4):370–375. doi: 10.1111/j.1365-2044.2006.04534.x. [DOI] [PubMed] [Google Scholar]
- 58.Figueredo E, Blanque R, García Villalba FJ, de Andrés J. Identification of the epidural space: usual practice among Spanish anesthesiologists. Revista Española de Anestesiología y Reanimación. 2005;52(9):521–528. [PubMed] [Google Scholar]
- 59.Cluff R, Mehio A-K, Cohen SP, Chang Y, Sang CN, Stoianovic MP. The technical aspects of epidural steroid injections: a national survey. Anesthesia and Analgesia. 2002;95(2):403–408. doi: 10.1097/00000539-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 60.Stojanovic MP, Vu T-N, Caneris O, Slezak J, Cohen SP, Sang CN. The role of fluoroscopy in cervical epidural steroid injections: an analysis of contrast dispersal patterns. Spine. 2002;27(5):509–514. doi: 10.1097/00007632-200203010-00011. [DOI] [PubMed] [Google Scholar]
- 61.Johnson BA, Schellhas KP, Pollei SR. Epidurography and therapeutic epidural injections: technical considerations and experience with 5334 cases. American Journal of Neuroradiology. 1999;20(4):697–705. [PMC free article] [PubMed] [Google Scholar]
- 62.Manchikanti L, Malla Y, Cash KA, Mcmanus CD, Pampati V. Fluoroscopic cervical interlaminar epidural injections in managing chronic pain of cervical postsurgery syndrome: preliminary results of a randomized, double-blind, active control trial. Pain Physician. 2012;15(1):13–26. [PubMed] [Google Scholar]
- 63.Manchikanti L, Cash KA, Pampati V, Wargo BW, Malla Y. The effectiveness of fluoroscopic cervical interlaminar epidural injections in managing chronic cervical disc herniation and radiculitis: preliminary results of a randomized, double-blind, controlled trial. Pain Physician. 2010;13(3):223–236. [PubMed] [Google Scholar]
- 64.Manchikanti L, Cash KA, Pampati V. Management of chronic pain of cervical disc herniation and radiculitis with fluoroscopic cervical interlaminar epidural injections. International Journal of Medical Sciences. 2012;9:424–434. doi: 10.7150/ijms.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abram SE, Hogan QH. Avoiding catastrophic complications from epidural steroid injections. APSF Newsletter. 2011 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of the hanging drop technique. As the needle enters the epidural space the pressure at the tip decreases below atmospheric level. This results in aspiration of the fluid from the hub.


