Abstract
Depression in temporal lobe epilepsy (TLE) is common, is a strong predictor of subjective disability, and may have unique pathophysiological characteristics. Previous studies showed that reduced hippocampal volume is associated with significant depressive symptoms in TLE patients. We utilized regions of interest analysis of high-resolution brain MRI and a reliable and valid measure of depressive symptoms to evaluate 28 consecutive adult subjects with video/EEG confirmed TLE. Regions of interest were based on prior human and animal studies of mood and behavioral dysfunction. Forty-three percent of the entire group had significant symptoms of depression, defined by a Beck Depression Inventory (BDI) score of greater than 15. Total hippocampal volumes were significantly smaller in the group with BDI< 15, (p< 0.007). None of the subjects in the quartile with the smallest left hippocampal volume had a BDI score greater than 15, compared to 57% of the subjects in the upper three quartiles (p< 0.008). No other limbic brain structures that we assessed: amygdala, subcallosal gyrus, subgenual gyrus, gyrus rectus, or total cerebral volume, were associated with depressive symptoms. Adequate hippocampal integrity may be necessary to maintain depression symptoms in mesial temporal lobe epilepsy. This finding also supports the possibility of a unique mechanism for depression in mesial temporal lobe epilepsy, such as hyerexcitable neuronal influence on the limbic network.
Keywords: hippocampus, depression, temporal lobe epilepsy, quantitative MRI, Beck Depression Inventory
1. Introduction
The hippocampus was initially described as a core component of the “emotional circuit” by Papez in 1937 [1]. He proposed a network with the concept that hippocampal hyperexcitation may activate negative mood states. Although smaller hippocampal volumes in humans have been associated with depression in psychiatric patients without other neurological disorders, the pathophysiology underlying these observations is not known [2, 3]. In the temporal lobe epilepsy (TLE), which is the most common type of epilepsy and often associated with depression [4], there is a much greater range of structural disturbances in the mesial structures, including the hippocampus [5]. An association of hippocampal volume and symptoms of depression remains controversial. Although a decreased hippocampal volume is associated with mood disorders in some but not all studies of TLE [6,7], recent studies indicate that left hippocampal volume reduction is associated with depression [8,9]. The neuronal cell loss and synaptic reorganization observed in hippocampal sclerosis [10,11,12], which correlates with hippocampal volume on MRI [5], may provide an opportunity to study the association of structural variability with depression in a specific patholophysiological state. Many investigators described frequent spiking as a characteristic of the injured, epileptic hippocampus. These data support the concept of hippocampal sclerosis related to adjacent hyperexcitable neurons [10]. If hippocampal volume in temporal lobe epilepsy is associated with severity of depressive symptoms, a model of hyperexcitable hippocampal input into the limbic network in depression could be postulated. We evaluated the relationship of neuroimaging evidence of hippocampal injury with severity of depressive symptoms in consecutive patients with TLE.
2. Methods
2.1 Participants
Patients with recurrent seizures receiving optimized pharmacological therapy in the outpatient clinic at the Washington University Comprehensive Epilepsy Center were offered presurgical evaluation that included long-term video/EEG monitoring and high resolution brain MRI. A neuropsychologist assessed the patients' clinical mood state during presurgical evaluations, and we used the Beck Depression Inventory BDI for reliable and valid quantification of their depression symptoms. We enrolled 28 consecutive adults with confirmed TLE based on clinical semiology of their seizures, interictal EEG, and ictal video/EEG. All participants signed an informed consent document that was approved by the local Human Studies Committee.
We determined demographic variables for age, gender, and epilepsy-related factors (seizure type, localization of epileptogenic region, seizure frequency, age at first seizure, and epilepsy duration). Seizure classification was based on the definitions proposed by the International League Against Epilepsy (ILAE) [13]. Seizure type and localization were determined by long-term video/EEG monitoring. Seizure frequency was defined as the average number of complex partial seizures per month for the previous six months. We also looked at type or number of antiepileptic drugs the subjects were taking at the time of their assessment.
2.2 Beck Depression Inventory
We utilized the BDI [14,15], which is a questionnaire commonly used for evaluation of depressive symptoms in persons with epilepsy [16]. A prior multicenter study determined that a cut score of 15 was based on an optimized receiver-operating characteristic curve of data for major depression in epilepsy [17].
2.3 MR acquisition
MR imaging was performed using an epilepsy-specific protocol on 1.5 T Siemens machine (Siemens Medical Systems, Erlangen, Germany). The imaging included coronal T1 weighted magnetization-prepared rapid gradient echo (MPRAGE) sequences [repetition time (TR) 11.4 ms, echo time (TE) 4.4 ms, inversion time (TI) 300 ms, flip angle (FA) 8°, resolution 1x1x1.5, matrix 256x256x108]. All parameters (calibration, window setting, etc.) were carefully monitored and optimized for best contrast and brightness. Sequence details of the coronal T1 weighted MPRAGE images were acquired from a high resolution clinical test. Inter- and intra-scan motion correction and averaging were accomplished off-line. Patients with hippocampal atrophy and/or signal change and patients with normal MRI were included. Since patients with normal MRI by visual and volumetric analysis may have hippocampal sclerosis on pathological evaluation, we elected to determine the association of MRI volumetric quantification with severity of depression.
2.4 Image Processing and Regions of Interest Measurement Procedures
Image processing prior to the region of interest (ROI) analysis included several image registration steps described earlier [2]. Multiple regions of interest were drawn using the Analyze AVW software (version 6.0, Biomedical Imaging Resource, Mayo Foundation) [18]. Images were displayed and each ROI was manually outlined (HH and JS), in consultation with a neuroanatomist (JP) and guided by the previous reports. Manual outline of the regions of interest was blinded to clinical and BDI data. The volume estimation was performed using the Cavalieri's principle [19].
2.5 Anatomical definitions of Regions of Interest
2.5.1 Hippocampus
Anatomical boundaries were identified using prior definitions [2, 20]. In the coronal plane the tail of the hippocampus continues as the indusium griseum, a thin strip of grey matter overlying the surface of the corpus callosum. The most posterior slice for measurement was defined as the slice where the hippocampus first appeared adjacent to the trigone of the lateral ventricle. The grey matter with a boundary superiorly defined by the fornix-fimbria white matter junction, inferiorly by the parahippocampal gyrus white matter, medially by the subarachnoid spaces of the cisterns, and laterally by the lateral ventricle was included. The subiculum extending medially to the edge of the dentate gyrus was included in the measurement, but the alveus with the surrounding white matter, including the thin white matter border with amygdala, was excluded. Orthogonal views were consulted for all slices.
2.5.2 Amygdala
The amygdala was first visualized in a coronal plane. The anterior boundary of this structure was the first section in which the temporal stalk was connected to the white matter of the insula. Dorsally, the border was defined in the anterior sections by the entorhinal sulcus between the basal forebrain and temporal lobe, and posteriorly, in the sagittal section, by a horizontal plane to the posteroinferior edge of the temporal stem with the temporal horn of the lateral ventricle. Ventrally, visualized in the sagittal section, the amygdala was restricted by the ventral-anterior edge of the hippocampus. Posteriorly, viewed also in the sagittal section, the amygdala was defined by its border with the hippocampus. Medially, best seen in the coronal section, the amygdala was bounded by a subarachnoid space. Laterally, also in the coronal section, the amygdala was bounded by the surrounding white matter [21].
2.5.3 Subcallosal gyrus (BA 25)
Tracing began at the slice where an anterior commissure was visible in a coronal plane. The mesial gray matter area of the prefrontal cortex in all slices anterior to the anterior commissure and extending to the last slice in which the internal capsule appeared was included.
2.5.4 Subgenual gyrus (BA 24)
This structure began as the first mesial gyrus in a superior to inferior plane in the prefrontal cortex on the coronal section. The grey matter included was between the first slice in which the internal capsule was visible and ended with the most anterior extension of the corpus callosum.
2.5.5 Gyrus rectus (BA 11)
The gyrus rectus was delineated in the coronal plane. It was defined by boundaries including the olfactory sulcus (lateral boundary), the olfactory trigone (posterior boundary), and, on the medial side, the cortex inferior to the horizontal line connecting the deepest point of the olfactory sulcus and the nearest point in the midline [22,23]. Tracing started in the plane where the most anterior part of the olfactory sulcus was visualized. Moving posteriorly, sagittal and axial views helped to identify the olfactory trigone. Tracing stopped at the point where the olfactory trigone was no longer visible.
2.5.6 Total cerebral volume
Brain tissue of the cerebral hemispheres, including both grey and white matters, was considered as the total cerebral volume. The midbrain superior to the pons was also included in the analysis. The volume was determined using an auto tracing module of the software in the coronal and transverse planes, with manual adjustments as needed to accurately define the border of neocortex.
Statistical analysis
Volume of each ROI was analyzed as an absolute volume and as a ratio, i.e. volume of the ROI as a proportion of the each subject's whole brain volume. The adjustment for the whole brain volume was done in order to minimize potential interindividual variations in the brain size due to individual and demographic characteristics (e.g. gender, age). Kolmogorov-Smirnov test confirmed that the distribution of the data was normal; thus student's t test and Pearson correlation analyzes were used to determine group differences for clinical and ROI variables. Assuming a sigma of 25% (as observed for most structures), the analysis had a power of 0.8 at an alpha of 0.05 to determine as significant any difference of greater that 25%. We elected to not use a formal correction procedure for multiple tests performed, such as the Bonferroni, which is in line with others [23]. This would have made the results highly susceptible to type II errors. Alpha level ≤.05 (two-tailed) was used as significant. All data were analyzed using the SPSS (SPSS Inc., Chicago, IL) statistical package.
3. Results
Patients with TLE were divided into two groups, based on the clinical significance of their depressive symptoms. The group of patients (n= 15) with BDI score < 15, indicating no or minimal depression symptoms had a mean total BDI of 3.9±3.8. The group of patients (n= 13) with the BDI≥ 15 had a mean score of 25.7±12.4, as shown in Table 1.
Table 1.
Demographic and clinical variables for two groups of temporal lobe epilepsy patients: euthymic or mildly depressed (BDI total score < 15), and moderate or severely depressed (BDI total score ≥ 15), mean±SD.
| Variable | BDI < 15 | BDI ≥ 15 |
|---|---|---|
| N | 15 | 13 |
| Age | 41±11 | 37±10 |
| Gender (male/female, %) | 40/60 | 61.5/38.5 |
| Age of seizure onset | 17±14 | 16±15 |
| Epilepsy duration (y) | 24±17 | 21±15 |
| Seizure frequency (per month) | 8.4±15.8 | 6.8±4.3 |
| Seizure focus lateralization (left/right/multiple, %) | 46.7/40.0/13.3 | 23.1/53.8/23.1 |
|
| ||
| Total BDI score | 3.9±3.8 | 25.7±12.4 |
The patient group with the lower BDI scores had significantly smaller left (p< .004), and total hippocampal volumes (p< .007). The level of significance was similar for the hippocampal volumes normalized to the whole brain volume (p< .007 and p<.01, respectively), as shown in Table 2. No difference was found between the two groups in the volumes of other limbic structures tested, including the amygdala, gyrus rectus, subgenual gyrus and subcallosal gyrus.
Table 2. Comparison of the region of interest relative volumes in the temporal lobe epilepsy patients with the BDI< 15 and BDI≥ 15.
| ROI | BDI < 15, Mean±SD | BDI ≥ 15, Mean±SD | t | p value |
|---|---|---|---|---|
| Hippocampus/WB ratioa | ||||
| Left | 2.00±0.52 | 2.50±0.32 | -2.93 | 0.007 |
| Right | 2.10±0.40 | 2.22±0.28 | -1.39 | 0.17 |
| Total | 4.10±0.76 | 5.12±.1.09 | -2.78 | 0.01 |
| Amygdala/WB ratioa | ||||
| Left | 1.46±0.49 | 1.55±0.51 | -0.47 | 0.63 |
| Right | 1.57±0.49 | 1.53±0.55 | 0.28 | 0.77 |
| Total | 2.70±0.83 | 2.94±0.91 | -0.60 | 0.55 |
| Subgenual gyrus/WB ratioa | ||||
| Left | 0.53±0.13 | 0.55±0.17 | -0.31 | 0.75 |
| Right | 0.53±0.15 | 0.51±0.16 | 0.43 | 0.67 |
| Total | 1.07±0.26 | 1.07±0.33 | 0.07 | 0.94 |
| Subcallosal gyrus/WB ratioa | ||||
| Left | 0.43±0.13 | 0.49±0.12 | -1,10 | 0.27 |
| Right | 0.43±0.12 | 0.47±0.08 | -0.10 | 0.91 |
| Total | 0.88±0.26 | 0.94±0.18 | -0.67 | 0.50 |
| Gyrus rectus/WB ratioa | ||||
| Left | 1.90±0.74 | 1.60±0.59 | 1,15 | 0.26 |
| Right | 2.06±0.78 | 1.66±0.57 | 1.43 | 0.15 |
|
| ||||
| Total | 4.37±0.08 | 3.87±0.05 | 1.70 | 0.10 |
WB, whole brain
Ratio × 1000
Left hippocampi and total hippocampi volumes of all subjects (n= 28), irrespective of their BDI score, were further analyzed after grouping into quartiles. The quartile of TLE patients with the smallest left hippocampi had a mean total BDI score of 6.5±4.2. This score was significantly lower than in the patients with the left hippocampal volumes in the upper three quartiles (n= 21), with the mean BDI score of 16.5±15.3 (p< .01), presented in Figure 1. No subjects in the quartile with the smallest hippocampal volumes had a BDI score of >15, compared to 57.1% of the subjects in the upper three quartiles (p< .008). Since the t-test analysis did not show an association of volume with depression for the right hippocampus, we did not do quartile analysis. There was no difference in the mean total BDI score in the subjects (n= 14) with normal MRI report reading (15±12) compared to patients (n= 14) with MRI-defined hippocampal sclerosis (15±19). The BDI scores were not associated with lateralization of the epileptogenic region defined by the results of long-term video/EEG monitoring (p=.56). We found no association of depression when we compared subjects taking one or more antiepileptic drugs concomitantly.
Figure 1.
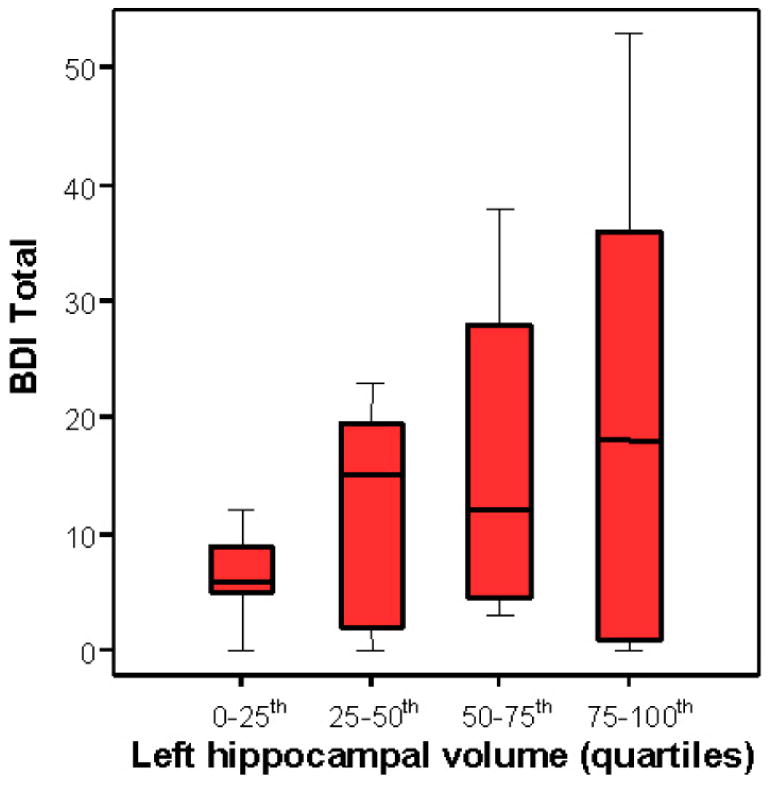
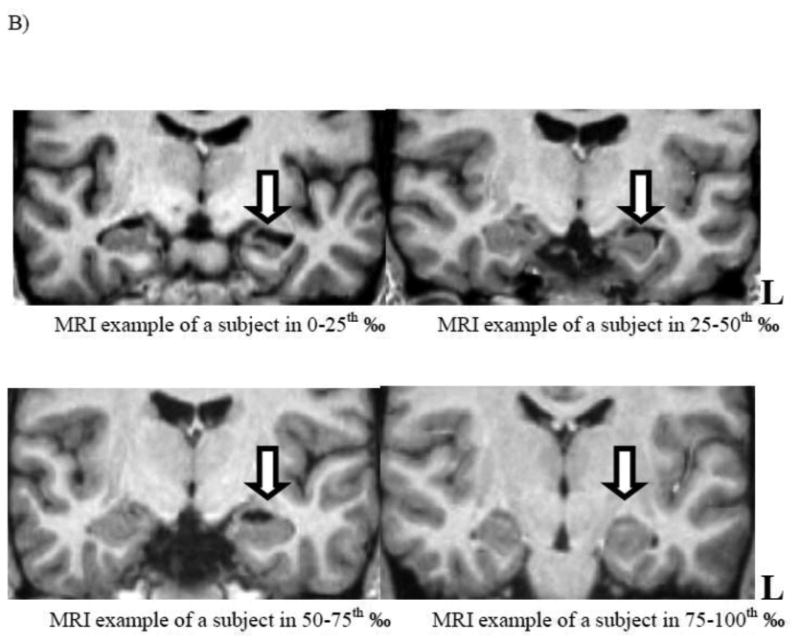
A) Boxplots with median lines and extremes for each quartile (n= 7) of the left hippocampal volume, in mm3, are compared with the total BDI score. B) Coronal sections of the representative MRI T1-weighted images show temporal structures and hippocampi for each quartile: the upper left and right, and lower left and right images show the left hippocampi in the lowest quartile (note the “ribbon” sign), second, third and the fourth quartile, respectively.
4. Discussion
In this study we found a high rate of significant depression symptoms, as previously reported [24] in other samples of patients with TLE, with 43% of our TLE patients scoring above 15 on the BDI. However, none of the subjects in the quartile with the smallest hippocampi had significant symptoms of depression. In contrast to the group with severely atrophic hippocampi, 57% of the subjects in the upper three quartiles had increased symptoms of depression (p< .008). We found no association between the epileptic region lateralization and severity of depressive symptoms.
Significant symptoms of depression are reported in 30-55% patients with medication refractory epilepsy, and possibly more prominent in TLE [17, 24], but the pathophysiology of the increased rate of depression symptoms is poorly understood. The most common pathological finding in TLE is mesial temporal sclerosis (MTS), and the severity of the sclerosis is highly variable. The combination of elevated rate of depression and high variability of hippocampal volume loss in TLE provides a unique opportunity to study the association of volume of the epileptic hippocampus with severity of depressive symptoms. Models of limbic system dysfunction in human depression [25] have consistently emphasized the role of the hippocampus, which could be predicted by Papez original description of the “circuit of emotion” that was defined by structures with known connections to the hippocampus [1]. A plausible hypothesis is that an adequately large aggregate of hyperexcitable hippocampal neurons is required to alter limbic network function and induce depressive symptoms. Hippocampal volumes in TLE have been directly correlated with the number of neurons present in the hippocampus [5]. Hippocampal atrophy and associated sclerosis in TLE is characterized by neuronal loss and gliosis in CA1 and CA3 regions of the hippocampus and the hilus of the dentate gyrus [11,12]. This pattern of neuronal injury is frequently associated with sprouting in the mossy fiber pathways, dispersion of granule cells in the dentate gyrus and aberrant neurogenesis [26]. Babb [10, 27] and others [28] suggested that cell reduction in the injured hippocampus initiates synaptic reorganization in adjacent epileptogenic neurons due to the existence of tonic hyperexcitable neuronal afferents. Using intrahippocampal electrodes during prolonged monitoring of patients with TLE, the group showed persistently increased frequency of spiking rates and seizures associated with a decrease in the hippocampal cell densities. These investigators hypothesized that neuronal cell loss in the injured hippocampus may contribute to the “feedforward” excitation of the neurons [27, 29]. Price [30] and others [28, 31] identified direct monosynaptic pathways from the hippocampus and subiculum to specific structures of the prefrontal cortex, such as Brodmann areas (BA) 11, 12, 13, 25 and 32 [30] This direct pathway is excitatory [32] and the terminal fields of these projections release glutamate as a neurotransmitter, with a direct excitatory effect on pyramidal cells of the prefrontal cortex. These neurons have mostly a-amino-3-hydroxy-5-methylisoxazole-4-priopionate (AMPA), and, to a lesser degree, N-methyl-D-aspartate (NMDA) receptors [32].
Based on these observations, we propose that the influence of chronic hippocampal hyperexcitability on the limbic network may play a key role in the modulation of depression in human TLE. Persistent hyperexcitability of the injured hippocampal neurons may activate prefrontal regions reported to influence depression symptoms [33]. Additional evidence supporting the association of hippocampal hyperexcitability and depression includes the observation that extent of hippocampal creatine/N-acetylaspartate ratio (Cr/NAA) abnormalities correlate with severity of depressive symptoms [34]. Other studies have associated electrophysiological evidence of the epileptogenic region with decreased NAA [35]. The combination of these observations indirectly supports that chronic hippocampal hyperexcitibility may alter limbic networks toward a depressed state.
Supplementary data are needed to better understand the role of the injured hippocampus in modulating expression of clinical depression in TLE. In our sample, the patients with very severe hippocampal atrophy reported no elevation of depressive symptoms. Kanner et al. [36] suggested that the absence of a psychiatric history, including depression, was an independent predictor of surgical outcomes. Recently, Adams et al. [37] studied a homogeneous group of 72 consecutive patients who underwent a temporal lobectomy for drug resistant TLE and had histopathologically proven MTS. A history of psychiatric disorder, in particular depression, was not associated with a poorer surgical outcome in patients with MTS. Others [38] also showed that depressive symptoms, identified by the BDI, do not seem to have a predictive value for postoperative seizure outcome in this highly selected patient population with MTS.
Several limitations of our study should be considered. Lack of a control group limits specific comments on the normalcy of the hippocampal volumes, but it is very likely that the lower three quartiles in our study have significant hippocampal dysfunction. Our conclusion that depression symptoms are not associated with severe volume loss, on the other hand, is not affected by the absence of control data. No formal psychiatric diagnosis was available on most patients, which limits statements regarding DSM-IV classification. However, the goals of our analysis were to evaluate the association of the severity of depressive symptoms with hippocampal volume. There may be relationships within the network that we could not identify with our approach, and acknowledge that a multivariate or neural network analysis could provide useful results. Other investigators found associations of depression with reduced hippocampal volumes or altered amygdala volumes; these differences with our results may be due to deferent approaches to volume measurment, investigator blinding, or other sample or methodologic differences. Observations from neurologically normal patients with major unipolar depression in the psychiatric literature describe smaller hippocampal volumes compared to nondepressed controls [2]. Smaller hippocampal volumes in these patients have been associated with early age of onset [39], number of previous episodes [40], or longer duration of untreated depression [41]. Severity of depression has not been clearly associated with hippocampal volumes. Some investigators have speculated that repeated stress during recurrent depressive episodes may result in cumulative hippocampal injury and volume loss [2], including neurotoxic effects of glucocorticoids, corticotrophin-releasing factor or decrease in trophic factors such as brain-derived neurotrophic factor [42]. Although potential mechanisms of hippocampal injury in depression have been studied, the pathophysiological relationship of hippocampal volumes and depression in the absence of a neurological disorder is not completely understood. Our observation suggests that a critical number of hyperexcitable hippocampal neurons may be required to maintain a state of clinical depression in TLE.
Highlights.
Depression is common in temporal lobe epilepsy
Region of interest MRI analysis was used to examine volumes of limbic structures
No subjects with the smallest hippocampal volume reported depressive symptoms
No other limbic brain structure volume was associated with depressive symptoms
Acknowledgments
We thank M.M. Rundle, J. Frye and K.W. Clark for help in collecting the neuroimaging data. This work was supported by the National Institutes of Health grants [NS40808 and NS047551 to F.G.] and the Epilepsy Foundation Fellowship to H.H.
Footnotes
Financial disclosures: The authors declare that they have no competing financial interests. The funding sources had no involvement in any aspect of study design, data collection, data analysis, data interpretation, writing the manuscript or decisions of publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. 1995;7:103–12. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- 2.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan RR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Gilliam F, Santos J, Vahle V, Carter J, Brown K, Hecimovic H. Depression in epilepsy: ignoring clinical expression of neuronal network dysfunction? Epilepsia. 2004;45(Suppl 2):28–33. 28–33. doi: 10.1111/j.0013-9580.2004.452005.x. [DOI] [PubMed] [Google Scholar]
- 5.Cascino GD, Jack CR, Jr, Parisi JE, Sharbrough FW, Hirschorn KA, Meyer FB, et al. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlations. Ann Neurol. 1991;30:31–6. doi: 10.1002/ana.410300107. [DOI] [PubMed] [Google Scholar]
- 6.Quiske A, Helmstaedter C, Lux S, Elger CE. Depression in patients with temporal lobe epilepsy is related to mesial temporal sclerosis. Epilepsy Res. 2000;39:121–5. doi: 10.1016/s0920-1211(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 7.Wrench JM, Wilson SJ, Bladin PF, Reutens DC. Hippocampal volume and depression: insights from epilepsy surgery. J Neurol Neurosurg Psychiatry. 2009;80:539–44. doi: 10.1136/jnnp.2008.152165. [DOI] [PubMed] [Google Scholar]
- 8.Baxendale SA, Thompson PJ, Duncan JS. Epilepsy and depression: the effects of comorbidity on hippocampal volume-a pilot study. Seizure. 2005;14:435–8. doi: 10.1016/j.seizure.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Shamim S, Hasler G, Liew C, Sato S, Theodore WH. Temporal lobe epilepsy, depression, and hippocampal volume. Epilepsia. 2009;50:1067–71. doi: 10.1111/j.1528-1167.2008.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babb TL, Lieb JP, Brown WJ, Pretorius J, Crandall PH. Distribution of pyramidal cell density and hyperexcitability in the epileptic human hippocampal formation. Epilepsia. 1984;25:721–8. doi: 10.1111/j.1528-1157.1984.tb03483.x. [DOI] [PubMed] [Google Scholar]
- 11.Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–50. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- 12.Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–21. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commission on Classification and Terminology of the International League Against Epilepsy, Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 14.Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. J Clin Psychol. 1984;40:1365–7. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Steer RA, Brown GK, Beck AT, Sanderson WC. Mean Beck Depression Inventory-II scores by severity of major depressive episode. Psychol Rep. 2001;88:1075–6. doi: 10.2466/pr0.2001.88.3c.1075. [DOI] [PubMed] [Google Scholar]
- 16.Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy Behav. 2003;4(Suppl 4):S26–30. S26–S30. doi: 10.1016/j.yebeh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Jones JE, Hermann BP, Woodard JL, Barry JJ, Gilliam F, Kanner AM, Meador KJ. Screening for major depression in epilepsy with common self-report depression inventories. Epilepsia. 2005;46:731–5. doi: 10.1111/j.1528-1167.2005.49704.x. [DOI] [PubMed] [Google Scholar]
- 18.Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433–54. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- 19.Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. Journal of neuroscience methods. 1990;35:115–24. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 20.Duvernoy HM. The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Berlin: Springer-Verlag; 1998. [Google Scholar]
- 21.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 23.Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior Cingulate, Gyrus Rectus, and Orbitofrontal Abnormalities in Elderly Depressed Patients: An MRI-Based Parcellation of the Prefrontal Cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 24.Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–98. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- 25.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 27.Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25:729–40. doi: 10.1111/j.1528-1157.1984.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 28.Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–46. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Babb TL, Pretorius JK, Mello LE, Mathern GW, Levesque MF. Synaptic reorganizations in epileptic human and rat kainate hippocampus may contribute to feedback and feedforward excitation. Epilepsy Res Suppl. 1992;9:193–202. 193–202. [PubMed] [Google Scholar]
- 30.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 31.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–86. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 32.Jay TM, Thierry AM, Wiklund L, Glowinski J. Excitatory Amino Acid Pathway from the Hippocampus to the Prefrontal Cortex. Contribution of AMPA Receptors in Hippocampo-prefrontal Cortex Transmission. Eur J Neurosci. 1992;4:1285–95. doi: 10.1111/j.1460-9568.1992.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 33.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Gilliam FG, Maton BM, Martin RC, Sawrie SM, Faught RE, Hugg JW, et al. Hippocampal 1H-MRSI correlates with severity of depression symptoms in temporal lobe epilepsy. Neurology. 2007;68:364–8. doi: 10.1212/01.wnl.0000252813.86812.81. [DOI] [PubMed] [Google Scholar]
- 35.Garcia PA, Laxer KD, van der GJ, Hugg JW, Matson GB, Weiner MW. Correlation of seizure frequency with N-acetyl-aspartate levels determined by 1H magnetic resonance spectroscopic imaging. Magn Reson Imaging. 1997;15:475–8. doi: 10.1016/s0730-725x(96)00327-x. [DOI] [PubMed] [Google Scholar]
- 36.Kanner AM, Byrne R, Chicharro A, Wuu J, Frey M. A lifetime psychiatric history predicts a worse seizure outcome following temporal lobectomy. Neurology. 2009;72:793–9. doi: 10.1212/01.wnl.0000343850.85763.9c. [DOI] [PubMed] [Google Scholar]
- 37.Adams SJ, Velakoulis D, Kaye AH, Corcoran NM, O'Brien TJ. Psychiatric history does not predict seizure outcome following temporal lobectomy for mesial temporal sclerosis. Epilepsia. 2012;53:1700–4. doi: 10.1111/j.1528-1167.2012.03569.x. [DOI] [PubMed] [Google Scholar]
- 38.Lackmayer K, Lehner-Baumgartner E, Pirker S, Czech T, Baumgartner C. Preoperative depressive symptoms are not predictors of postoperative seizure control in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Epilepsy and Behavior. 2013;26:81–6. doi: 10.1016/j.yebeh.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 39.MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC Med. 2004;2:2. doi: 10.1186/1741-7015-2-2. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 42.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. JNeurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


