Abstract
There is a clear and pressing need to expand pharmacotherapy options for substance use disorders (SUDs) in order to improve sustained abstinence outcomes. Preclinical literature has demonstrated the role of glutamate in addiction, suggesting that new targets for pharmacotherapy should focus on the restoration of glutamatergic function. Glutamatergic agents for SUDs may span multiple addictive behaviors and help demonstrate potentially overlapping mechanisms in addiction. The current review will focus specifically on N-acetylcysteine (NAC), a safe and well-tolerated glutamatergic agent, as a promising potential pharmacotherapy for the treatment of SUDs across several substances of abuse. Building on recently published reviews of the clinical efficacy of NAC across a broad range of conditions, this review will more specifically discuss NAC as a pharmacotherapy for SUDs, devoting particular attention to the safety and tolerability profile of NAC, the wealth of preclinical evidence that has demonstrated the role of glutamate dysregulation in addiction, and the limited but growing clinical literature that has assessed the efficacy of NAC across multiple substances of abuse. Preliminary clinical studies show the promise of NAC in terms of safety, tolerability, and potential efficacy for promoting abstinence from cocaine, nicotine, and cannabis. Results from randomized clinical trials have been mixed, but several mechanistic and methodological factors are discussed to refine the use of NAC in promoting abstinence and relapse prevention across several substances of abuse. Further preclinical and clinical investigation into the use of NAC for SUDs will be vital in addressing current deficits in the treatment of SUDs.
1 Novel Pharmacotherapies for Substance Use Disorders (SUDs)
Substance use disorders (SUDs) represent a significant and pressing public health concern. Estimated costs in the form of healthcare expenditures, drug-related crime, and lost productivity total approximately $621 billion per year when considering both illicit and licit (i.e. tobacco and alcohol) substances in the US (illicit drugs and tobacco) and globally (alcohol) [1-3]. In 2011, approximately 20.6 million Americans aged 12 years or older reported substance dependence or abuse of illicit drugs and/or alcohol, while 68.2 million Americans aged 12 years or older reported use of a tobacco product in the past month [4]. Additional estimates indicate that 43.8 million (19.0 %) adults (≥18 years of age) in the US were current cigarette smokers [5]. Despite advancements in the treatment of SUDs, many individuals fail to access treatment resources, and even fewer receive treatment that results in sustained, long-term abstinence. Treatment efforts for SUDs require significant refinement in order to maximize the chances of successful recovery from these chronic and relapsing disorders.
As the complex and multifaceted mechanisms contributing to SUDs have more closely aligned with a medical model of disease [6], first-line pharmacotherapeutic treatment interventions have been explored extensively in research and clinical practice. In the search for effective pharmacotherapeutic medications to treat SUDs, a number of promising agents have been identified throughout the years. For example, in the mid-1960s agonist replacement therapies for opioid addiction (i.e. methadone maintenance) were demonstrated to be effective in improving treatment outcomes [7], thus representing a pivotal moment in the treatment of SUDs. Agonist replacement therapies for opioid addiction, such as methadone and buprenorphine, continue to be widely accepted and enduring evidence-based pharmacotherapies for opioid-dependent patients [8]. Replacement pharmacotherapy has also been a key strategy in treatment guidelines for nicotine dependence [9]. A major advantage to replacement pharmacotherapies is that they are substance-specific, but this may also prove to be a weakness when considering the potential need to apply pharmacotherapy across multiple substances of abuse.
Many first-line pharmacotherapies improve the likelihood of sustained abstinence, but vary greatly in treatment outcomes, and many still suffer from generally poor outcomes. Several first- and second-line pharmacotherapeutic options exist for substance-specific SUDs and pathological gambling, and are discussed at length in a recent review by van den Brink [10]. Within the smoking cessation literature, nicotine replacement therapy (NRT), bupropion, and varenicline have been shown to increase the odds of quitting smoking by two to threefold compared with placebo [11]. However, even with varenicline, arguably the most efficacious smoking cessation pharmacotherapy, abstinence rates after 12 weeks of treatment are still only approximately 40 % [12-14]. Modest effect sizes have been demonstrated for pharmacotherapies for alcohol dependence that function to reduce the reinforcing effects of alcohol and reduce craving, such as naltrexone and acamprosate [15, 16]. Several pharmacotherapies for cannabis dependence have been explored with mixed results, and few positive controlled clinical trials exist to guide clinical practice [17, 18]. Finally, there are currently no US FDA-approved medications for cocaine and other stimulant dependence, although some have suggested that agonist replacement approaches may be a fruitful avenue to explore [19]. Unfortunately, the abuse potential of stimulant pharmacotherapy has dampened enthusiasm for agonist replacement therapies for stimulant dependence. Even in the case that highly effective pharmacotherapies are developed for specific substances of abuse, this does not address the issue that many individuals report using multiple substances of abuse [4], and some may use multiple substances concurrently. Use of other substances (particularly licit substances) during a treatment episode may persist and even increase during the course of medication-assisted treatment.
There is a need to improve and expand pharmacotherapy options for SUDs. Extensive preclinical literature exists highlighting the role of glutamate in addiction. More specifically, chronic drug exposure in non-human animal models leads to enduring alterations in glutamatergic signaling and the downregulation of the cystine-glutamate exchanger [20, 21]. Glutamatergic agents may restore that functioning, thus providing ample justification to target glutamatergic signaling in the treatment of SUDs in clinical populations. New targets for pharmacotherapy that span multiple addictive behaviors would be beneficial and may help to demonstrate potentially overlapping mechanisms in SUDs. The current review will focus specifically on N-acetylcysteine (NAC) as a promising potential pharmacotherapy for the treatment of SUDs across several substances of abuse. NAC is an N-acetyl prodrug that acts primarily on the glutamatergic system. Preclinical studies have demonstrated the efficacy of NAC in restoring normal glutamate signaling and reversing addiction pathology. The clinical literature focusing on NAC for SUDs is relatively limited, but preliminary work to date has shown the promise of NAC in terms of safety, tolerability, and potential efficacy for promoting abstinence from cocaine, nicotine, and cannabis. Building on several excellent reviews that have been published recently on the utility of NAC in the treatment of a range of conditions [22-24], this review will more specifically discuss NAC as a pharmacotherapy for SUDs, devoting particular attention to the safety and tolerability profile of NAC, the preclinical evidence that has demonstrated the role of glutamate dysregulation in addiction, and the limited but growing clinical literature that has assessed the efficacy of NAC across multiple substances of abuse. We conducted an extensive PubMed literature search through December 2013. The following keywords were used in our search: ‘N-acetylcysteine’, ‘substance use disorders’, ‘addiction’, ‘pharmacotherapy’, ‘glutamate’, ‘translational’, ‘relapse’, and ‘abstinence’. We also explored references cited in relevant articles and review papers.
2 Overview of N-Acetylcysteine (NAC)
NAC is an N-acetyl prodrug of the naturally occurring amino acid cysteine. The chemical structure of NAC is shown in Fig. 1. NAC is available by prescription in intravenous, oral, and nebulizer forms. It is also sold orally as an over-the-counter product in health food stores. Oral bioavailability of NAC has been shown to range from 4–10 % [25, 26]. NAC has a long-established safety record in adults and children, with FDA approval since 1963. NAC has clinical efficacy as a mucolytic agent for bronchopulmonary disorders [27] and in the treatment of chronic obstructive pulmonary disease (COPD) [28]. It is also used as an oral or intravenous antidote to treat acetaminophen poisoning, acting as a precursor for the synthesis of the antioxidant glutathione (GSH), and forming complexes with toxic reactive oxidative metabolites of acetaminophen, ultimately preventing hepatic cell necrosis [29]. A meta-analysis of studies evaluating long-term oral treatment with NAC for the prevention of chronic bronchitis found that NAC was well-tolerated. Adverse events reported in those trials were generally mild and most commonly included gastrointestinal events that did not require medication termination [27]. Local irritation has also been reported when NAC is administered as a mucolytic [30]. Systemic allergic reactions to NAC have been observed, but only with intravenous administration [31, 32], and seizures have been reported at very high intravenous doses [33]. NAC may be appropriate among varying age groups since it has been used safely for several decades in adults and children, often at doses greatly exceeding those typically used in research studies [34, 35].
Fig. 1.
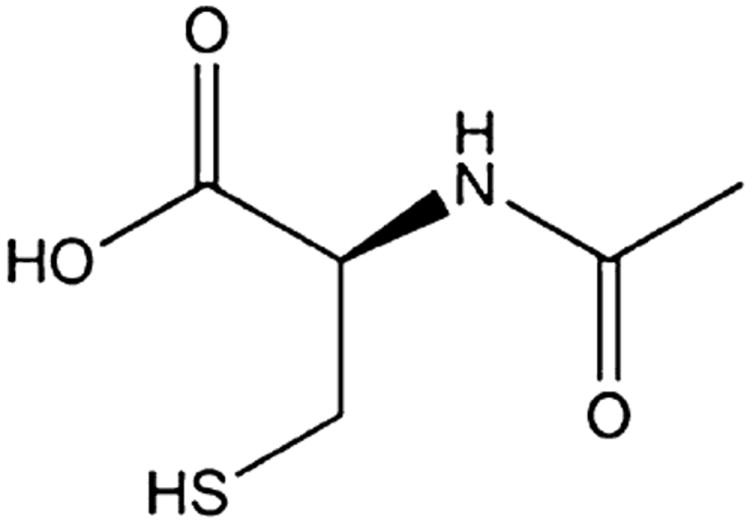
Molecular structure of N-acetylcysteine
Research interest in NAC has grown amid increasing evidence of its clinical efficacy in the treatment of several psychiatric disorders. A recent review by Berk and colleagues [22] summarized the literature on the uses of NAC in neuropsychiatry, specifically in the treatment of SUDs, pathological gambling, obsessive-compulsive disorder (OCD) and other compulsive disorders, schizophrenia, unipolar depression, bipolar disorder, and autism, as well as neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases. That review noted the promising efficacy of NAC as a pharmacotherapy among these diverse disorders; however, it was also noted that many trials with NAC were preliminary and further study through controlled efficacy trials is required. Additionally, NAC has been studied for the treatment of psychiatric disorders based on its antioxidant properties. It has been suggested that oxidative stress may function as a mechanism underlying psychiatric disorders, including bipolar and anxiety disorders, depression, and SUDs, in addition to other diagnoses such as autism and attention-deficit hyperactivity disorder [36]. GSH is depleted during oxidative stress, which can be reversed with NAC treatment [37], and providing evidence to pursue GSH as a novel therapeutic target for psychiatric disorders [38]. Furthermore, in rodent models NAC has been shown to reduce myocardial oxidative stress due to chronic ethanol administration [39, 40].
Specific to the treatment of SUDs, there is increasing evidence to support the role of glutamatergic signaling in addiction [20, 21, 41-43], which will be discussed in greater detail in this review. NAC may be a particularly attractive candidate pharmacotherapy for SUDs given its favorable safety profile and the preclinical evidence of its reversal of (i) glutamatergic dysregulation and (ii) GSH depletion and oxidative stress. In the following sections, the role of glutamate in addiction will be discussed, as well as the preclinical literature assessing the influence of NAC on neural plasticity, restoring cystine-glutamate exchange, drug self-administration, and reinstatement of drug seeking in an animal model.
3 Dysregulation of Glutamatergic Signaling in Addiction
Animal models mimicking aspects of human addiction allow deep exploration of the neurobiological changes induced with prolonged administration of substances of abuse. In these models, neurobiological alterations have been examined following various timepoints of withdrawal from a substance of abuse. Following periods of withdrawal (i.e. 1–14 days, up to 3 months) from self-administration of a substance of abuse such as cocaine, heroin, or nicotine, dysregulation of glutamatergic signaling has been found within a corticostriatal circuit [prefrontal cortex (PFC)–nucleus accumbens (NA)] [41, 44-46]. Extensive evidence indicates that chronic exposure to addictive drugs decreases the expression of the excitatory amino-acid transporter-2 (EAAT2), also known as glial glutamate transporter-1 (GLT1), in the NA [44, 47-49]. This decrease in turn reduces glutamate elimination from the extracellular space and contributes to the spillover of synaptically released glutamate during reinstated drug seeking [50-52]. Activity in this impaired state is represented in Fig. 2a. After withdrawal from chronic administration of cocaine, reduced basal extracellular glutamate occurs in conjunction with a decrease in expression and function of both glial proteins, including the catalytic subunit of the cystine-glutamate exchanger system (Xc− system; xCT, glial catalytic subunit) and GLT1 in the NA (note that nicotine and cocaine reduce system Xc−, while all addictive drugs tested to date elicit enduring reductions in GLT1) [44, 50-52]. This results in decreased tone on presynaptic inhibitory metabotropic glutamate 2/3 (mGluR2/3) receptors. Basal tone on these receptors provides a means of feedback inhibition and control of synaptic transmission. However, in the absence of this inhibitory tone, excessive glutamatergic transmission occurs [53]. The excessive release of extracellular glutamate during reinstated drug seeking arises from the decrease in GLT1 and stimulates extrasynaptic N-methyl-d-aspartate (NMDA) receptors and metabotropic glutamate receptor 5 [43, 54]. This sequence of events leads to a rapid, transient increase in synaptic plasticity rendering increased relapse vulnerability [55-57]. Indeed, GLT1 is strategically expressed near the synaptic cleft to minimize glutamate spillover into the extrasynaptic space [58]. Presentation of a drug or a cue associated with drug taking reinstates drug seeking in a preclinical model of relapse and this behavior is associated with elevated extracellular glutamate derived from synaptic activity in the PFC-NA pathway [44, 45, 59].
Fig. 2.
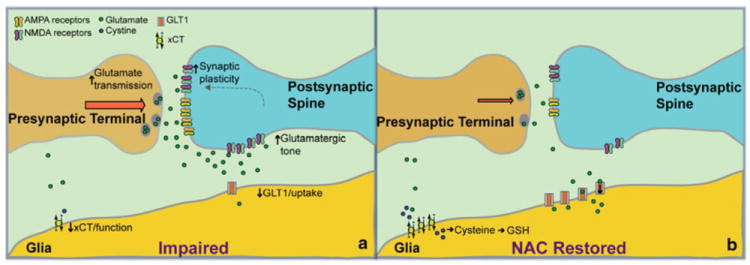
a Representation of a synapse in the NAcore with glutamatergic input from the PFC during an impaired state caused by withdrawal from drugs of abuse such as cocaine, nicotine, and heroin. b Representation of the same neural areas and glutamate restoration due to the administration of NAC. Following chronic NAC administration, upregulation of both the cysteine-glutamate xCT and the GLT1 occurs, restoring uptake of glutamate from the extracellular space. This leads to less tone on extrasynaptic NMDA receptors, and possibly inhibits postsynaptic relapse-induced synaptic plasticity. AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, GLT1 glial glutamate transporter-1, GSH glutathione, NA nucleus accumbens, NAC N-acetylcysteine, NMDA N-methyl-d-aspartate, PFC prefrontal cortex, xCT exchanger catalytic subunit
In addition to the glutamate overflow that occurs during a withdrawal state after chronic drug administration, recent evidence shows that reinstatement of drug seeking prompted by drug cues (i.e. cue-induced reinstatement) in cocaine- and nicotine-extinguished rats is associated with a rapid, transient long-term potentiation (LTP)-like plasticity in the NAcore [44, 56]. The LTP-like changes observed in these studies were quantified as increases in dendritic spine head diameter (dh) and in the ratio of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor to NMDA-mediated excitatory postsynaptic currents (EPSCs; AMPA/NMDA) [60-62]. Importantly, both dh and AMPA/NMDA were significantly correlated with reinstated cocaine seeking. Since presynaptic and non-synaptic glutamate release regulates synaptic plasticity in both primary cell cultures and in vivo [63, 64], the LTP-like changes may arise from the reinstatement-associated increase in glutamate release and overflow [44, 45, 65]. Accordingly, the LTP-like plasticity was prevented by inhibiting PFC glutamatergic input to the NAcore [66]. Within the corticostriatal circuit, the NAcore is thought to serve as a ‘gateway’ through which information concerning behavioral output is processed from the limbic cortex to motor subcircuits [67, 68]. Throughout the addiction cycle, it is thought that the switch to compulsive drug seeking from controlled intake (i.e. recreational use) arises from an impaired ability of this circuit to successfully process negative environmental contingencies, leading to decreased capacity to inhibit prepotent drug-associated responses [41, 69]. This process thereby leads to heightened vulnerability to relapse. Although the NAcore is heavily implicated in relapse vulnerability, other striatal regions have been implicated in the extinction of drug seeking. Specifically, input to the shell subcompartment of the NA from the infralimbic cortex [70], and similar neuro-biological alterations, have been found in the NA shell as well as the core [47, 71]. Given the extensive disruption of glutamatergic input from the PFC to the NAcore, NAC may serve to restore this dysregulation. The following sections expand on the preclinical research on NAC that suggests its role in reversing addiction pathology.
4 Preclinical Literature Review
4.1 NAC to Restore Cystine-Glutamate Exchange
NAC is a cysteine prodrug with antioxidant properties via activation of the Xc− system [72, 73]. NAC enters cells after it is deacetylated to cysteine and rapidly oxidized to cystine, primarily through cysteine transporters. Once transported by Xc− into cells, cystine is reduced to cysteine for synthesis of GSH [72] (see Fig. 2b). High concentrations of NAC in tissue slices lead to glutamate release from xCT that potentiates EPSCs in NA medium spiny cells [74].
Following withdrawal from cocaine and nicotine, the xCT subunit of the Xc− system is reduced both in function and content [47, 48]. NAC activates the glial system Xc− to exchange extracellular cystine for intracellular glutamate in a 1:1 stoichiometry, thereby elevating non-synaptic glutamate and restoring levels of both xCT and GLT1 [59]. This increase in non-synaptic glutamate counters the reduced extrasynaptic levels after withdrawal from a drug of abuse [75]. The restoration of glutamate then activates the presynaptic mGluR2/3, which then reduces synaptic glutamate release and drug seeking [76]. Visual representation of the system restored through NAC treatment is shown in Fig. 2b. In contrast to previous work on the effects of NAC on xCT, recent work has found an important action of NAC on GLT1 in the reduction of drug seeking. Restoration of GLT1, but not xCT, was found critical for chronic NAC to inhibit cue-induced cocaine seeking [Reissner et al., unpublished], although the mechanism by which NAC restores GLT1 is currently unknown. It is possible that restoration of GLT1 after treatment with NAC will inhibit glutamate spillover into the extrasynaptic space via increased glutamate uptake [77], thus inhibiting activation of the primarily extrasynaptic NMDA receptor subunit, GluN2B [78]. This in turn may inhibit the rapid synaptic plasticity that has been demonstrated during drug seeking. The reduction in activation of GluN2B NMDA receptors could influence insertion of calcium-permeable AMPA subunit receptors (GluA1), which are increased during incubation of cocaine craving [79]. The reduction in GluA1-containing AMPA receptors could inhibit the rapid increase in dh shown to be linked to increased relapse vulnerability [55, 56]. Although not yet shown, it would also be of interest to examine the effect of NAC on reducing the basal potentiated resting state of NAcore medium spiny neurons (MSNs) after withdrawal from chronic drug self-administration [63].
4.2 NAC to Inhibit Drug Seeking
Systemic administration of NAC has been shown to robustly decrease reinstatement of cocaine, heroin, and nicotine seeking in preclinical models [44, 45, 63, 80-83]. This reduction in relapse vulnerability has been attributed to the ability of chronic NAC treatment to reverse drug-induced metaplasticity and may serve as a mechanism to chronically restore prefrontal to accumbens glutamate transmission, which serves to provide enduring relapse protection [63, 84]. In support of this, daily NAC treatment given prior to cocaine self-administration sessions has been shown to prevent escalation of cocaine intake, behavioral sensitization, and cocaine-induced reinstatement of cocaine seeking [85]. It has also been shown to reduce cocaine-induced cocaine seeking in rodents [86]. In animals being withdrawn from chronic heroin administration, chronic NAC administration was shown to decrease extinction responding and inhibit cue-induced heroin seeking up to 40 days after discontinuing NAC treatment [82]. Acute administration of NAC has been shown to be effective at decreasing nicotine self-administration and nicotine seeking that is prompted by a previously paired cue [83]. Finally, NAC has been shown to reduce alcohol-induced oxidative stress in rats [39, 40], and therefore may be of interest in the examination of NAC in the treatment of alcohol abuse and dependence. This exploration would also be supported by the literature, as increased glutamate levels have been found in the NAcore during cue-induced alcohol seeking [87].
5 Clinical Literature Review
5.1 Safety/Tolerability in SUD Populations
The wealth of preclinical data demonstrating the role of glutamate in addiction, and the overall safety and tolerability of NAC among adults and children when administered orally, has contributed to the preliminary investigation of NAC as a pharmacotherapy for SUDs, specifically in cocaine-dependent adults. To assess the safety and tolerability of oral NAC in SUD populations, LaRowe and colleagues [88] conducted an inpatient, laboratory-based, placebo-controlled, crossover study. This study involved two separate inpatient stays in which non-treatment-seeking, cocaine-dependent adults (N = 13) received NAC 600 mg or placebo every 12 h for 3 days. Two hours after each dose administration, vital signs and adverse events related to the medication were assessed. No differences were found in the number of participants reporting adverse events in the placebo and NAC conditions. Adverse events reported in this study included pruritus (itching), headache, flatulence/diarrhea, abdominal cramps, local rash, fatigue, increased blood pressure, sweating, ears popping, increased appetite, canker sores, chest pain, crying, and dizziness. None were serious and study results demonstrated that NAC was safe and well-tolerated among study participants.
In an outpatient context, two open-label studies assessed safety and tolerability among SUD populations. Several doses of NAC were assessed in an open-label, between-subjects study that recruited treatment-seeking cocaine-dependent adults (N = 23) [89]. Total daily doses of NAC were 1,200 mg (600 mg twice daily), 2,400 mg (1,200 mg twice daily), and 3,600 mg (1,200 mg thrice daily) during a 4-week treatment period with two clinic visits per week. All doses were shown to be safe and well-tolerated among this outpatient population, with the most common adverse events experienced being pruritus, headache, and elevated blood pressure. Retention rates through the 4-week study were higher among the 2,400 mg and 3,600 mg dosing groups, and self-reported cocaine use (biologically confirmed through a urine drug screen) was reduced among participants who were retained in the study. Another open-label investigation of NAC was conducted with cannabis-dependent young adults (N = 24; aged 18–21 years) [90] over a 4-week treatment period with a dose of 2,400 mg/day (1,200 mg twice daily). Similar to previous studies, results showed that NAC was safe and well-tolerated. Adverse events (from most common to least common) included abdominal discomfort, muscle pains/aches, insomnia, headache, nasal congestion, nausea, weight decrease, restlessness, and dizziness. Additionally, reductions in self-reported cannabis craving and use over the 4-week trial were reported among study participants.
These aforementioned studies not only provided much needed safety and tolerability data for NAC among SUD populations, both during periods of active use and abstinence, but also provided preliminary efficacy data to support further investigation. Additional studies have assessed the neural mechanism of NAC in humans through imaging techniques, effects on craving, withdrawal, and drug reward, and, finally, the potential efficacy of NAC in promoting abstinence.
5.2 Neural Mechanism
Only one study thus far has used imaging techniques in humans to assess the neural mechanisms affected by NAC administration. Schmaal and colleagues [75] recruited cocaine-dependent adult males (N = 10) and healthy controls (N = 14) to assess glutamate activity in the dorsal anterior cingulate cortex (dACC) using proton magnetic resonance spectroscopy. This was an open-label, randomized, crossover study in which participants received either a single 2,400 mg dose of NAC or no compound. Each scan was separated by 1–2 weeks. This study showed reductions in glutamate activity (quantified individually, in addition to glutatmate+glutamine) in the left dACC due to NAC administration, but only in cocaine-dependent patients (not in healthy controls). This demonstrated normalization of cortical glutamate levels in cocaine-dependent humans is generally consistent with preclinical work showing excessive glutamatergic signaling after chronic cocaine self-administration. Thus, NAC may work to restore glutamate homeostasis in cocaine-dependent humans.
5.3 Craving/Withdrawal/Reward
Several studies have explored the influence of NAC on craving, withdrawal, and the rewarding properties of substances of abuse to determine if these processes, thought to contribute to relapse, are modified under NAC administration. As part of the same inpatient trial that assessed the safety and tolerability of NAC [88], another study was published that assessed the effects of NAC on cue-induced cocaine craving [91]. Two additional participants were included within that data set (N = 15). Cue reactivity assessments included the presentation of slides depicting cocaine, neutral, pleasant, and unpleasant images. Physiological measures were taken (i.e. heart rate and skin conductance) and, following slide presentations, participants were asked about their craving for cocaine, desire to use, and interest in using. Results showed that with NAC, compared with placebo, participants self-reported less desire to use cocaine and less interest when presented with cocaine-related slides. However, no medication effects were found for ratings of craving.
A translational study was conducted that utilized preclinical and clinical designs to assess the influence of repeated administration of NAC on cocaine seeking (rodents) and craving (humans) [86]. The clinical component assessed cocaine craving and rewarding properties following intravenous cocaine administration through an inpatient, laboratory-based, crossover study design with doses of NAC [1,200 mg/day (400 mg thrice daily) or 2,400 mg/day (800 mg thrice daily)] or baclofen for 3 days. Participants were cocaine-dependent adult males (N = 4). This study found that NAC, compared with baclofen, reduced cocaine craving following an intravenous cocaine injection, but did not alter the reinforcing properties of cocaine. No differences between the 1,200 mg and 2,400 mg NAC doses were found.
Finally, an outpatient, laboratory-based, placebo-controlled study recruited nicotine-dependent young adults (N = 22) [92]. Participants were randomized to receive 3,600 mg/day of NAC (1,800 mg twice daily) or placebo for 3.5 days. Abstinence from smoking was confirmed using breath carbon monoxide (CO) each morning of medication administration, and craving and nicotine withdrawal symptoms were assessed. A laboratory session was conducted on day 4 of the study, in which participants were asked to smoke one cigarette and report on the subjective rewarding effects of that cigarette. Results showed that NAC had no effect on cigarette craving, and only a trend towards reduced withdrawal with NAC. With NAC administration, compared with placebo, the first cigarette smoked after 3.5 days of abstinence was rated as less rewarding. Only mild stomach problems were reported as adverse events in this study in both the placebo and NAC groups.
5.4 Efficacy
All studies discussed above provided sufficient preliminary data to conduct pilot, as well as larger, fully-powered clinical trials to determine the potential efficacy of NAC across several substances of abuse. A pilot randomized controlled trial (RCT) was conducted as part of a translational study to assess glutamate disruption caused by nicotine dependence, and to determine if control could be restored through cystine-glutamate exchange [47]. Specific to the RCT component of this study, nicotine-dependent adult smokers (N = 29) were recruited and were randomized to 2,400 mg/day (1,200 mg twice daily) of NAC or matched placebo for 4 weeks. This study showed a reduction in self-reported cigarettes per day across the treatment period in the NAC group, but only when two participants were excluded due to alcohol and cigarette consumption that was greater than two standard deviations above the mean. However, there was no effect of NAC on CO levels, craving, or withdrawal ratings.
Previous to the exploration of NAC as a pharmacotherapy for SUDs, work was conducted with NAC in smokers, but mainly due to its oxidative and protective properties [93]. Healthy adult smokers (N = 41) were recruited and were randomized to 1,200 mg/day (600 mg twice daily) of NAC versus placebo for 6 months. At the 6-month timepoint, results showed that NAC had reduced tobacco smoke carcinogenicity, modulated cancer-associated biomarkers, and decreased oxidative damage in a generally positive manner. However, NAC did not appear to reduce smoking (verified by cotinine levels).
Another pilot RCT recruited non-treatment seeking methamphetamine-dependent adults (N = 31) to test the efficacy of NAC + naltrexone versis placebo on methamphetamine craving and use [94]. Doses of NAC were steadily increased throughout the study (i.e. 600, 1,200, 1,800, and 2,400 mg/day) in the absence of significant clinical improvement (remained at the current dose if improvements were seen). The intervention phase lasted for 8 weeks. There was no reduction in methamphetamine craving or use throughout the 8-week treatment period, but there was greater dropout from study procedures among the placebo group. Adverse events included nausea and lethargy and did not differ across experimental groups.
Finally, two fully-powered RCTs testing NAC for SUDs have been completed. Gray and colleagues [95] conducted an 8-week, placebo-controlled RCT with cannabis-dependent adolescents (N = 116; aged 15–21 years) and tested the efficacy of NAC (2,400 mg/day; 1,200 mg twice daily) versus placebo, added to contingency management and brief cessation counseling, in achieving cannabis abstinence. In this trial, NAC doubled the odds of having negative urine cannabinoid tests during treatment. The medication was also well-tolerated and no differences were found in the occurrence of adverse events between treatment groups. Adverse events included upper respiratory infection, vivid dreams, insomnia, irritability, and heartburn. Interestingly, although NAC was efficacious in promoting cannabis abstinence, a secondary analysis from this data set found no differential reductions in cannabis craving between the NAC and placebo groups, although craving did decrease over the course of the trial, regardless of treatment group [96]. Therefore, craving seemed to decrease as a function of some undefined variable, but was not attributable to NAC. The second clinical trial recruited treatment-seeking cocaine-dependent adults (N = 111) [97] and tested the efficacy of NAC in promoting cocaine abstinence. Participants were randomized to receive 1,200 or 2,400 mg/day (600 or 1,200 mg twice daily, respectively) of NAC or placebo, added to cognitive-behavioral therapy, during an 8-week treatment phase. Treatment with NAC was not found to reduce cocaine use or craving. Exploratory analyses found that participants abstinent from cocaine at the beginning of the trial and randomized to the 2,400 mg dose of NAC had longer time to relapse and lower ratings of craving. Tolerability of study medication was favorable, with no differences in adverse events between groups. The most commonly reported adverse event was gastrointestinal problems (i.e. heartburn, flatulence, abdominal cramps). This study further supported the safety of NAC, and suggested unique conditions (i.e. abstinence) under which NAC may be most effective.
6 Discussion
The trajectory of research on NAC as a pharmacotherapy for SUDs has been well-informed through the preclinical investigation of mechanism. The role of glutamate dysregulation, the target of NAC treatment, has been proposed as a ubiquitous finding and underlying feature across SUDs [20, 21, 98]. This lends strong support to the strategy of employing glutamatergic agents in the treatment of SUDs across multiple substances of abuse. NAC has been shown to be a safe and well-tolerated medication [27] with a long history of use for several conditions, making it a promising candidate for use in the treatment of SUDs. Despite preclinical evidence to suggest the efficacy of NAC, and early phase I and II clinical studies demonstrating safety, tolerability, and potential efficacy, not all pilot trials and fully-powered RCTs have yielded positive results. Of the three pilot or smaller-scale RCTs reviewed above [47, 93, 94], two of the three failed to demonstrate robust reductions in the substance of abuse being targeted. The study conducted by van Schooten and colleagues [93] was unique given the emphasis on measuring oxidative stress due to cigarette smoking, rather than targeting smoking itself. Of the two fully-powered clinical trials discussed above, only one showed positive results in the form of increased likelihood of abstinence from cannabis [95]. Given the promising preclinical and preliminary clinical work on NAC for SUDs, the results of these RCTs are curious, although several alternatives have been suggested to refine the use of NAC in treating SUDs and improve its efficacy.
6.1 Improving the Efficacy of NAC
The majority of the preclinical work conducted with NAC has used a model of reinstatement of drug seeking meant to mimic relapse vulnerability, and many of the most robust findings with NAC have been in the context of a reinstatement model to predict relapse. This suggests that NAC may be most effective under conditions of abstinence, rather than to promote initial cessation. This possibility was discussed by LaRowe and colleagues [97] to account for the finding that participants who were abstinent from cocaine at study entry had better outcomes in the NAC condition compared with placebo. This is an important consideration for efficacy trials. NAC may prove to be an ideal relapse prevention aid when given after periods of abstinence or when combined with other forms of pharmacological and/or behavioral treatments to promote abstinence. It may be that the positive findings in the cannabis cessation trial by Gray and colleagues were a function of the synergy of NAC with contingency management, an established abstinence-targeted behavioral treatment platform [95]. In the cases of forced abstinence from substances of abuse (i.e. inpatient drug treatment, hospitalization, incarceration, etc.), NAC may potentially be used during periods of abstinence and following release to promote continued abstinence. This will be challenging to incorporate into clinical trials, given that methodologies typically administer investigational medication during periods of active use. As above, concurrent treatment with abstinence-targeted pharmacotherapies or behavioral treatments may be an avenue to address this issue and explore NAC as a complement to established cessation interventions.
A notable limitation to the use of NAC for SUDs is its poor oral bioavailability, which is approximately 4–10 % [25, 26]. It is possible that participants are not receiving doses sufficient to demonstrate efficacy in all cases. Recommended doses of 1,200 or 2,400 mg are often given across two to four pills taken two to three times per day, and some studies suggest that higher doses may be preferable for treatment outcomes [89]. Medication dosing at multiple times per day is burdensome for patients, especially those with SUDs, who may also be dealing with psychiatric comorbidity, unstable housing, interpersonal, economic, and/or professional consequences due to their substance use, etc. Improved medication regimens and forms of NAC that are more bioavailable would allow for less response effort on the part of the participant. This would be an important advancement for its use in the treatment of SUDs and may serve to improve compliance rates. With this goal in mind, a recent study discussed the synthesis of an amide derivative NAC amide (NACA) [99]. Preliminary results suggest that this compound has more membrane permeability and bioavailability, which may result in improved clinical outcomes. Alternate formulations of NAC that would only require once a day dosing would also be an important advancement for the clinical viability of NAC.
It is possible that NAC alone is not a sufficient pharmacotherapy to promote abstinence, and it must be part of a more comprehensive treatment plan or combination pharmacotherapy. Treatment for nicotine dependence is an example of how NAC could be used in combination with an already approved smoking cessation pharmacotherapy. Improvements to smoking cessation pharmacotherapy are greatly needed as even the most effective pharmacotherapies only promote abstinence in approximately 40 % of patients [12-14]. Combination pharmacotherapies may target the initiation of quit attempts or initial abstinence through reduced smoking reward or reduction in craving and withdrawal, and may also provide more enduring relapse protection through normalization and restoration of glutamate homeostasis during periods of withdrawal, as is seen with NAC treatment in non-human animal models. A potentially synergistic interaction between NAC and other smoking cessation pharmacotherapies may therefore be achieved. It is also possible that NAC as a pharmacotherapy for SUDs will only demonstrate efficacy when paired with psychosocial support, education and skills-based training, or contingency management. Future research is greatly needed with NAC in combination with other forms of treatment, including as a combination pharmacotherapy, especially in cases of distinct, yet complementary, neurobiological mechanisms.
6.2 Future Directions for Research
Many questions remain regarding the use of NAC for SUDs, both in preclinical and clinical investigation. Preclinically, it will be of interest to examine the ability of NAC to restore basal synaptic plasticity of glutamatergic NAcore synapses via a possible neuron-glial interaction, as well as the ability to inhibit the rapid, transient increase in synaptic plasticity found during cue-induced cocaine seeking. Additionally, it will be important to examine the mechanism contributing to the ability of NAC to restore GLT1, inhibit glutamate overflow, and inhibit activation of extrasynaptic NMDA receptors across different drugs of abuse. Among clinical investigators, more studies are needed to determine the conditions under which NAC is an efficacious pharmacotherapy for SUDs. NAC may be most effective when administered under conditions of abstinence, rather than during active use. NAC as a relapse prevention pharmacotherapy in abstinent cocaine-dependent adults is being explored in a recently funded study sponsored by the National Institute on Drug Abuse (R01DA034054, PI Malcolm). Conversely, studies with cannabis-dependent adolescents have demonstrated the efficacy of NAC during periods of active use [95]. This distinction could be based on differences in the substances of abuse being targeted by the study interventions, the behavioral support used in these clinical trials (e.g. abstinence-targeted contingency management in the cannabis trial), actual medication adherence rates, etc. RCTs assessing the efficacy of NAC that have yielded negative findings highlight the importance of careful consideration of conditions that may contribute to the efficacy of NAC. These and other possibilities are worthy of further inquiry.
The restoration of glutamatergic activity due to NAC following withdrawal in preclinical models has been demonstrated across various substances, including opioids [82]. Despite the consistencies across various drugs of abuse, no studies have been conducted in human participants to determine the efficacy of NAC in opioid dependence, either as a stand-alone treatment or adjunct to opioid-replacement therapies or behavioral treatment. Additionally, the effects of NAC on alcohol withdrawal and other alcohol-induced aversive effects in a rodent model have been explored [39, 40, 100], with promising results. A recent review dedicated to new pharmacotherapies for alcohol abuse and dependence suggested that medications targeting the glutamatergic system may be a promising avenue to explore, including those agents that reduce excess extracellular glutamate (i.e. NAC, among others) [101]. These and other results contribute to the preclinical evidence suggesting that NAC may be an effective pharmacotherapy for several substances of abuse, and that more clinical investigation across substances is needed.
Among the limited clinical work to date with NAC for SUDs, a number of ongoing studies aim to further address the efficacy of NAC. NAC as a treatment for cannabis dependence in adults is being explored through an ongoing, multisite RCT through the Clinical Trials Network of the National Institute of Drug Abuse (NCT01675661). Another RCT is assessing the effects of NAC on cannabis withdrawal (NCT01439828). A third study will assess NAC as a relapse prevention pharmacotherapy in cocaine-dependent participants (R01DA034054, PI Malcolm), while an ongoing inpatient study will assess the effects of NAC on the rewarding properties of cocaine and changes in glutamate activity during abstinence (NCT01392092). Another RCT focusing on comorbidity is assessing the efficacy of NAC on nicotine dependence and pathological gambling (NCT00967005). Finally, an RCT exploring NAC plus naltrexone for the treatment of alcohol dependence is ongoing (NCT01214083). These trials will provide further clarification on the role of NAC in the treatment of SUDs. The number of ongoing studies with NAC is even greater when the scope is extended to psychiatric and compulsive disorders more generally, which may eventually lead to the study of NAC in patients with comorbid SUDs and psychiatric disorders.
An additional area of future research focused on the clinical utility of NAC is in its influence on minimizing damage caused by oxidative stress. Based on evidence supporting the role of oxidative stress as a mechanism underlying psychiatric disorders [36], it will be important to study this specifically for the treatment of SUDs. Oxidative stress as an underlying mechanism of psychiatric disorders, and treatment with NAC, represents a preliminary and promising direction for future research focused on poly-substance use and psychiatric comorbidities.
7 Conclusions
Preclinical evidence supporting the role of glutamatergic dysregulation in addiction has led to clinical investigation exploring glutamatergic targets for the treatment of SUDs. These results have been mixed, but generally show promise. Over 50 years, NAC has been shown to be safe and well tolerated in adults and adolescents. It is being explored as a pharmacotherapy for several indications, including psychiatric disorders, making it a promising agent for those experiencing psychiatric comorbidity or struggling with multiple substances of abuse. The economic implications for effectively treating SUDs and other psychiatric disorders are substantial. NAC has FDA approval and is widely available, while also being relatively inexpensive compared with other pharmacotherapeutic options. If NAC is shown to be efficacious for promoting abstinence and preventing relapse, either alone or as part of a combination pharmacotherapy regimen, the cost savings of preventing future treatment episodes and related healthcare costs would be well worth the additional cost of medication. Future clinical studies should pursue research focused on the necessary conditions required for NAC to be effective in the treatment of SUDs. This may involve the use of NAC as part of a combination pharmacotherapy or as part of a comprehensive treatment plan that works to promote the initiation of quit attempts or initial periods of abstinence. These and other questions will be vital in addressing the deficits that currently exist in the treatment of SUDs and the potential that NAC holds to provide improved treatment outcomes.
Acknowledgments
The authors wish to acknowledge the funding sources for this study. Funding was provided by the National Institute on Drug Abuse grants DA031779 (Kevin M. Gray, Erin A. McClure), DA013727 (Kevin M. Gray, Erin A. McClure), DA033690 (Cassandra D. Gipson), and DA003906, DA012513, and DA015369 (Peter W. Kalivas). The funding source had no role other than financial support.
Footnotes
Conflicts of interest Erin A. McClure, Cassandra D. Gipson, Robert J. Malcolm, Peter W. Kalivas, and Kevin M. Gray have no relevant conflicts of interest to report. The authors alone are responsible for the content and writing of this paper. All authors have read and approved the manuscript.
Contributor Information
Erin A. McClure, Email: mccluree@musc.edu, Clinical Neuroscience Division, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 125 Doughty St., Suite 190, Charleston, SC 29407, USA; Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St, Charleston, SC 29425, USA.
Cassandra D. Gipson, Department of Neurosciences, Medical University of South Carolina, 96 Jonathan Lucas St, MSC 606, Charleston, SC 29425, USA
Robert J. Malcolm, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St, Charleston, SC 29425, USA
Peter W. Kalivas, Department of Neurosciences, Medical University of South Carolina, 96 Jonathan Lucas St, MSC 606, Charleston, SC 29425, USA
Kevin M. Gray, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, 67 President St, Charleston, SC 29425, USA
References
- 1.Centers for Disease Prevention and Control. Smoking-attributable mortality, years of potential life lost, and productivity losses: United States, 2000–2004. MMWR. 2008;57(45):1226–8. [PubMed] [Google Scholar]
- 2.National Drug Intelligence Center. The economic impact of illicit drug use on American society. Washington, D.C.: United States Department of Justice; 2011. [Google Scholar]
- 3.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teera-wattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: summary of national findings. Rockville: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2012. [Google Scholar]
- 5.Centers for Disease Prevention and Control. Vital signs: current cigarette smoking among adults aged ≥18 years: United States, 2005–2010. MMWR. 2011;60(35):1207–12. [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- 7.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (Heroin) addiction: a clinical trial with methadone hydrochloride. JAMA. 1965;193:646–50. doi: 10.1001/jama.1965.03090080008002. Journal Article. [DOI] [PubMed] [Google Scholar]
- 8.van den Brink W, Haasen C. Evidenced-based treatment of opioid-dependent patients. Can J Psychiatry. 2006;51(10):635–46. doi: 10.1177/070674370605101003. [DOI] [PubMed] [Google Scholar]
- 9.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; May, 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 10.van den Brink W. Evidence-based pharmacological treatment of substance use disorders and pathological gambling. Curr Drug Abuse Rev. 2012;5(1):3–31. doi: 10.2174/1874473711205010003. [DOI] [PubMed] [Google Scholar]
- 11.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5 doi: 10.1002/14651858.CD009329.pub2. CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Int Med. 2006;166(15):1571–7. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 15.Rosner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev. 2010;(9) doi: 10.1002/14651858.CD004332.pub2. CD004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, Soyka M. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010;(12) doi: 10.1002/14651858.CD001867.pub3. CD001867. [DOI] [PubMed] [Google Scholar]
- 17.Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23(7):543–53. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein AM, Gorelick DA. Pharmacological treatment of cannabis dependence. Curr Pharm Design. 2011;17(14):1351–8. doi: 10.2174/138161211796150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoops WW, Rush CR. Agonist replacement for stimulant dependence: a review of clinical research. Curr Pharm Design. 2013;19(40):7026–35. doi: 10.2174/138161281940131209142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167–77. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Brown RM, Kupchik YM, Kalivas PW. The story of glutamate in drug addiction and of N-acetylcysteine as a potential pharmacotherapy. JAMA Psychiatry. 2013;70:895–7. doi: 10.1001/jamapsychiatry.2013.2207. [DOI] [PubMed] [Google Scholar]
- 24.Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Expert Opin Biol Ther. 2008;8(12):1955–62. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 25.Borgstrom L, Kagedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217–22. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- 26.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- 27.Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebocontrolled clinical trials. Clin Ther. 2000;22(2):209–21. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 28.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Resp Crit Care Med. 1997;156(2 Pt 1):341–57. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 29.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose: analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319(24):1557–62. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 30.Dippy JE, Davis SS. Rheological assessment of mucolytic agents on sputum of chronic bronchitis. Thorax. 1969;24(6):707–13. doi: 10.1136/thx.24.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Ann Emerg Med. 1998;31(6):710–5. doi: 10.1016/s0196-0644(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 32.Mant TG, Tempowski JH, Volans GN, Talbot JC. Adverse reactions to acetylcysteine and effects of overdose. Br Med J (Clinical Res Ed) 1984;289(6439):217–9. doi: 10.1136/bmj.289.6439.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey B, Blais R, Letarte A. Status epilepticus after a massive intravenous N-acetylcysteine overdose leading to intracranial hypertension and death. Ann Emerg Med. 2004;44(4):401–6. doi: 10.1016/j.annemergmed.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Marzullo L. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Curr Opin Pediatr. 2005;17(2):239–45. doi: 10.1097/01.mop.0000152622.05168.9e. [DOI] [PubMed] [Google Scholar]
- 35.Mucomyst [package insert] New York City (NY): Bristol-Myers Squibb Company; 2007. [Google Scholar]
- 36.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 37.Atkuri KR, Mantovani JJ, Herzenberg LA. N-Acetylcysteine: a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7(4):355–9. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berk M, Ng F, Dean O, Dodd S, Bush AI. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29(7):346–51. doi: 10.1016/j.tips.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Seiva FR, Amauchi JF, Rocha KK, Ebaid GX, Souza G, Fernandes AA, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649–56. doi: 10.1016/j.alcohol.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Ozaras R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J Gastroenterol. 2003;9(1):125–8. doi: 10.3748/wjg.v9.i1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10(8):561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 42.Olive MF, Cleva RM, Kalivas PW, Malcolm RJ. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol Biochem Behav. 2012;100(4):801–10. doi: 10.1016/j.pbb.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology. 2008;33(9):2139–47. doi: 10.1038/sj.npp.1301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013;110(22):9124–9. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531–7. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67(1):81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr Med Chem. 2012;19(30):5148–56. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW. N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci. 2003;1003:349–51. doi: 10.1196/annals.1300.023. [DOI] [PubMed] [Google Scholar]
- 51.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16(4):1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29(12):3715–9. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30(23):7984–92. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict Biol. 2013;18(1):40–9. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gipson CD, Kupchik YM, Kalivas PW. Rapid, transient synaptic plasticity in addiction. Neuropharmacology. 2014;76(Pt B):276–86. doi: 10.1016/j.neuropharm.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77(5):867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen HW, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. doi: 10.1038/npp.2013.318. Epub 15 Nov 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, Lingwood BE, et al. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49(4):520–41. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- 59.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6(7):743–9. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 60.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28(4):182–7. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 61.De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008;6(9):e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang Y, Zhou Q. Spine modifications associated with long-term potentiation. Neuroscientist. 2009;15(5):464–76. doi: 10.1177/1073858409340800. [DOI] [PubMed] [Google Scholar]
- 63.Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108(1):385–90. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D. Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci. 2003;1003:169–75. doi: 10.1196/annals.1300.009. [DOI] [PubMed] [Google Scholar]
- 66.Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–72. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–76. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 68.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 69.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 70.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008;28(23):6046–53. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11(3):344–53. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 72.Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, et al. The cystine/glutamate antiporter system x(c)(−) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal. 2013;18(5):522–55. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dringen R, Gutterer JM, Gros C, Hirrlinger J. Aminopeptidase N mediates the utilization of the GSH precursor CysGly by cultured neurons. J Neurosci Res. 2001;66(5):1003–8. doi: 10.1002/jnr.10042. [DOI] [PubMed] [Google Scholar]
- 74.Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, et al. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry. 2012;71(11):978–86. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology. 2012;37(9):2143–52. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–50. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 77.Trantham-Davidson H, Lalumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci. 2012;32(36):12406–10. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150(3):633–46. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 79.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther. 2011;337(2):487–93. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murray JE, Everitt BJ, Belin D. N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol. 2012;17(2):437–40. doi: 10.1111/j.1369-1600.2011.00330.x. [DOI] [PubMed] [Google Scholar]
- 82.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63(3):338–40. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez-Nino AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–82. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–9. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27(51):13968–76. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871–8. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF. Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors. Addict Biol. 2011;16(2):215–28. doi: 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15(1):105–10. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389–94. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Gray KM, Watson NL, Carpenter MJ, Larowe SD. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187–9. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, et al. Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry. 2007;164(7):1115–7. doi: 10.1176/ajp.2007.164.7.1115. [DOI] [PubMed] [Google Scholar]
- 92.Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addict Res. 2011;17(4):211–6. doi: 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- 93.Van Schooten FJ, Besaratinia A, De Flora S, D’Agostini F, Izzotti A, Camoirano A, et al. Effects of oral administration of N-acetyl-l-cysteine: a multi-biomarker study in smokers. Cancer Epidemiol Biomarkers Prev. 2002;11(2):167–75. [PubMed] [Google Scholar]
- 94.Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823–8. doi: 10.1016/j.euroneuro.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 95.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–12. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788–91. doi: 10.1016/j.addbeh.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443–52. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sunitha K, Hemshekhar M, Thushara RM, Santhosh MS, Yariswamy M, Kemparaju K, et al. N-Acetylcysteine amide: a derivative to fulfill the promises of N-acetylcysteine. Free Radic Res. 2013;47(5):357–67. doi: 10.3109/10715762.2013.781595. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira Seiva FR, Amauchi JF, Ribeiro Rocha KK, Souza GA, Ebaid GX, Burneiko RM, et al. Effects of N-acetylcysteine on alcohol abstinence and alcohol-induced adverse effects in rats. Alcohol. 2009;43(2):127–35. doi: 10.1016/j.alcohol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology. 2013;229(3):539–54. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


