Abstract
Background
We examined the interaction of illicit drug use and depressive symptoms, and how they affect the subsequent likelihood of highly active antiretroviral therapy (HAART) use among women with HIV/AIDS.
Methods
Subjects included 1,710 HIV-positive women recruited from six sites in the U.S. including Brooklyn, Bronx, Chicago, Los Angeles, San Francisco/Bay Area, and Washington, DC. Cases of probable depression were identified using depressive symptom scores on the Centers for Epidemiologic Studies Depression Scale. Crack, cocaine, heroin, and amphetamine use were self-reported at 6-month time intervals. We conducted multivariate logistic random regression analysis of data collected during sixteen waves of semiannual interviews conducted from April 1996 through March 2004.
Results
We found an interaction effect between illicit drug use and depression that acted to suppress subsequent HAART use, controlling for virologic and immunologic indicators, socio-demographic variables, time, and study site.
Conclusions
This is the first study to document the interactive effects of drug use and depressive symptoms on reduced likelihood of HAART use in a national cohort of women. Since evidence-based behavioral health and antiretroviral therapies for each of these three conditions are now available, comprehensive HIV treatment is an achievable public health goal.
Keywords: HIV, depression, HAART, drug use
1. Introduction
The prevalence of illicit drug use among sub-populations of HIV-positive individuals is well documented, as is the association of drug use with lower utilization of the latest combination antiretroviral therapies (ART). In a prospective population-based probability sample of HIV-infected persons receiving medical care, ART was less likely among those with drug dependence, severe drug abuse, and injection drug use (Turner et al., 2001). The research of Sambamoorthi and colleagues (Sambamoorthi et al., 2000) found that those engaged in current injection drug use were less likely to use ART; moreover, among users of ART those injecting drugs were less likely to use ART consistently. In a prospective European cohort of 7331 HIV-positive men, intravenous drug users were significantly less likely to be receiving highly active ART (HAART) at time of study recruitment and followup (Mocroft et al., 1999). In a nationally representative probability sample of HIV-infected adults receiving care in the United States (HCSUS), drug users had significantly lower odds of current HAART use than homosexual men (Cunningham et al., 2000). Similarly, a longitudinal study of 565 injection drug users who were clinically eligible for HAART found that those continuing injection drug use (IDU) were significantly less likely to initiate HAART, and more likely to remain HAART-naïve longer, than those who ceased IDU (Celentano et al., 2001).
The association between illicit drug use and lower HAART utilization has been found in cohorts of HIV-positive women as well. In an early study of injection drug users with universal access to ART in Canada, women were over twice as likely as men not to use ART (Strathdee et al., 1998). Research on HIV-positive women in the Women and Infants Transmission Study found that prenatal hard drug use was negatively associated with receiving HAART as well as non-HAART combination therapy (Cooper et al., 2002). In the nationally representative HCSUS cohort, women with drug use exposure had significantly lower proportions on HAART than other groups of study participants (Cunningham et al., 2000). In a longitudinal study of HIV-positive women in the Women's Interagency HIV Study (WIHS), those using marijuana, hashish, crack/cocaine, heroin, or amphetamines were significantly less likely to be on ART before the advent of protease inhibitors, as well as less likely to use HAART regimens after their introduction, controlling for clinical factors, health insurance status, and demographic variables (Cook et al., 2002b).
HIV-positive individuals also have high rates of depression and depressive symptoms, and this is particularly true of women whose rates of depression are double those of men (Cook et al., 2002a; Golub et al., 2003; Ickovics et al., 2001; Kaplan et al., 1997; Moore et al., 1999; Morrison et al., 2002;Turner et al., 2003; Vedhara et al., 1999). Some investigators have speculated that psychiatric disorders interfere with a patient's ability to initiate or continue multi-drug ART regimens (Boland, 1997). This notion is supported by evidence that patients with a history of depression have significant delays in beginning protease inhibitor (PI) treatment (Fairfield et al., 1999). In addition, a prior study of the WIHS cohort found that women with high levels of depressive symptoms who injected drugs were significantly more likely to discontinue use of HAART than others (Ahdieh-Grant et al., 2005).
There is also evidence that receiving treatment for psychiatric problems has the potential to increase use of HAART. Analysis of data from the HCSUS cohort found that HIV-positive patients who received mental health care were over 50% more likely to report HAART use than other HIV patients, controlling for possible confounding factors (Turner et al., 2001). HIV+ women with depression who were receiving either antidepressants and psychotherapy, or psychotherapy alone, were significantly more likely to use HAART, compared to those receiving no depression treatment (Cook et al., 2006). There is also some evidence that HAART initiation may improve later mental health status. Among one group of 453 HIV-positive men and women, the number of depressive symptoms decreased significantly one year after initiation of protease inhibitors (Low-Beer et al., 2000). Similarly, in a nationally representative sample of 2,466 people in treatment for HIV in the United States, those initiating or maintaining combination ART had significantly fewer psychiatric symptoms than those not on combination ART (Chan et al., 2003).
In addition to drug use and depression, other factors have been shown to influence HAART. Prior research has found HAART use to be lower among African American women and men than those of other races/ethnicities (Anderson and Mitchell, 2000; Ghani et al., 2003; Palacio et al., 2002; Shapiro et al., 1999), and among individuals with lower vs. higher levels of education (Mouton 1997). Skepticism of African Americans toward Western medicine is well-documented (Corbie-Smith, 1999; Gamble, 1997), and research has found that African American patients may have difficulty expressing their HIV treatment preferences to health care providers (Mouton, 1997). Low literacy and education levels may negatively impact people's ability to understand complex antiretroviral regimens (Cargill et al., 2004). Another influence on HAART use is physician experiences and attitudes. One study of U.S. infectious disease physicians treating HIV patients found that patients' alcohol and drug use were as important as disease severity in influencing physicians' prescriptions of HAART regimens (Bogart et al., 2000). It has also been suggested that willingness to prescribe HAART to people who are using illicit drugs is greater among providers with more experience treating HIV (Celentano et al., 2001).
Despite what is known about these associations, there is far less understanding of the interaction of depression and drug use, and its potential association with HAART utilization, particularly among HIV-positive women. In the prospective population-based probability sample of HIV-infected men and women receiving medical care cited earlier (Turner et al., 2001), no significant interactions were found between depression and severe drug abuse and decreased likelihood of HAART. The purpose of the present study was to restrict the analysis to women, given their significantly higher rates of depression and lack of prior research attention, in order to explore the interaction of illicit drug use and depression, and examine their association with HAART use over time.
2. Methods
2.1. Sample
Starting in October 1994, HIV-positive and seronegative women were enrolled in the Women's Interagency HIV Study (WIHS) at six medical and university consortia sites nationwide: Brooklyn, NY; the Bronx, NY; Chicago, IL; Los Angeles, CA; San Francisco/Bay Area, CA, and Washington, DC. Over the next nine years, participants completed WIHS study visits at 6-month intervals that included a physical and OB/GYN exam, blood draws, and administration of an extensive battery of questions regarding their mental and physical health, use of medical and social services, and current demographic statuses (e.g., housing, employment, marital, education). As part of the interviews, HIV+ women were asked to report the names of their HIV ART drugs and identify their medications from pictures shown to them by interviewers. In order to examine relationships between illicit drug use and use of HAART longitudinally, WIHS study visits after HAART was generally available in the community (April 1, 1996) were included in the analysis of HIV-positive women (n=1,710).
2.2. Measures
2.2.1. Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) was used to identify cases of probable depression. The CES-D is a 20 item, self-report measure, scored 0-60, with higher scores indicating higher levels of depressive symptoms. It has demonstrated excellent validity, reliability, and factor structure in prior research, and is commonly used in studies of HIV-positive populations (Moore et al., 1999), including women with HIV (Ickovics et al., 2001). Its sensitivity for DSM-III major depression is excellent, in the 80%-90% range, with somewhat lower specificity in the 70%-80% range (Low-Beer et al., 2000; Richardson et al., 2001). In published research on the WIHS cohort (Cook et al., 2002a) different ways of computing scores on the CES-D included: 1) use of the standard cutoff of 16; and 2) use of a more stringent cutoff of 23. In the present study, both cutoffs were used to operationalize depression.
2.2.2. Drug Use
At each study visit, women were asked whether they had used crack, cocaine, heroin, or amphetamines via any means of administration since the time of their last interview, a period of roughly 6 months. For this study, such reports are considered as “recent illicit drug use,” in keeping with prior research on this cohort (Landay et al., 2003) and that on another multi-site cohort of women with HIV (Ickovics et al., 2001). We chose these drugs for special emphasis over others (e.g., marijuana) due to their closer association with routes of HIV infection, disease progression, and poor medical outcomes (Cook et al., 2004; Golub et al., 2003).
2.2.3. Dependent Variable
The dependent variable of HAART use was assessed at each interview from women's reports of the names of all antiretroviral medications taken currently. All drugs were later coded and classified as PIs, nucleoside reverse transcriptase inhibitors (NRTIs), NNRTIs, and other HIV-related drug therapies (e.g., hydroxyurea). The definition of HAART was determined by the Department of Health and Human Services guidelines (2006) and defined as: 1) 2 or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor (PI) or 1 non-nucleoside reverse transcriptase inhibitor (NNRTI); 2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI; 3) a regimen containing ritonavir and saquinavir in combination with 1 NRTI and no NNRTIs; and 4) an abacavir or tenofovir containing regimen of 3 or more NRTIs in the absence of both PIs and NNRTIs, except for the 3 NRTI regimens consisting of abacavir + tenofovir + lamivudine, or didanosine + tenofovir + lamividune. All other antiretroviral regimens were classified as non-HAART regimens. Participants reporting no HAART use at a particular study visit may have reported the therapy at prior visits or later ones; the time-varying nature of the analysis allowed us to include all reports while controlling for the effects of time.
2.2.4. Covariates
CD4 counts were measured by immunofluorescence using flow cytometry at laboratories participating in the AIDS Clinical Trials quality assurance program. P lasma HIV-RNA levels were measured using a nucleic acid sequence-based amplification technique (Organon Teknika, Durham, USA). To measure poorest level of immune functioning, each woman's nadir CD4 was coded as “high” if greater than 500, and “medium” if 200-500, with less than 200 as the reference category. To measure peak viral concentration, apex HIV-RNA was coded as “low” (<=10,000) or “high” (>10,000) based on National Institutes of Health (2006) guidelines for HAART use that were in effect at the study's midpoint, with the latter used as the reference category. Ethnicity was assessed as African American, Hispanic/Latina, and Caucasian/other. Those who had completed high school or a General Equivalency Degree (GED) were coded as “1” with all others coded as “0.” Age at baseline was measured in decades, per ten-year increase. Finally, each of the six sites was represented by an indicator variable, with Chicago serving as the arbitrary reference category.
2.3. Statistical Analysis
Sixteen waves of semiannual data from the seropositive cohort of the WIHS were analyzed, including all study visits from April 1996 through March 2004. Descriptive, univariate, and bivariate statistics were computed for all variables. The probability of utilizing HAART (vs. not) at all study visits was calculated by fitting random effects logistic regression models (Gibbons and Hedeker, 1996). The models tested included illicit drug use, probable depression, and their interaction, along with control variables for clinical factors (CD4, HIV-RNA), sociodemographic features (age, race/ethnicity, education), and time (study visit number). This model included both fixed covariates (age at baseline, ethnicity, high school education, nadir CD4, apex HIV-RNA), and time varying variables (HAART use, illicit drug use, probable depression, study visit). Because we were interested in the longitudinal association between drug use/depressive symptoms and subsequent HAART use, we examined associations between drug use and depressive symptoms prior to each study visit and related these to HAART use at the time of the subsequent study visit. Thus, temporal ordering was structured into the analysis by the nature of the variables included in the model. Average proportional odds ratios were calculated for each model variable from MIXOR parameter estimates.
Random effects regression modeling (using MIXOR 2.0) was chosen because of its sensitivity to serial correlation, ability to handle missing data, and the need to include both fixed and time-varying variables. Serial correlation resulting from repeated measures of the same individual is a critical issue in longitudinal data analysis, often resulting in unrealistically low standard errors of the fixed effects of interest, and biased tests of hypotheses due to type I or “false positive” errors (Gibbons et al., 1993). Individuals in a study may also be expected to have varying propensities toward the outcomes of interest, due to varying predispositions and other unobserved influences (Hu et al., 1998). The random intercept statistically accounts for such heterogeneity, and can be thought of as representing each individual's baseline propensity toward use of HAART. Missing observations can be of particular concern in longitudinal data analysis, since participants commonly miss assessments, either sporadically or consistently. RRM models assume that data are “missing at random” and are, therefore, in the category of ignorable nonresponse, which has been shown to result in valid statistical inferences for the model parameters in RRM (Hedeker & Gibbons, 1997).
3. Results
3.1. Background Characteristics
Table 1 presents the background characteristics of the 1,710 women included in the analysis at study baseline and 8 years later at their last follow up visit. At the time of the last study visit, 62% (n=1,058) of the cohort was still active, defined as having had a study visit within the prior 12 months. Of the 652 women no longer active, 405 had died (24% of the initial cohort), and these deaths accounted for the majority of study attrition (62%). About half of the deaths (49%) were ascertained to be AIDS-related, 33% were non-AIDS related, and the remaining 18% were unknown or indeterminate (Cohen et al., 2002).
Table 1. Characteristics of HIV-Positive Women (N=1710) with HIV/AIDS in 1996 and 2004.
| Characteristic | 1996 N=1,710 |
Percent | 2004 N=1058 |
Percent | |
|---|---|---|---|---|---|
| African American | 956 | 55.9 | 587 | 55.5 | |
| Hispanic/Latina | 372 | 21.8 | 241 | 22.8 | |
| Caucasian, Other | 382 | 22.3 | 230 | 21.7 | |
| High School Graduate | 1071 | 62.6 | 676 | 63.9 | |
| Crack/cocaine/heroin/amphetamine use | 339 | 19.8 | 116 | 11.1 | |
| HAART | 160 | 9.4 | 707 | 66.8 | |
| Probable Depression (CES-D>=16) | 945 | 55.3 | 564 | 54.4 | |
| Probable Depression (CES-D>=23) | 572 | 33.5 | 269 | 25.8% | |
| Nadir CD4 | <200 | 470 | 27.5 | 213 | 20.1 |
| 200-500 | 764 | 44.7 | 469 | 44.3 | |
| >500 | 443 | 25.9 | 393 | 34.3 | |
| Apex HIV-RNA Viral Load | 10,000 | 831 | 48.6 | 278 | 26.3 |
| 10,000+ | 879 | 51.4 | 780 | 73.7 | |
| Average (s.d.) | Minimum-maximum | Average (s.d.) | Minimum-maximum | ||
| Age in years | 38.1 (7.8) | 18-74 | 44.8 (7.8) | 25-81 |
Note: Percentages may not add to 100% due to missing data.
Source: Women's Interagency HIV Study-1996-2004
3.2. Frequency of Illicit Drug Use
We began by examining the frequency of self-reported illicit drug use in the cohort over time. Starting with the first study visit, crack use was more common (13.2%) than use of heroin (7.4%), cocaine (6.9%) or amphetamines (4.3%), and this relative difference between the three drugs was maintained throughout the study. Reported use of illicit drugs also declined over time for all four substances. At the most recent study visit, crack use was reported by 7.6%, heroin by 3.5%, cocaine by 1.8%, and amphetamines by 1.2%.
3.3. Associations Between Drug Use, Depressive Symptoms, and HAART Likelihood
Next, we examined the relationship between illicit drug use and HAART use. We plotted the proportions of women on HAART at each study visit by whether or not they reported illicit drug use since the prior study visit. At every study visit, the unadjusted proportions of women on HAART were greater for women not reporting recent use of illicit drugs than for those reporting use of these drugs (not shown).
We then turned to the relationship between depressive symptoms and HAART use. We examined the proportion of women using HAART by whether or not their depressive symptom levels indicated probable depression in the month prior to each study visit. At every visit, the unadjusted proportions of women on HAART were greater for women not reporting recent depression than those reporting depressive symptoms (not shown). This was true using the traditional CES-D cutoff 16, as well as the more stringent cutoff of 23.
3.4. Relationships Between Drug Use and Depressive Symptoms
To explore the relationship between illicit drug use and depression (again using both CES-D cutoffs) we calculated bivariate correlations between depression and recent crack, cocaine, heroin, or amphetamine use for all study visits (not shown). Significant zero-order correlations between the two were found beginning with visit 3 and continuing throughout all subsequent time points. These showed that women reporting illicit drug use in the past six months were more likely to report depression at the next study visit.
3.5. Multivariate Analysis of Drug Use, Depressive Symptoms and HAART
To test for an interaction between depressive symptoms and recent crack, cocaine, heroin or amphetamine use, we classified the women into 4 categories at each study visit: 1) probable depression (using the more stringent CES-D>= 23 cutoff) + recent illicit drug use; 2) probable depression + no recent illicit drug use; 3) recent illicit drug use + no probable depression; and 4) no probable depression + no illicit drug use. The first 3 categories were used as time-varying variables in the same random regression model (with no use/no probable depression as the contrast) controlling for the same covariates. Results, presented in Table 3, showed that, compared to women with no depression or illicit drug use, HAART use was significantly less likely among women with depression plus illicit drug use, and also less likely among women with recent illicit drug use alone. The difference between women with depression but no drug use compared to those with neither was not statistically significant. To test this model with a less stringent definition of probable depression, it was re-run using the traditional cutoff of 16. Results (not shown) were virtually identical, with the same interaction terms remaining significant in the model.
To examine the interaction visually, we graphed the proportion on HAART at each study visit, by the 4 categories of recent illicit drug and depression using the CES-D cutoff of 23. As shown in Figure 1, with some small variations in early study visits, the lowest proportions of women on HAART were those with depression plus drug use throughout the time period studied. The next lowest proportions were among women who used illicit drugs but did not screen positive for depression. Conversely, the largest proportions of women on HAART were the group of women reporting neither depression nor recent illicit drug use, followed closely by those women who screened positive for depression, but reported no illicit drug use.
Figure 1.
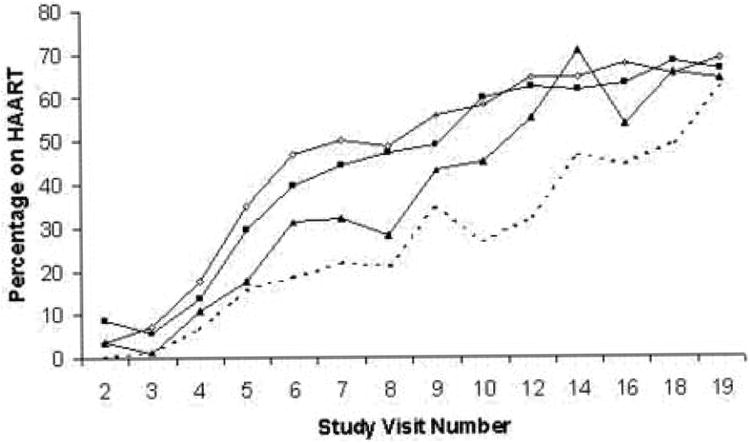
Proportion on HAART by illicit drug use and probable depression over time. Illicit drug use = crack, cocaine, heroin, and amphetamine use in past 6 months. Probable depression = Centers for Epidemiologic Studies – Depression Scale (CES-D) score >=23.

4. Discussion
The results of our analysis suggest that illicit drug use and high levels of depressive symptoms are significantly associated with one another. We also found that the effects of depression interacted with those of illicit drug use to suppress the initiation and use of HAART over time. Another important finding is that illicit drug use without co-occurring depression also decreases subsequent HAART use in comparison to the occurrence of neither. However, in the absence of illicit drug use, high levels of depressive symptoms alone did not reduce the likelihood of later HAART use. Thus, when considering the relative effects of illicit drugs and depression, while illicit drug use alone is significantly associated with reduced likelihood of HAART, depression alone is not.
One potential way that illicit drug use may affect HAART likelihood is through its effects on health service utilization. In previous studies of the WIHS cohort, current injection drug use was associated with missed primary care appointments (Palacio et al., 2004), while recent crack, cocaine and heroin use was associated with less optimal patterns of health service utilization such as not seeing the same provider consistently and infrequent primary care visits (Burke-Miller et al., 2006). In a study of injection drug users with HIV infection, Bouhnik et al. (2004) found a significant association between cessation of injection drug use and engagement in treatment for HIV, including use of ART. Since optimal patterns of health care such as more outpatient visits, less use of inpatient and emergency care, and seeing the same provider consistently have been associated with greater HAART use in prior studies of the WIHS and other cohorts (Burke-Miller et al., 2006; Fleishman et al., 2005; Gebo et al., 2005), it may be that illicit drug use affects health care utilization in ways that lower HAART likelihood.
Beyond issues of access to care and utilization of health services, the finding that HIV-positive users of illicit drugs are less likely to be on HAART has been hypothesized to be the result of providers withholding HAART from active drug-users because of concerns about adherence or other issues (Bangsberg and Moss, 1999; Celentano et al., 2001; Crisp et al., 2004). While we were unable to address the effects of provider experience and expertise in this study, it is possible that these influences were operating in the study population. If so, medical provider education specifically focused on management of HAART among active drug users has the potential to enhance HAART utilization in this and other cohorts.
This study's results also suggest that efforts to assess HIV-seropositive women for depression should be encouraged among health service providers in a wide variety of settings, as others have proposed (Ickovics et al., 2001; Sherbourne et al., 2000). Since individuals from various cultures experience and express depressive symptoms differently (Chamberlain et al., 2000; Goldberg and Lecrubier 1995; Weissman et al., 1996), the finding of lower HAART likelihood among African American women in this study suggests that assessment and referral must be culturally sensitive to individuals of African heritage (Abas et al., 1998; Chamberlain et al., 2001) as well as those who are illicit drug users (Golub et al., 2004). Others have suggested that clinicians can optimize the care they provide for minority patients by using a cultural competence framework that explores the meaning of the illness to the patient, and enhancing patient-provider communication by negotiating treatment plans across the patient-physician culture, as well diversifying their clinical staff (Stone, 2004). Still others note that physician-patient interaction should take into account the co-occurrence of physical and sexual abuse, substance use, and depression among African American women at risk for or infected with HIV (Johnson et al., 2003).
Another need suggested by this study's results is for depression therapies that have been proven effective among women with a history of illegal drug use. For example, pharmacotherapy plus psychotherapy has been shown to be effective in treating active injection drug users with both major depression and substance-induced depression (Stein et al., 2004). Cognitive behavioral therapy may be especially useful in this population when immediate changes in lifestyle and behavior are called for (Dean et al., 2000). The previous finding in the WIHS cohort of greater HAART likelihood among depressed women who receive psychopharmacolgic and psychotherapeutic treatment for depression bolsters this proposal (Cook et al., 2006).
Some caveats to our findings include the fact that our sample is a longitudinal cohort and not a representative sample of all U.S. women with HIV infection. Thus, our results are not necessarily generalizable to the larger population of HIV-positive women. Another study limitation concerns the use of the CES-D depression rather than research quality diagnostic instruments such as the Structured Clinical Interview for the Diagnostic and Statistical Manual, or the Composite International Diagnostic Interview. Our inability to assess the level of HIV experience of women's HIV care providers is another study limitation that bears specific mention. We are unable to say whether lower use of HAART among users of illicit drugs is due to the woman's choice, her physician's unwillingness to prescribe HAART given her drug use, physician inexperience, or some combination of these factors.
While ours is not the first study to connect depression and illicit drug use to lower use of HAART, our finding of an interaction effect between the two can be considered a call to action. Given the accumulated evidence from our research and that of others, concerted efforts to address both depression and substance abuse are needed, especially among women who remain untreated with HAART over long periods of time. We now possess the means to treat all three of these conditions with evidence-based behavioral health and antiretroviral therapies. What remains is the will, the funding, and the training needed to make an approach to genuinely comprehensive HIV treatment a reality.
Table 2. Random effects logistic regression analysis of longitudinal HAART use among women with HIV (N=1710) from 4/96-3/04: Effects of interaction of probable depression1 and recent crack, cocaine, heroin, or amphetamine use, controlling for study site.
| HAART Use | |||
|---|---|---|---|
| Characteristic | Estimate2 (Standard Error) | Odds3 Ratio | p-value |
| Time (study visit) | 0.24 (.00) | 1.27 | .000 |
| Probable depression + crack, cocaine, heroin use | -0.72 (.13) | 0.49 | .000 |
| Probable depression + no crack, cocaine, or heroin use | -0.07 (.06) | 0.93 | .ns |
| Crack, cocaine, or heroin use + no probable depression | -0.29 (.12) | 0.75 | .01 |
| No probable depression + no crack, cocaine, or heroin use | (reference) | (reference) | -- |
| Age (in years) | 0.01 (.01) | 1.01 | ns |
| African American | -0.41 (.13) | 0.67 | .000 |
| Hispanic/Latina | -0.10 (.16) | 0.91 | ns |
| Caucasian | (reference) | (reference) | -- |
| High school education | 0.25 (.11) | 1.28 | .003 |
| High CD4 (>500) | 0.26 (.09) | 1.30 | .000 |
| Medium CD4 (200-500) | 0.00 (.07) | 1.00 | ns |
| Low CD4 (<200) | (reference) | (reference) | -- |
| High RNA viral load (>=10,000) | -1.76 (.06) | 0.17 | .000 |
| Low RNA viral load (< 10,000) | (reference) | (reference) | -- |
Probable depression = CES-D score >=23. Highly similar results were obtained with this model using the traditional CES-D cutoff of 16.
Unstandardized random regression coefficient (MIXOR), sign indicates direction of effect
Average proportional odds ratio
Source: Women's Interagency HIV Study-1996-2004
Acknowledgments
Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Alvaro Munoz). The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute, the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Dental Research, the Agency for Health Care Policy and Research, and the Centers for Disease Control and Prevention. U01-AI-35004, U01-AI-31834, U01-AI-34994, AI-34989, U01-HD-32632 (NICHD), U01-AI-34993, U01-AI-42590, N01-AI-35161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abas MA, Phillips C, Carter J, Walter J, Banerjee S, Levy R. Culturally sensitive validation of screening questionnaires for depression in older African-Caribbean people living in south London. Br J Psychiatry. 1998;173:249–254. doi: 10.1192/bjp.173.3.249. [DOI] [PubMed] [Google Scholar]
- Ahdieh-Grant L, Tarwater PM, Schneider MF, Anastos K, Cohen M, Khalsa A, Minkoff H, Young M, Greenblatt R. Factors and temporal trends associated with highly active antiretroviral therapy discontinuation in the Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2005;38:500–503. doi: 10.1097/01.qai.0000138160.91568.19. [DOI] [PubMed] [Google Scholar]
- Anderson KH, Mitchell JM. Differential access in the receipt of antiretroviral drugs for the treatment of AIDS and its implications for survival. Arch Intern Med. 2000;160:3114–3120. doi: 10.1001/archinte.160.20.3114. [DOI] [PubMed] [Google Scholar]
- Bangsberg DR, Moss A. When should we delay highly active antiretroviral therapy? J Gen Intern Med. 1999;14:446–448. doi: 10.1046/j.1525-1497.1999.05109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LM, Kelly JA, Catz SL, Sosman JM. Impact of medical and nonmedical factors on physician decision making for HIV/AIDS antiretroviral treatment. J Acquir Immune Defic Syndr. 2000;23:396–404. doi: 10.1097/00126334-200004150-00006. [DOI] [PubMed] [Google Scholar]
- Boland R. HIV and depression. Am J Psychiatry. 1997;154:1632–1633. doi: 10.1176/ajp.154.11.1632-a. [DOI] [PubMed] [Google Scholar]
- Bouhnik AD, Carrieri MP, Rev D, Spire B, Gastaut JA, Gallais H, Obadia Y MANIF 2000 Study Group. Drug injection cessation among HIV-infected injecting drug users. Addict Behav. 2004;29:1189–1197. doi: 10.1016/j.addbeh.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Burke-Miller JK, Cook JA, Cohen MH, Hessol NA, Wilson TE, Richardson JL, Williams P, Gange SJ. Longitudinal Relationships Between Use of Highly Active Antiretroviral Therapy and Satisfaction with Care Among Women Living with HIV/AIDS. Am J Public Health. 2006;96(4):1044–1051. doi: 10.2105/AJPH.2005.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill VA, Stone VE, Robinson MR. HIV treatment in African Americans: challenges and opportunities. Journal of Black Psychology. 2004;30:24–39. [Google Scholar]
- Celentano DD, Galai N, Sethi AK, Shah NG, Strathdee SA, Vlahov D, Gallant JE. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- Chamberlain D, Muntaner C, Walrath C, LaVeist T, Nickerson K. Racial differences in attitudes toward professional mental health care and in the use of services. Am J Orthopsychiatry. 2000;70:455–464. doi: 10.1037/h0087736. [DOI] [PubMed] [Google Scholar]
- Chamberlain D, Muntaner C, Walrath C, Nickerson K, LaVeist T, Leaf P. Racial and ethnic differences in attitudes toward seeking professional mental health services. Am J Public Health. 2001;91:805–807. doi: 10.2105/ajph.91.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KS, Orlando M, Joyce G, Gifford AL, Burnam MA, Tucker JS, Sherbourne CD. Combination antiretroviral therapy and improvements in mental health: Results from a nationally representative sample of persons undergoing care for HIV in the United States. J Acquir Immune Defic Syndr. 2003;33:104–111. doi: 10.1097/00126334-200305010-00015. [DOI] [PubMed] [Google Scholar]
- Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, Minkoff H, Hessol NA. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Burke J, Grey DD, Anastos K, Kirstein L, Palacio H, Richardson J, Wilson TE, Young M. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002a;30:401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Grey DD, Kirstein L, Burke J, Anastos K, Palacio H, Richardson J, Wilson TE, Young M. Use of highly active antiretroviral therapy in a cohort of HIV-seropositive women. Am J Public Health. 2002b;92:82–87. doi: 10.2105/ajph.92.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Grey DD, Burke-Miller J, Anastos K, Gandhi M, Richardson J, Wilson T, Young M. Effects of treated & untreated depressive symptoms on highly active antiretroviral therapy use in a U.S. multi-site cohort of HIV-positive women. AIDS Care. 2006;18:93–100. doi: 10.1080/09540120500159284. [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, Wilson TE, Young MA, Hessol NA. Relationship of depressive symptoms to AIDS-related mortality in a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, Hayani K, Handelsman E, Smeriglio V, Hoff R, Blattner W. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- Corbie-Smith G. The Continuing Legacy of the Tuskegee Syphilis Study: Considerations for Clinical Investigation. Am J Med Sci. 1999;317:5–8. doi: 10.1097/00000441-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Crisp BR, Williams M, Timpson S, Ross MW. Medication compliance and satisfaction with treatment for HIV disease in a sample of African-American crack cocaine smokers. AIDS Behav. 2004;8:199–206. doi: 10.1023/B:AIBE.0000030250.33931.af. [DOI] [PubMed] [Google Scholar]
- Cunningham WE, Markson LE, Andersen RM, Crystal SH, Fleishman JA, Golin C, Gifford A, Liu HH, Nakazono TT, Morton S, Bozzette SA, Shapiro MF, Wenger NS. Prevalence and predictors of highly active antiretroviral therapy use in patients with HIV infection in the United States. J Acquir Immune Defic Syndr. 2000;25(2):115–123. doi: 10.1097/00042560-200010010-00005. [DOI] [PubMed] [Google Scholar]
- Dean C, Antoni MH, Schneiderman N, Ironson G, McCabe P, Fernandez JB, Cruess SE, Klimas N, Kumar M. Cognitive-behavioral stress management increases free testosterone and decreases psychological distress in HIV-seropositive men. Health Psychol. 2000;19:12–20. doi: 10.1037//0278-6133.19.1.12. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [Accessed January 31, 2006]; Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL10292004002.pdf.
- Dilorio C, Hartwell T, Hansen N. Childhood sexual abuse and high risk behaviors among men at high risk for HIV infection. Am J Public Health. 2002;92:214–219. doi: 10.2105/ajph.92.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield KM, Libman H, Davis RB, Eisenberg DM. Delays in protease inhibitor use in clinical practice. J Gen Intern Med. 1999;14:395–401. doi: 10.1046/j.1525-1497.1999.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleishman JA, Gebo KA, Reilly ED, Conviser R, Mathews WC, Korthuis PT, Hellinger J, Rutstein R, Keiser P, Rubin H, Moore RD. Hospital and outpatient health services utilization among HIV-infected adults in care 2000-2002. Med Care. 2005;43:III40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- Gamble VN. Under the shadow of Tuskegee: African Americans and health care. Am J Public Health. 1997;87:1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, Hellinger J, Keiser P, Rubin HR, Crane L, Hellinger FJ, Mathews WC. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- Ghani AC, Donnelly CA, Anderson RM. Patterns of antiretroviral use in the United States of America: analysis of three observational databases. HIV Med. 2003;4:24–32. doi: 10.1046/j.1468-1293.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D. MIXOR: a computer program for mixed-effects ordinal regression analysis. Comput Methods Programs Biomed. 1996;49:157–176. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux HC, Kraemer JB, Greenhouse MT, Shea SD, Imber SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data: Application of NIMH Treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Goldberg DP, Lecrubier Y. Form and frequency of mental disorders across centres Mental illness in general health care: An international study. John Wiley; Chichester: 1995. p. 323. [Google Scholar]
- Golub ET, Astemborski JA, Hoover DR, Anthony JC, Vlahov D, Strathdee SA. Psychological distress and progression to AIDS in a cohort of injection drug users. J Acquir Immune Defic Syndr. 2003;32:429–434. doi: 10.1097/00126334-200304010-00013. [DOI] [PubMed] [Google Scholar]
- Golub ET, Latka M, Hagan H, Havens JR, Hudson SM, Kapadia F, Campbell JV, Garfein RS, Thomas DL, Strathdee SA. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the Beck Depression Inventory. J Urban Health. 2004;81:278–290. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol. 1998;147:694–703. doi: 10.1093/oxfordjournals.aje.a009511. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J HIV Epidemiology Research Study Group. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV epidemiology research study. JAMA. 2001;285:1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Cunningham-Williams RM, Cottler LB. A tripartite of HIV-risk for African American women: the intersection of drug use, violence, and depression. Drug Alcohol Depend. 2003;70:169–175. doi: 10.1016/s0376-8716(02)00345-9. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Marks G, Mertens SB. Distress and coping among women with HIV infection: Preliminary findings from a multiethnic sample. Am J Orthopsychiatry. 1997;67:80–91. doi: 10.1037/h0080213. [DOI] [PubMed] [Google Scholar]
- Landay A, Benning L, Bremer J, Weiser B, Burger H, Nowicki M, Kovacs A. Correlates of immune activation marker changes in human immunodeficiency virus (HIV)-seropositive and high risk HIV-seronegative women who use illicit drugs. J Infect Dis. 2003;188:209–218. doi: 10.1086/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low-Beer S, Chan K, Yip B, Wood E, Montaner JSG, O'Shaughnessy MV, Hogg RS. Depressive symptoms decline among persons on HIV protease inhibitors. J Acquir Immune Defic Syndr. 2000;23:295–301. doi: 10.1097/00126334-200004010-00003. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Madge S, Johnson AM, Lazzarin A, Clumeck N, Goebel FD, Viard JP, Gatell J, Blaxhult A, Lundgren JD. A comparison of exposure groups in the EuroSIDA study: Starting highly active antiretroviral therapy (HAART), response to HAART, and survival. J Acquir Immune Defic Syndr. 1999;22(4):369–378. doi: 10.1097/00126334-199912010-00008. [DOI] [PubMed] [Google Scholar]
- Moore J, Schuman P, Schoenbaum E, Boland B, Solomon L, Smith D. Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. AIDS. 1999;13:2459–2468. doi: 10.1097/00002030-199912030-00018. [DOI] [PubMed] [Google Scholar]
- Morrison MF, Petitto JM, Have TT, Gettes DR, Chiappini MS, Weber AL, Brinker-Spence P, Bauer RM, Douglas SD, Evans DL. Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry. 2002;159:789–796. doi: 10.1176/appi.ajp.159.5.789. [DOI] [PubMed] [Google Scholar]
- Mouton C, Teno JM, Mor V, Piette J. Communication of preferences for care among human immunodeficiency virus-infected patients: Barriers to informed decisions? Ann Fam Med. 1997;6:342–347. doi: 10.1001/archfami.6.4.342. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. [Accessed January 30, 2006]; Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL01282000010.pdf.
- Palacio H, Kahn JG, Richards TA, Morin SF. Effect of race and/or ethnicity in use of antiretrovirals and prophylaxis for opportunistic infection: A review of the literature. Public Health Rep. 2002;117:233–251. doi: 10.1093/phr/117.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacio H, Shiboski CH, Yelin EH, Hessol NA, Greenblatt RM. Access to and utilization of primary care services among HIV-infected women. J Acquir Immune Defic Syndr. 2004;32:293–300. doi: 10.1097/00126334-199908010-00006. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Richardson J, Barkan S, Cohen M, Back S, FitzGerald G, Feldman J, Young M, Palacio H. Experience and covariates of depressive symptoms among a cohort of HIV infected women. Soc Work Health Care. 2001;32:93–111. doi: 10.1300/J010v32n04_05. [DOI] [PubMed] [Google Scholar]
- Sambamoorthi U, Walkup J, Olfson M, Crystal S. Antidepressant treatment and health services utilization among HIV-infected Medicaid patients diagnosed with depression. J Gen Intern Med. 2000;15:311–320. doi: 10.1046/j.1525-1497.2000.06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MF, Morton SC, McCaffrey DF, Senterfitt JW, Fleishman JA, Perlman JF, Athey LA, Keesey JW, Goldman DP, Berry SH, Bozzette SA. Variations in the care of HIV- infected adults in the United States: Results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–2315. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Hays RD, Fleishman JA, Vitiello B, Magruder KM, Bing EG, McCaffrey D, Burnam A, Longshore D, Eggan F, Bozzette SA, Shapiro MF. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. Am J Psychiatry. 2000;157:248–254. doi: 10.1176/appi.ajp.157.2.248. [DOI] [PubMed] [Google Scholar]
- Stein MD, Solomon DA, Herman DS, Anthony JL, Ramsey SE, Anderson BJ, Miller IW. Pharmacotherapy plus psychotherapy for treatment of depression in active injection drug users. Arch Gen Psychiatry. 2004;61:152–159. doi: 10.1001/archpsyc.61.2.152. [DOI] [PubMed] [Google Scholar]
- Stone VE. Optimizing the care of minority patients with HIV/AIDS. Clin Infect Dis. 2004;38:400–404. doi: 10.1086/380969. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PGA, Yip B, O'Shaughnessy MV, Montaner JSG, Schechter MT, Hogg RS. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Fleishman JA, Wenger N, London AS, Burnam MA, Shapiro MF, Bing EG, Stein MD, Longshore D, Bozzette SA. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16:625–633. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Cosler L, Hauck WW. Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. J Gen Intern Med. 2003;18:248–257. doi: 10.1046/j.1525-1497.2003.20122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Giovanni S, McDermott M. Disease progression in HIV-positive women with moderate to severe immunosuppression: The role of depression. Behav Med. 1999;25:43–47. doi: 10.1080/08964289909596738. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]


