Abstract
A recent line of inquiry has examined how an observer’s experience with action changes the neural processing of similar actions when they are subsequently observed. The current study used electroencephalography (EEG) to test the hypothesis that giving participants different types and amounts of experience with specific objects would lead to differential patterns of sensorimotor rhythms during the observation of similar actions on those objects. While EEG was recorded, three groups of participants (n = 20 in each group; mean age = 22.0 years, SD = 2.7) watched video clips of an actor reaching, grasping, and lifting two objects. Participants then received information about differences in weight between the two objects. One group gained this information through extended sensorimotor experience with the objects, a second group received much briefer sensorimotor experience with the objects, and the third group read written information about the objects’ weights. Participants then viewed the action sequences again. For participants who had sensorimotor experience with the objects, the EEG response to viewing the actions was differentially sensitive to the anticipated weight of the objects. We conclude that this sensitivity was based on the participant’s prior sensorimotor experience with the objects. The participants who only received semantic information about the objects showed no such effects. The primary conclusion is that even brief experience with actions affects sensorimotor cortex activity during the subsequent observation of similar actions.
Keywords: alpha, beta, EEG, sensorimotor, experience, action processing
1. Introduction
There is substantial support for the notion that observing another person’s action is associated with activation of the observer’s sensorimotor system (for review, see Avenanti, Candidi, & Urgesi, 2013). A significant part of this work has concerned patterns of brain activation during action observation, with various findings pointing to the existence of what has been termed an “action observation network” (AON) in the human brain (Caspers, Zilles, Laird, & Eickhoff, 2010). The activation of sensorimotor areas within this network during action observation has sometimes been termed “neural mirroring”, although there is a good deal of debate about the nature and function of these purported mirroring mechanisms in relation to action understanding (Rizzolatti & Sinigaglia, 2010). Partly related to this debate, key open questions concern the determinants of sensorimotor system activity during the observation of similar actions being carried out by others, including the role of self-experience with actions on the nature of this activation. Recent studies have illuminated the temporal dynamics of mirroring responses during the observation of hand actions (e.g., Berchio et al., 2013; Streltsova, Berchio, Gallese, & Umilta, 2010), and here we extended that work by examining the effect of gaining experience with an abstract property (weight) of the specific objects being acted on.
It is well established that prior experience with carrying out a particular action influences processing during action observation (Hamilton, Wolpert, & Frith, 2004; Hecht, Vogt, & Prinz, 2001; Schutz-Bosbach & Prinz, 2007). For example, being trained to perform pulling movements causes observers to identify others’ ambiguous movements as pulling movements, likely as a result of motor-to-visual adaptation (Cattaneo et al., 2011). This type of evidence strongly suggests that when observing actions, prior experiences with carrying out similar actions may be highly influential. A related line of research has examined whether greater expertise with certain actions might result in more activity in the AON during later observation of another person performing a similar action (e.g., Calvo-Merino, Glaser, Passingham, & Haggard, 2005; Cross et al., 2012; Orgs, Dombrowski, Heil, & Jansen-Osmann, 2008). More recent research has suggested that the relation between action experience and AON activity is nonlinear (Diersch et al., 2013; James, Oechslin, Van De Ville, Hauert, Descloux, & Lazeyras, 2014; Liew, Sheng, Margetis, & Aziz-Zadeh, 2013). Increased AON activation following action experience may relate to greater engagement of predictive processes (Kilner, Friston, & Frith, 2007) or a richer understanding of the observed actions (Rizzolatti & Sinigaglia, 2010), whereas decreased AON activity following experience may imply more efficient neural processing of familiar actions (Babiloni et al., 2010).
In recent work we investigated how sensorimotor experiences associated with acting upon specific objects result in expectations about the specific sensory consequences when others carry out actions directed toward those objects. For example, in a recent study we found that differing somatosensory experiences when reaching into a box contributed uniquely to changes in sensorimotor EEG rhythms during observation of someone else reaching into a similar-looking box (Quandt, Marshall, Bouquet, & Shipley, 2013). In another study, we reported that experience with lifting heavy and light objects leads to differential responses of sensorimotor EEG rhythms when seeing another person gesture toward similar-looking objects (Quandt, Marshall, Shipley, Beilock, & Goldin-Meadow, 2012). Specifically, participants showed greater alpha- and beta-range suppression during observation of gestures toward objects predicted to be light, based on the participant’s own prior experiences. Findings such as these suggest that activity in the AON may be modulated by specific experience-based predictions about observed actions. However, while these studies suggest that sensorimotor experiences with acting on specific objects play a role in the processing of others’ actions on those objects, it is unknown precisely how much, or what type, of experience is needed to bring about such sensitivity to the predicted sensorimotor consequences of action.
In addition to learning about the sensorimotor characteristics of actions by means of performing them, it is also possible to gain relevant information by other means, for instance by receiving verbal information about the objects being acted on. Studies in this area have had mixed results, and it remains to be determined whether the acquisition of relevant descriptive semantic information (i.e., “this object is heavy”) results in sensitivity of sensorimotor cortex to the predicted characteristics of an observed action (i.e., different patterns of activation for “heavy” compared with “light” objects). In one transcranial magnetic stimulation (TMS) experiment, there was no difference in motor cortex excitability when participants observed actions upon objects labeled as “heavy” versus “light”, suggesting that expectations about object weight did not affect how the actions were processed (Senot et al., 2011). However, in a recent study using functional magnetic resonance imaging, different patterns of brain activity were found during the viewing of knots that participants had learned to tie, compared to knots they had only learned names for. Observation of knots that were learned by name was associated with a modest increase in activation of the superior parietal lobule, whereas observation of knots learned via tying experience was associated with robust increases in activation along the intraparietal sulcus (Cross et al., 2012).
Cognitive neuroscience studies investigating the effects of action experience on the response of sensorimotor cortex during action observation have employed a variety of methods, including the measurement of EEG rhythms (Pineda, 2005). Alpha-range rhythms (8-13 Hz) and beta rhythms (14-22 Hz) are uniquely suited for investigating the determinants of action processing due to their involvement in sensorimotor processing. In particular, the mu rhythm recorded over central electrode sites has proven especially useful in this area, due to its sensitivity to sensorimotor stimuli (Hari, 2006; Muthukumaraswamy, Johnson, & McNair, 2004; Quandt et al., 2013; Quandt et al., 2012). The mu rhythm oscillates in the same frequency range (8-13 Hz) as the classical posterior alpha rhythm, but has distinct sources in sensorimotor regions of the AON (Arnstein, Cui, Keysers, Maurits, & Gazzola, 2011; Ritter, Moosmann, & Villringer, 2009).
The EEG beta rhythm is thought to originate in sensory and motor cortex (Gaetz & Cheyne, 2006; Jensen et al., 2005; Ritter et al., 2009), and its amplitude is modulated by both the production of movement as well as motor imagery (McFarland, Miner, Vaughan, & Wolpaw, 2000). Recent research has further explored the utility of beta in the study of action processing, with the finding that this rhythm is sensitive to both the production and observation of action (Puzzo, Cooper, Cantarella, & Russo, 2011; Quandt et al., 2012). The beta rhythm appears to be particularly sensitive to variation in the somatosensory aspects of observed actions (Mizelle, Forrester, Hallett, & Wheaton, 2010; van Ede, de Lange, Jensen, & Maris, 2011; van Ede, Jensen, & Maris, 2010).
A primary goal of the current study was to examine how much sensorimotor experience with specific actions is needed to induce changes in subsequent neural mirroring. Prior studies have looked at brief (Marshall et al., 2009) or moderate (Quandt, Marshall, Bouquet, Young, & Shipley, 2011) experience with imitating drawing actions, multiple days of dance training (Orgs et al., 2008), or moderate amounts of knot-naming or tying experience (Cross et al., 2012). A major limitation of these studies is that they present subjects with a single type of action experience, often compared to a control condition receiving no experience, but they do not examine different amounts of experience (e.g., brief vs. extensive) or types of experience (e.g., sensorimotor vs. semantic). The study aimed to answer the following two questions: 1) Does gaining experience with or receiving semantic information about the characteristics of specific objects lead to greater activation of sensorimotor cortex during subsequent observation of actions on those objects?; 2) Does physically performing an action lead to increased sensitivity to the anticipated sensorimotor consequences of an observed action, compared to learning semantic information about the action?
In order to address these questions, EEG was recorded during action observation before and after the acquisition of information about the sensorimotor characteristics of acting on specific objects. At the beginning of the experiment, participants observed video clips of an actor reaching for, grasping, and lifting two objects that were visually identical except for color (yellow or blue). Participants then received either: 1) Brief experience with acting on the objects so that they learned the different weights of the two objects (heavy or light); 2) Extended experience with acting on the objects, again indicating the different weight of the objects; or 3) Written information about the weights of the objects. Participants then viewed the same video clips as in the beginning of the experiment. EEG rhythmic activity during action observation was analyzed for differences before and after experience, and for differences based on the expected weights of the objects following experience.
2. Methods
2.1. Participants
The sample comprised 60 undergraduate participants (42 females) between the ages of 18 and 29 years (mean = 22.08; SD = 2.67) who received course credit in exchange for participation. Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971). No left-handed participants were included in the study (mean handedness score = 77; SD = 20.35). All participants had normal or corrected-to-normal vision, and reported no history of neurological abnormalities.
2.2. Stimuli
2.2.1. Apparatus
Four cylindrical objects (15.5 cm tall × 7.0 cm diameter) were created. The objects varied by both color (yellow or blue) and weight (1300 g or 130 g), such that there was one of each possible color and weight combination. The objects were organized into two sets containing one of each color and one of each weight: e.g., heavy yellow and light blue. Gaining information about these objects (during an initial experience block) created an association for each participant between each object’s color and its weight, and therefore, the somatosensory and motor consequences associated with acting upon each object. This type of color-weight association has been used successfully in past studies (Quandt et al., 2012).
Two “distracter objects” were also included in the experiment—a small plastic cup (48 g), and a small rubber toy (27 g). Both objects were easy to grasp and lift. These objects were placed on the table during the experience phase of the protocol, and were used to control for the amount and duration of activity performed by the participant (see Section 2.3 for details). All participants performed a total of 20 actions, divided between the assigned objects and the distracter objects.
2.2.2. Videos
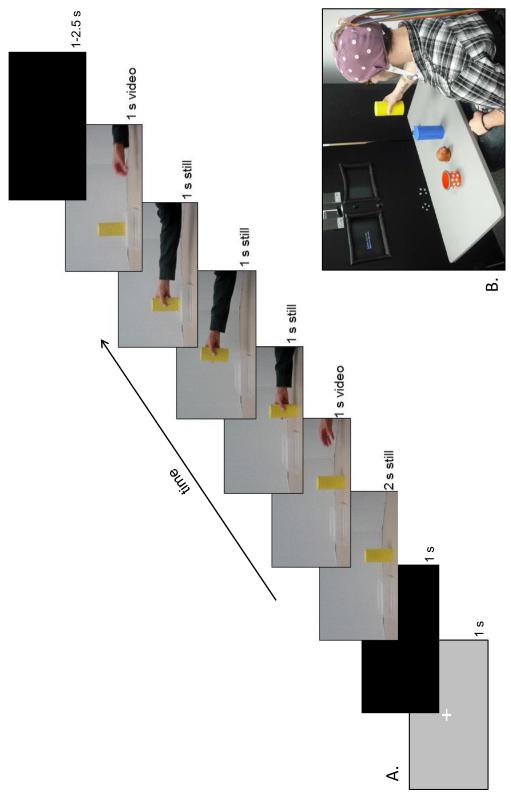
We created four video clips (recorded at 30 fps with a resolution of 1280 × 720 pixels) depicting an actor grasping and lifting yellow and blue objects, with two video clips being created for each color object. The video clips began with a fixation point (1 s), followed by a black screen (1 s). The yellow or blue object was then shown resting on a table with a small platform nearby (see Figure 1). After 2 s of the static scene, an actor’s hand entered from the right side of the screen and reached toward the object as if to grasp it (reach duration = 1 s). At the point in which the fingers made contact with the object, a still frame of the hand grasping the object remained on the display for 1 s. Another still frame was then displayed (1 s duration) showing the hand transporting the object towards the platform. A third still image then showed the hand placing the object on the platform (1 s). The hand then retracted away from the object in a continuous motion and disappeared from view, leaving the object on the platform. A black screen (between 1 and 2.5 s in duration) was then shown before the next video clip began. The objects used during the creation of the video clips were a third set of objects (never available to any participant) with a weight (585 g) that was exactly halfway between the weights of the “heavy” and “light” objects. However, because we used still images rather than continuous video to depict the lifting and grasping aspects, the video clips contained no kinematic information that might indicate the actual weight of the objects used during filming.
Figure 1. Video stimuli and experimental setup.
A) Each trial began with a fixation point and a black screen. Then, video stimuli depicted an actor reaching for, transporting, and placing an object on a small platform. Half of the videos depicted actions upon yellow objects (shown) and half depicted actions upon blue objects (not shown). In all videos, the actor’s hand entered from the right side of the screen. B) Experimental setup. During the Experience phase of the protocol, a participant sits at a table in front of a screen displaying instructions. Four objects are present; from left to right: the cup (distracter object), the toy (distracter object), the blue object, and the yellow object. For explanation of the distracter objects, see text.
An additional 2-4 video clips were included in each observation block in which the video clip froze for an additional 2 seconds when the actor’s fingers came in contact with the object. During the breaks following each sub-block, participants were asked to verbally report how many of these extended freeze-frame trials they had observed. This requirement ensured that participants focused on the reaching and grasping actions, rather than becoming distracted from watching the video stimuli. The trials with the longer freeze-frame were excluded from further analyses.
2.3. Experimental procedure
Upon arrival at the laboratory, each participant was randomly assigned into one of three conditions: Brief Experience (BE; n = 20, mean age = 22.5, SD = 2.57), Extended Experience (EE; n = 20, mean age = 21.8, SD = 2.46), or Semantic Information (SI; n = 20; mean age = 21.7, SD = 2.94). The groups did not differ significantly in age (F(2,57) = .55, p = .58) or sex (F(2,57) = .23, p = .80). Participants were also randomly assigned to one pair of objects: heavy yellow and light blue or heavy blue and light yellow. The unassigned pair of objects was not seen or touched by the participant at any time.
Each experimental session involved participants observing the video clips as well as gaining experience with their assigned pair of objects. EEG and video signals were recorded during the entire experiment. Each participant sat 125 cm in front of a monitor (31 cm × 23 cm), and completed the following parts of the experiment:
Part 1: participants observed 60 trials of the video clips depicting the reaching and grasping actions directed toward yellow or blue objects (30 of each color). The trials were organized into 3 blocks of 20 trials and were presented in a random order.
-
Experience: participants received experience depending on their group assignment. For all groups, the experimenter placed the two assigned objects on the table in front of the participant, next to the two distracter objects (see Figure 1).
The Brief Experience (BE) group lifted the distracter objects nine times each, then lifted each assigned object one time.
The Extended Experience (EE) group lifted the assigned objects ten times each. Participants in this group did not lift the distracter items at all.
The Semantic Information (SI) group first lifted the distracter objects ten times each. They then received written information regarding the sensorimotor characteristics of the assigned objects. The screen displayed the words “The blue object is heavy. It weighs about 3 pounds. It is filled with concrete.”, “The yellow object is light. It weighs about one third of a pound. It is filled with air.”, and “The blue object is 10 times heavier than the yellow object” (depending on the color-weight association to which they had been assigned).
Following the acquisition of information regarding the objects’ weights, each participant was asked by the experimenter which object was heavy and which was light, to confirm that they understood and remembered the association between color and weight. All participants (100% in each of the three groups) answered both questions correctly.
Part 2: all participants saw 60 trials (3 blocks of 20) depicting the same reaching, grasping, and lifting actions seen in Part 1. The order of trials was random.
2.4. EEG Acquisition
EEG was collected from 26 electrode sites plus the left and right mastoids using a custom-made Lycra stretch cap (Electro-Cap, Eaton, OH) in combination with Electro-Gel conducting gel. The sites were Fp1, Fp2, F3, F4, Fz, FC1, FC2, FC5, FC6, F7, F8, C3, C4, CP1, CP2, CP5, CP6, T7, T8, P3, P4, Pz, P7, P8, O1, O2. The EEG signals were amplified by optically isolated, high input impedance (>1 GΩ) bioamplifiers from SA Instrumentation (San Diego, CA). The signals were digitized at 512 Hz onto the hard drive of a PC running Snap-Master data acquisition software from HEM Data Corp. (Southfield, MI) using a 16-bit A/D converter (±5 V input range). Scalp electrode impedances were kept under 25 kΩ. Bioamplifier gain was 4000, and the hardware filter settings were .1 Hz (high-pass) and 100 Hz (low-pass), with a 12 dB/octave rolloff. The EEG signals were collected referenced to Cz with an AFz ground, and were re-referenced offline to the average of the left and right mastoids (Luck, 2005). Eyeblink artifacts were removed with independent component analysis using the JadeR algorithm (Cardoso, 1999), based on extraction and removal of the characteristic signal produced by blinks (Hoffmann & Falkenstein, 2008). Event marks were recorded along with the EEG signal using the same method as reported in Quandt et al. (2013).
2.5 Data processing and reduction
EEG analysis epochs were time-locked to the moment at which the actor’s hand made contact with the object (time 0 ms). Each analysis epoch began at −2000 ms, when the object was visible on the table but the hand had not yet entered the screen. The epoch extended through the hand entering the screen and reaching toward the object, and continued until the end of the video clip (+5000 ms). The epoch of interest was analyzed in comparison to a baseline period defined as −4000 to −2050 ms. The baseline period included the display of the black screen and part of the time the object was resting on the table (see Figure 1), with the latter included in order to compute changes in band power during action observation relative to viewing of the static object. Epochs were removed from analysis if there was significant artifact in the EEG signal. There was no significant difference in rejection rate between the two trial types (heavy vs. light; means = 94.47% and 93.89% of trials accepted, respectively; t(59) = 1.08, p = .28).
2.6 Data analysis
A MATLAB (The Mathworks Inc., Natick, MA) toolbox, EEGLAB, was used to compare the event-related spectral perturbation (ERSP; Delorme & Makeig, 2004) between conditions. ERSP was computed over a frequency range that encompassed the alpha (8-13 Hz) and beta (14-22 Hz) sensorimotor rhythms. ERSP was calculated by means of a Morlet wavelet decomposition, applied over 200 overlapping windows, starting with a 4-cycle wavelet at the lowest frequency. Time-frequency decompositions were created for each condition (e.g., heavy and light), and averaged across all participants. In order to compare one epoch (e.g., observation of light object) with another epoch (e.g., observation of heavy object), bootstrap significance tests were performed based on random resampling of the data. These significance tests compared ERSP between two conditions, with the results visualized on time-frequency plots indicating the points where significant differences arose.
Based on the literature and our own prior work, planned statistical analyses tested the following predictions concerning EEG suppression over sensorimotor regions in the alpha and beta frequency range:
There would be greater suppression of power (i.e., a more negative ERSP) during the second block of observation trials for all three groups, due to greater visual experience and familiarity with the objects, as well as increased knowledge about the sensorimotor characteristics of the objects (whether in the form of sensorimotor experience or semantic information).
During the first block of observation trials, there would be no difference in suppression during observation of reaches toward the different-colored objects, since participants will have no expectations regarding the sensorimotor characteristics of the objects.
During the second block of observation trials, the extended experience group will show a significant difference in suppression depending on the expected weight of the object, such that objects expected to be lighter will elicit a greater suppression of band power (i.e., a more negative ERSP) than objects expected to be heavier. These effects will be particularly evident for the sensorimotor mu rhythm over central electrode sites.
The effect of weight will be present in the same direction for the brief experience group, but may be a weaker suppression effect than for the extended experience group.
The semantic information group will show no effect of expected weight during the second observation epoch.
Based on these predictions, planned parallel group comparisons were performed for each group to assess whether band power suppression differed between conditions (for a similar approach see Ono, Kimura, & Ushiba, 2013). We conducted a limited number of a priori statistical significance tests in order to test our specific predictions, and to avoid spurious findings as a result of large numbers of comparisons across time and frequency domains. Specifically, since particular effects were predicted within each of the three groups, the effect of Weight (heavy vs. light) was analyzed for each Group (Extended Experience, Brief Experience, and Semantic Information), as was the effect of Part (Part 1 vs. Part 2).
A scalp Region of Interest (ROI) for the EEG analyses was defined as the electrodes over sensorimotor cortex. The ROI was made up of seven electrodes: FC1, FC2, C3, C4, Cz, CP1, and CP2. Data were also analyzed at frontal (F3, F4, and Fz), parietal (P3, P4, and Pz), and occipital (O1 and O2) electrodes, to provide information about the topographic extent of any significant effects (see Figure 2). Given that our predictions only concerned activity in the ROI, the analyses performed at frontal, parietal, and occipital electrodes enabled us to assess whether any effects were specific to central regions, which would suggest that sensorimotor cortex was uniquely sensitive to the task manipulations. In order to correct for comparisons across multiple electrodes and lower the risk of committing a Type I error (Kilner, 2013), all statistical tests were held to a Bonferroni corrected p-value threshold (.05 alpha level / 7 electrodes in the ROI = adjusted p value of .007). In addition, in order to correct for comparisons across thousands of time and frequency points, all results were corrected using the False Discovery Rate correction, as originally described by Benjamini and Hochberg (1995) and implemented in EEGLAB.
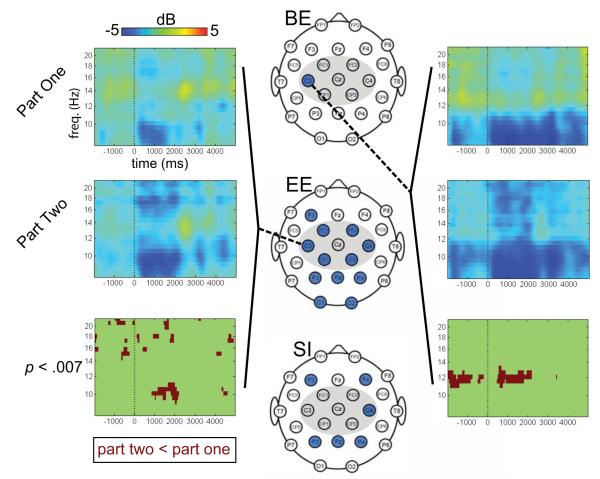
Figure 2. Differences in alpha and beta suppression between Part 1 and Part 2 of the experiment, for each group.
The three scalp maps depict the electrode montage (central ROI highlighted with gray). For each group (Brief Experience, BE; Extended Experience, EE; and Semantic Information, SI), the highlighted electrodes (blue) indicate electrodes at which there were significant differences between conditions (p < .007, FDR) at any time or frequency within the analysis epoch. The epoch spanned from −2000 to +5000 ms (time 0 = start of grasping phase), and frequencies from 8-22 Hz were analyzed. Time-frequency plots and statistical maps are shown for selected electrodes of interest. On these plots, averaged ERSPs from Part 1 and Part 2 are shown, as are the significant differences between the conditions. Cool colors indicate a decrease in power relative to baseline; warm colors indicate an increase in power. On the statistical map, dark red indicates that power was lower during observation of trials in Part 2 than in Part 1.
3. Results
3.1. Behavioral Performance
The behavioral distracter task embedded within the experimental protocol ensured that participants were paying attention to the videos and watching the reaching actions. At the end of each of the six observation blocks, participants were asked to report how many freeze-frame reaches they had seen. Participants performed well on the distracter task (mean correct responses out of six: BE group = 5.05 [SD = .83], EE group = 5.10 [SD = .91], SI group = 5.10 [SD = .79]), and there was no significant difference in accuracy between groups (F(2,59) = .023, p = .977).
3.2. Overall Effects of Action Experience on Alpha and Beta Suppression
Initial time-frequency analyses compared alpha and beta power during action observation before and after experience (Part 1 vs. Part 2) for each Group (Extended Experience, Brief Experience, and Semantic Information), regardless of the color of the object. This analysis used bootstrap significance testing as implemented in EEGLAB, to test whether participants’ increased experience with the objects influenced suppression during action observation in Part 2. For details of the statistical analysis, refer to Section 2.6.
For the EE group, there was significantly more alpha and beta suppression at six central electrode sites at various time points during observation of the actions in Part 2, compared to observation of the same actions in Part 1 (ps < .007). Similar alpha-range effects were seen at parietal and occipital electrodes, and beta suppression was significantly greater during Part 2 at one frontal electrode, three parietal electrodes, and both occipital electrodes (ps < .007). Figure 2 depicts the distribution of these significant effects for the three groups, along with time-frequency plots for selected electrodes.
For the BE group, there was significantly greater alpha suppression at the left central electrode (C3) during Part 2 compared to Part 1, with this effect being apparent during observation of reaching, grasping, and lifting (p < .007).
For the SI group, analyses showed that at one central electrode and at several frontal and parietal electrodes, there was significantly greater alpha suppression during Part 2 compared to Part 1 at various time points throughout the observation epoch (ps < .007). The SI group also showed significantly greater beta-range suppression at one central electrode and one parietal electrode during Part 2 compared to Part 1.
3.3. Effects of Expected Object Weight on Alpha and Beta Suppression
During Part 1, all participants viewed the reaching/grasping video clips. Analysis of the data from Part 1 tested the predictions that prior to receiving experience with acting upon the objects, there would be no differences in suppression based on expected object weight (heavy vs. light, based on random assignment for each participant) or group. This prediction was confirmed. There were no effects of group or expected weight on alpha or beta suppression at any frontal, central, parietal, or occipital electrode sites during Part 1 of the experiment, for any group (all ps > .007).
Time-frequency analyses of EEG collected during Part 2 of the experiment tested whether suppression in the alpha and beta frequency ranges would be related to the expected weight of the objects, within each of the groups. Any expectation regarding object weight during this portion of the experiment would be based on the color-weight associations formed during the acquisition of experience with the objects. During Part 2, no significant effects were found for expected weight at frontal, parietal, or occipital electrodes for any group (ps > .007). Given this, the results outlined below only concern electrodes within the ROI over the central region.
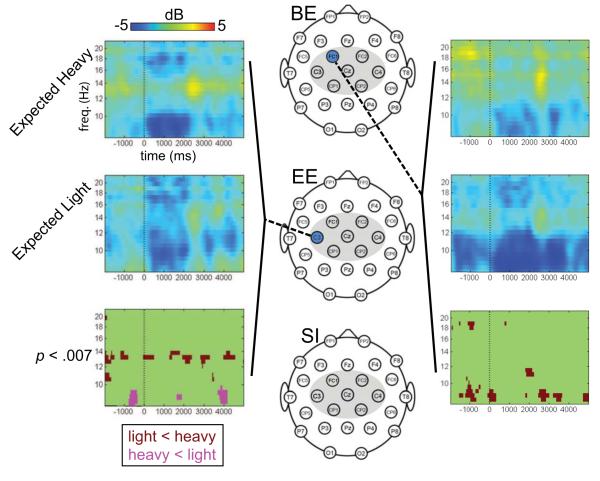
In the extended experience (EE) group, significant differences in suppression between the expected weight conditions were apparent throughout the analysis epoch. The time-frequency analyses indicated that at frequencies above 10 Hz, there was significantly greater suppression at the left central mid-electrode (C3) during observation of trials showing objects expected to be light, compared to objects expected to be heavy (p < .007). In the lower alpha range, between 8-10 Hz, there was a significant effect in the opposite direction—with greater alpha suppression during the observation of trials showing objects expected to be heavy (p < .007). Figure 3 depicts the spatial distribution of these effects as well as associated time-frequency plots.
Figure 3. Differences in alpha and beta suppression between heavy and light objects during Part 2 of the experiment, for each group.
The three scalp maps depict the electrode montage (central ROI highlighted with gray). For each group (Brief Experience, BE; Extended Experience, EE; and Semantic Information, SI), the highlighted electrodes (blue) indicate electrodes at which there were significant differences between conditions (p < .007, FDR) at any time or frequency within the analysis epoch. The epoch spanned from −2000 to +5000 ms (time 0 = start of grasping phase), and frequencies from 8-22 Hz were analyzed. Time-frequency plots and statistical maps are shown for selected electrodes of interest. On these plots, averaged ERSPs from observation of heavy and light trials are shown, as are the significant differences between the conditions. Cool colors indicate a decrease in power relative to baseline; warm colors indicate an increase in power. On the statistical map, dark red indicates that power was lower during observation of light trials compared to heavy trials; pink indicates that power was lower during observation of heavy trials than compared to light trials.
For the brief information (BE) group, there was significantly more alpha and beta suppression throughout the entire observation epoch at the left fronto-central electrode (FC1) during observation of actions directed toward an expected light object compared to an expected heavy object (p < .007). These effects were particularly evident in the 8-10 Hz range.
The semantic information (SI) group showed no significant differences for expected object weight following experience (all ps > .007).
4. Discussion
Prior research suggests that cortical activity during action observation may be modulated by one’s own prior experience with the observed action. However, to this point it has been unclear how much and what kind of experience brings about these changes. In addition, researchers have debated whether more experience with action increases the extent of neural mirroring, or alternatively whether it introduces greater efficiency into the action processing system (e.g., Babiloni et al., 2010). The current study was designed in part to test whether different amounts of sensorimotor experience with acting on certain objects, or the acquisition of semantic knowledge about those objects, would alter the neural oscillations involved in action observation.
The results of the time-frequency analyses comparing Part 1 and Part 2 for each group show that to some extent, all three types of experience (Brief Experience, Extended Experience, and Semantic Information) led to greater alpha and beta suppression during subsequent action observation. These findings support previous work suggesting that action experience leads to greater AON activation (Behmer, Jr. & Jantzen, 2011; Calvo-Merino et al., 2005; Cross et al., 2012; Cross, Kraemer, Hamilton, Kelley, & Grafton, 2008; Haslinger, Erhard, & Altenmüller, 2005; Kim et al., 2011; Marshall, Bouquet, Shipley, & Young, 2009; Orgs et al., 2008). However, it is important to note that much of this prior work was focused on long-term action experience, whereas in the current study, participants only had limited amounts of experience with acting on the specific objects used. Our findings suggest that even this relatively brief amount of exposure to the objects depicted in the videos changed subsequent action processing. More generally, the differences in suppression between Part 1 and Part 2 support the notion of motor-to-visual adaptation (Cattaneo et al., 2011), in that prior experience with carrying out an action, or learning about the sensorimotor consequences of an action, modulates how subsequent actions are perceived.
The increase in alpha and beta suppression was present at a number of electrodes for the extended experience and semantic information groups, while the brief experience group showed this effect only at one central electrode. The indistinct topography of these effects suggests that alpha and beta rhythms during action observation may be quite broadly affected by prior action experience. It is possible that these widespread changes reflect not only the acquisition of knowledge about the sensorimotor consequences of acting upon the objects, but also increased visual experience, familiarity with the objects, and possible changes in attention to the stimuli. In addition, for the semantic information group, the experience of reading about the objects’ weights may have primed the participants toward processing the imagined weights of the objects, as suggested by the semantic priming literature (Bargh, Chen, & Burrows, 1996; Springer & Prinz, 2010).
Overall, our first analysis suggested that gaining knowledge about actions leads to greater suppression of the alpha and beta rhythms during subsequent activation, whether or not the knowledge stems from direct experience with sensorimotor characteristics of the object. The next analysis tested the prediction that sensorimotor experience with objects of different weights would lead to differential mu rhythm suppression during subsequent observation, based on the expected weights of the objects. The most salient finding in support of this prediction is that alpha- and beta-range suppression over the left central electrode (C3) was sensitive to the predicted consequences of acting upon different objects, following sensorimotor experience with those objects. In particular, participants in the extended experience group showed a significant difference in suppression depending on whether the object they were seeing was expected to be heavy or light. Since this effect was significant at C3 only, we suggest that it is specific to the mu rhythm, and that it reflects differential involvement of sensorimotor cortex in processing the actions directed toward objects expected to be heavy or light. These significant differences occurred throughout the alpha frequency range and into the beta range, with the most sustained effect occurring at around 12-14 Hz. This difference was apparent during essentially the entire epoch, from when the object was visible resting on the table to when the hand was retracting away from the object, suggesting that the sensitivity to the sensorimotor consequences of action occurs in anticipation of upcoming action, as well as during the observation of the unfolding action.
Following experience with the objects, the brief experience group showed greater suppression during the observation of expected light versus expected heavy objects at the left fronto-central electrode (FC3). This significant difference was apparent throughout much of the analysis epoch and was present mostly within the 8-10 Hz frequency range, which is lower than the effect reported above for the extended experience group. This difference in frequency-band sensitivity between groups suggests that the brief and extended experience conditions affected different aspects of subsequent action processing, but it remains unknown exactly what those might be. It is also possible that for the participants in the brief experience group, the greater amount of experience with the relatively light-weight distracter objects (the cup and the toy) could have biased these participants toward the lighter of the test objects.
There was no effect of predicted weight during observation in Part 2 for the semantic information (SI) group, meaning that the participants who learned about the objects’ weights by means of written material did not subsequently show any sensitivity in EEG suppression to the anticipated sensorimotor consequences of observed actions. The SI group received relatively rich information concerning the sensorimotor characteristics of the yellow and blue objects—learning, for example, that a blue object weighed three pounds and was filled with concrete, whereas the yellow object weighed one third of a pound and was filled with air. Even with these elaborate descriptions of weight, alpha and beta suppression was not significantly affected during Part 2 for this group. Our results therefore extend the work of Senot et al. (2011) who found that labeling objects as "heavy" and "light" did not affect motor evoked potentials during observation of an actor lifting the objects.
As initially noted we predicted that the observation of an action directed toward a light object would result in greater mu rhythm suppression. One may wonder why, then, the extended experience group showed some significant differences going in the opposite direction (see Figure 3). For instance, in the lower alpha band (8-10 Hz) the observation of action toward an object expected to be heavy was associated with greater suppression. It is possible that this finding reflects greater involvement of sensorimotor cortex during the observation of a more physically demanding action such as lifting a heavy weight (Alaerts, Senot, et al., 2010; Alaerts, Swinnen, & Wenderoth, 2010). At higher frequencies (in the upper alpha and to some extent the beta band) the significant results went in the opposite, and predicted, direction. At these frequencies, there was greater suppression during the observation of actions directed towards objects expected to be lighter. These results in the upper alpha and beta bands are consistent with the results from a previous study (Quandt et al., 2012) of EEG suppression involving the same objects as used in the current study. In that study, both the observation of gesture towards lighter objects and the execution of action on lighter objects were associated with greater alpha and beta suppression.
Increased alpha and beta suppression during the observation of actions upon objects with lighter weights may be due to anticipation of the increased level of motor control needed to lift a light object. Lifting a heavy object (in this case, a relatively heavy weight of around three pounds) requires more brute force, whereas lifting the lighter object may require more delicate control of one’s action, in order to avoid thrusting the object suddenly into the air. This hypothesis is supported by other findings that alpha suppression decreases as weight-bearing load increases (Mizelle et al., 2010), and that this pattern may take a U-shaped form, with greatest suppression for mid-weight objects. Other research also found a non-linear relation between inertial load and EEG measures of sensorimotor activation (Kristeva, Cheyne, Lang, Lindinger, & Deecke, 1990). The results of the current study, in combination with prior research, therefore suggest that the relations between sensorimotor characteristics of observed action and the activity of sensorimotor EEG rhythms are not entirely straightforward. In particular, the current findings suggest that power in different frequency ranges–even within the same overall band–may be differentially sensitive to variations in expected object weight. Future research should continue to explore this topic, perhaps by through parametric variations in object weight.
5. Conclusion
The human sensorimotor cortex is involved in the processing of observed actions, and it has been proposed that the extent of this activation is sensitive to the types of experiences an observer has had with the observed actions. The current study demonstrates two particular ways that prior experiences with actions can change subsequent action processing. First, after receiving experience with actions, suppression of sensorimotor EEG rhythms was increased. Secondly, the analysis of EEG oscillations during action observation following experience showed that brief or extended experience with specific objects is associated with sensitivity to the predicted sensorimotor consequences of observed actions on those objects. Taken together, these results contribute to the current conceptualization of how neural mirroring processes link our experiences and our perceptions.
Changes in EEG were measured during action observation.
Alpha/beta EEG was compared before and after experience with actions.
After sensorimotor experience, alpha/beta rhythms were sensitive to specific characteristics of action.
Learning semantic information about actions did not bring about similar changes.
Acknowledgements
The authors are grateful to Angelique Frazier, Josh Froberg, Melissa Goodwin, Reanna Serafine, Nhi Tran, and Amanda Viands for help with data collection, and to Daniel Appel for assistance with data collection and EEG processing.
Abbreviations
- AON
Action Observation Network
- BE
Brief Experience
- EE
Extended Experience
- ERSP
Event-Related Spectral Perturbation
- SI
Semantic Information
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Mailing address: Center for Cognitive Neuroscience University of Pennsylvania 3720 Walnut Street, Room B51 Philadelphia, PA 19104 434-294-6458
References
- Alaerts K, Senot P, Swinnen S, Craighero L, Wenderoth N, Fadiga L. Force requirements of observed object lifting are encoded by the observer's motor system: a TMS study. European Journal of Neuroscience. 2010;31:1144–1153. doi: 10.1111/j.1460-9568.2010.07124.x. doi: 10.1111/j.1460-9568.2010.07124.x. [DOI] [PubMed] [Google Scholar]
- Alaerts K, Swinnen SP, Wenderoth N. Observing how others lift light or heavy objects: Which visual cues mediate the encoding of muscular force in the primary motor cortex? Neuropsychologia. 2010;48:2082–2090. doi: 10.1016/j.neuropsychologia.2010.03.029. doi: 10.1016/j.neuropsychologia.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. Mu suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. Journal of Neuroscience. 2011;31:14243–14249. doi: 10.1523/JNEUROSCI.0963-11.2011. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Candidi M, Urgesi C. Vicarious motor activation during action perception: Beyond correlational evidence. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00185. doi: 0.3389/fnhum.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargh JA, Chen M, Burrows L. Automaticity of social behavior: Direct effects of trait construct and stereotype activation on action. Journal of personality and social psychology. 1996;71:230. doi: 10.1037//0022-3514.71.2.230. [DOI] [PubMed] [Google Scholar]
- Babiloni C, del Percio C, Rossini PM, Marzano N, Iacoboni M, Infarinato F, Eusebi F. Judgment of actions in experts: A high-resolution EEG study in elite athletes. Neuroimage. 2009;45:512–521. doi: 10.1016/j.neuroimage.2008.11.035. doi: 10.1016/j.neuroimage.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Marzano N, Infarinato F, Iacoboni M, Rizza G, Aschieri P, Del Percio C. "Neural efficiency" of experts' brain during judgment of actions: A high-resolution EEG study in elite and amateur karate athletes. Behavioural Brain Research. 2010;207:466–475. doi: 10.1016/j.bbr.2009.10.034. doi: 10.1016/j.bbr.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Behmer LP, Jr., Jantzen KJ. Reading sheet music facilitates sensorimotor mu-desynchronization in musicians. Clinical Neurophysiology. 2011;122:1342–1347. doi: 10.1016/j.clinph.2010.12.035. doi: 10.1016/j.clinph.2010.12.035. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995:289–300. [Google Scholar]
- Berchio C, Rihs TA, Michel CM, Brunet D, Apicella F, Muratori F, Umiltà MA. Parieto-frontal circuits during observation of hidden and visible motor acts in children. A high-density EEG source imaging study. Brain Topography. 2013 doi: 10.1007/s10548-013-0314-x. advance online publication. doi: 10.1007/s10548-013-0314-x. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser D, Grezes J, Passingham R, Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cerebral Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Cardoso JF. High-order contrasts for independent component analysis. Neural computation. 1999;11:157–192. doi: 10.1162/089976699300016863. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–1167. doi: 10.1016/j.neuroimage.2009.12.112. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Barchiesi G, Tabarelli D, Arfeller C, Sato M, Glenberg AM. One's motor performance predictably modulates the understanding of others' actions through adaptation of premotor visuo-motor neurons. Social Cognitive and Affective Neuroscience. 2011;6:301–310. doi: 10.1093/scan/nsq099. doi: 10.1093/scan/nsq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Cohen N, Hamilton A, Ramsey R, Wolford G, Grafton S. Physical experience leads to enhanced object perception in parietal cortex: Insights from knot tying. Neuropsychologia. 2012;50:3207–3217. doi: 10.1016/j.neuropsychologia.2012.09.028. doi: 10.1016/j.neuropsychologia.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross ES, Kraemer D, Hamilton A, Kelley W, Grafton S. Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex. 2008;19:315–326. doi: 10.1093/cercor/bhn083. doi: 10.1093/cercor/bhn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diersch N, Mueller K, Cross ES, Stadler W, Rieger M, Schütz-Bosbach S. Action prediction in younger versus older adults: Neural correlates of motor familiarity. PloS ONE. 2013;8:e64195. doi: 10.1371/journal.pone.0064195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Cheyne DO. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. Neuroimage. 2006;30:899–908. doi: 10.1016/j.neuroimage.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Wolpert D, Frith U. Your own action influences how you perceive another person's action. Current Biology. 2004;14:493–498. doi: 10.1016/j.cub.2004.03.007. doi: http://dx.doi.org/10.1016/j.cub.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hari R. Action-perception connection and the cortical mu rhythm. Progress in Brain Research. 2006;159:253–260. doi: 10.1016/S0079-6123(06)59017-X. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Altenmüller E. Transmodal sensorimotor networks during action observation in professional pianists. Journal of Cognitive Neuroscience. 2005;17:282–293. doi: 10.1162/0898929053124893. [DOI] [PubMed] [Google Scholar]
- Hecht H, Vogt S, Prinz W. Motor learning enhances perceptual judgment: A case for action-perception transfer. Psychological Research. 2001;65(1):3–14. doi: 10.1007/s004260000043. doi: 10.1007/s004260000043. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS ONE. 2008;3:e3004. doi: 10.1371/journal.pone.0003004. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CE, Oechslin MS, Van De Ville D, Hauert CA, Descloux C, Lazeyras F. Musical training intensity yields opposite effects on grey matter density in cognitive versus sensorimotor networks. Brain Structure and Function. 2014;219:353–366. doi: 10.1007/s00429-013-0504-z. doi: 10.1007/s00429-013-0504-z. [DOI] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Kilner JM. Bias in a common EEG and MEG statistical analysis and how to avoid it. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2013;124:2062–2063. doi: 10.1016/j.clinph.2013.03.024. doi: 10.1016/j.clinph.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cognitive processing. 2007;8:159–166. doi: 10.1007/s10339-007-0170-2. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-T, Seo J-H, Song H-J, Yoo D-S, Lee HJ, Lee J, Chang Y. Neural correlates related to action observation in expert archers. Behavioural Brain Research. 2011;223:342–347. doi: 10.1016/j.bbr.2011.04.053. doi: 10.1016/j.bbr.2011.04.053. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Cheyne DO, Lang W, Lindinger G, Deecke L. Movement-related potentials accompanying unilateral and bilateral finger movements with different inertial loads. Electroencephalography and Clinical Neurophysiology. 1990;75:410–418. doi: 10.1016/0013-4694(90)90086-y. [DOI] [PubMed] [Google Scholar]
- Liew SL, Sheng T, Margetis JL, Aziz-Zadeh L. Both novelty and expertise increase action observation network activity. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00541. doi: 10.3389/fnhum.2013.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. An Introduction to the Event-Related Potential Technique. A Bardford Book, MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Marshall PJ, Bouquet CA, Shipley TF, Young T. Effects of brief imitative experience on EEG desynchronization during action observation. Neuropsychologia. 2009;47:2100–2106. doi: 10.1016/j.neuropsychologia.2009.03.022. doi: 10.1016/j.neuropsychologia.2009.03.022. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR. Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topography. 2000;12:177–186. doi: 10.1023/a:1023437823106. [DOI] [PubMed] [Google Scholar]
- Mizelle JC, Forrester L, Hallett M, Wheaton LA. Electroencephalographic reactivity to unimodal and bimodal visual and proprioceptive demands in sensorimotor integration. Experimental Brain Research. 2010;203:659–670. doi: 10.1007/s00221-010-2273-8. doi: 10.1007/s00221-010-2273-8. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S, Johnson B, McNair N. Mu rhythm modulation during observation of an object-directed grasp. Cognitive Brain Research. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono T, Kimura A, Ushiba J. Daily training with realistic visual feedback improves reproducibility of event-related desynchronisation following hand motor imagery. Clinical Neurophysiology. 2013;124:1779–1786. doi: 10.1016/j.clinph.2013.03.006. doi: 10.1016/j.clinph.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Orgs G, Dombrowski J-H, Heil M, Jansen-Osmann P. Expertise in dance modulates alpha/beta event-related desynchronization during action observation. European Journal of Neuroscience. 2008;27:3380–3384. doi: 10.1111/j.1460-9568.2008.06271.x. doi: 10.1111/j.1460-9568.2008.06271.x. [DOI] [PubMed] [Google Scholar]
- Pineda JA. The functional significance of mu rhythms: translating "seeing" and "hearing" into "doing". Brain Research Reviews. 2005;50:57–68. doi: 10.1016/j.brainresrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Puzzo I, Cooper NR, Cantarella S, Russo R. Measuring the effects of manipulating stimulus presentation time on sensorimotor alpha and low beta reactivity during hand movement observation. Neuroimage. 2011;57:1358–1363. doi: 10.1016/j.neuroimage.2011.05.071. doi: 10.1016/j.neuroimage.2011.05.071. [DOI] [PubMed] [Google Scholar]
- Quandt LC, Marshall PJ, Bouquet CA, Young T, Shipley TF. Experience with novel actions modulates frontal alpha EEG desynchronization. Neuroscience Letters. 2011;499:37–41. doi: 10.1016/j.neulet.2011.05.028. doi: 10.1016/j.neulet.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Quandt LC, Marshall PJ, Bouquet C, Shipley TF. Somatosensory experiences with action modulate alpha and beta power during subsequent action observation. Brain Research. 2013;1534:55–65. doi: 10.1016/j.brainres.2013.08.043. doi: 10.1016/j.brainres.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt LC, Marshall PJ, Shipley TF, Beilock SL, Goldin-Meadow S. Sensitivity of alpha and beta oscillations to sensorimotor characteristics of action: an EEG study of action production and gesture observation. Neuropsychologia. 2012;50:2745–2751. doi: 10.1016/j.neuropsychologia.2012.08.005. doi: 10.1016/j.neuropsychologia.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms' strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping. 2009;30:1168–1187. doi: 10.1002/hbm.20585. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Reviews Neuroscience. 2010;11:264–274. doi: 10.1038/nrn2805. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Schütz-Bosbach S, Prinz W. Perceptual resonance: Action-induced modulation of perception. Trends in cognitive sciences. 2007;11:349–355. doi: 10.1016/j.tics.2007.06.005. doi: http://dx.doi.org/10.1016/j.tics.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Senot P, D’Ausilio A, Franca M, Caselli L, Craighero L, Fadiga L. Effect of weight-related labels on corticospinal excitability during observation of grasping: a TMS study. Experimental Brain Research. 2011;211:161–167. doi: 10.1007/s00221-011-2635-x. doi: 10.1007/s00221-011-2635-x. [DOI] [PubMed] [Google Scholar]
- Springer A, Prinz W. Action semantics modulate action prediction. The Quarterly Journal of Experimental Psychology. 2010;63:2141–2158. doi: 10.1080/17470211003721659. doi: 10.1080/17470211003721659. [DOI] [PubMed] [Google Scholar]
- Streltsova A, Berchio C, Gallese V, Umilta MA. Time course and specificity of sensory-motor alpha modulation during the observation of hand motor acts and gestures: a high density EEG study. Experimental Brain Research. 2010;205:363–373. doi: 10.1007/s00221-010-2371-7. doi: 10.1007/s00221-010-2371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen O, Maris E. Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor alpha- and beta-band oscillations. Journal of Neuroscience. 2011;31:2016–2024. doi: 10.1523/JNEUROSCI.5630-10.2011. doi: 10.1523/JNEUROSCI.5630-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Jensen O, Maris E. Tactile expectation modulates pre-stimulus β-band oscillations in human sensorimotor cortex. Neuroimage. 2010;51:867–876. doi: 10.1016/j.neuroimage.2010.02.053. doi: 10.1016/j.neuroimage.2010.02.053. [DOI] [PubMed] [Google Scholar]
- Vogt S, Buccino G, Wohlschlager A, Canessa N, Shah N, Zilles K, Fink G. Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage. 2007;37:1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]