Abstract
While symptoms of deficient emotional self-regulation (DESR) such as low frustration tolerance, temper outbursts, emotional impulsivity, and mood lability are commonly associated with attention deficit hyperactivity disorder (ADHD), little is known about their nature. The main aim of this post hoc study was to examine the correlates of DESR in a large sample of adults with and without ADHD. Subjects were 206 adults with ADHD and 123 adults without ADHD from a family study of ADHD. Emotional impulsivity was operationalized using items from the Barkley Current Behavior Scale. Subjects were comprehensively assessed for psychiatric comorbidity using structured diagnostic interview methodology. We used the Quality of Life, Enjoyment, and Satisfaction Questionnaire-Short Form (QLES-Q-SF) and Social Adjustment Scale-Self-report (SAS-SR) to assess quality of life and psychosocial functioning. DESR was more common among ADHD compared with non-ADHD adults, and 55 % of adults with ADHD reported extreme DESR of greater severity than 95 % of control subjects. The association of ADHD and DESR was not entirely accounted for by either current or lifetime comorbid disorders. DESR was also associated with significant functional impairment as evaluated by the QLES-Q-SF and SAS-SR, and with reduced marital status, as well as higher risk for traffic accidents and arrests. DESR adversely impacts quality of life in adults with ADHD. More work is needed to further evaluate DESR in clinical and investigational studies of subjects with ADHD.
Keywords: Attention deficit hyperactivity disorder, Emotion, Comorbidity, Adult
Introduction
Attention deficit hyperactivity disorder (ADHD) is a prevalent neurobiological disorder estimated to affect up to 10 % of children and 5 % of adults worldwide (Faraone and Biederman 2005; Kessler et al. 2006; Faraone et al. 2006a). It is associated with high levels of morbidity and disability across the lifecycle (Biederman and Faraone 2005). Although conceptualized as a disorder of deficient self-regulation (Barkley 1997a), current conceptualizations of the disorder have focused on cognitive (attention) and behavioral (hyperactivity/impulsivity) aspects of deficient self-regulation.
While symptoms of deficient emotional self-regulation (DESR) have been long associated with ADHD, there has been limited investigation into this aspect of the clinical picture of the disorder. The early concept of minimal brain dysfunction included emotional symptoms as a central aspect of the disorder (Barkley 2006). Dr. Paul Wender highlighted the association between ADHD and affective lability as well as stress intolerance, and the Utah Criteria for ADHD include temper, mood instability, and emotional overreactivity as core features of ADHD (Wender 1995). Conceptions of ADHD represented by the Brown Adult ADHD rating scale (Brown 1995) and Conners’ Adult ADHD rating scale (Conners et al. 1999) also include emotional symptoms. Since 1968 versions of the DSM have included deficits in emotional regulation as associated symptoms of ADHD but not as core features (Barkley 2006; American Psychiatric Association 1968, 1987, 2000). In DSM-IV-TR, emotional symptoms associated with ADHD include “low frustration tolerance,” “temper outbursts,” and “mood lability” (American Psychiatric Association 2000). Although these traits may be a source of significant morbidity, there has been little research on the subject.
Barkley (1997a) proposed that DESR traits should be considered a core feature of ADHD rather than associated symptoms. This would be consistent with conceptualizations of ADHD as a disorder of self-regulation of affect, as well as of attention, motivation, and arousal (Nigg 2005). Barkley et al. (2008) reported on the prevalence of such traits in adults with ADHD presenting to an ADHD clinic. They found that impatience, being quick to anger, being easily frustrated, overreacting emotionally, and being easily excited by nearby activities were each endorsed by over 60 % of adults with ADHD and less than 15 % of control subjects. Similar findings have been identified in one-third of adults with ADHD participating in registration trials for atomoxetine (Reimherr et al. 2005), and over half of adults in a smaller clinical trial of OROS methylphenidate (Reimherr et al. 2007). These trials, and a large double-blind trial of OROS methylphenidate, suggest that emotional dysregulation symptoms are sensitive to treatment (Rosler et al. 2010). ADHD presenting with DESR may represent a subtype of ADHD, based on our recent analysis of familial inheritance of DESR in adults with ADHD (Surman et al. 2011).
However, because ADHD in adults is associated with high rates of comorbid psychiatric disorders that are also associated with DESR, it is unclear whether symptoms of DESR in subjects with ADHD are manifestations of psychiatric comorbidity or ADHD itself. While data from the two clinical trials of adults with ADHD suggested that symptoms of DESR frequently occur in ADHD subjects without current major DSM-IV Axis I comorbidity (Reimherr et al. 2005, 2007), these findings may not extrapolate to adults with ADHD in the community, who have higher rates of comorbidity than those in clinical trials (Surman et al. 2010).
To further characterize the frequency and clinical significance of DESR in adults with ADHD, we analyzed data from a large study of well-characterized community adults with and without ADHD. We hypothesized that symptoms of DESR would be overrepresented in adults with ADHD and that their presence will be associated with higher levels of morbidity.
Methods
Participants
We conducted a secondary analysis of a study of subjects between 18 and 55 years of age, of both sexes, ascertained to systematically characterize adults with ADHD. We have previously reported several studies from this sample, including analyses related to age of onset criteria in adults (Faraone et al. 2006b, c, 2007, 2009). Subjects with major sensorimotor handicaps, psychosis, inadequate facility with English, or a full-scale intelligence quotient (IQ) less than 80 were excluded (as measured by the IQ estimated from the block design and vocabulary subtests of the Wechsler Adult Intelligence Scales—Revised). Subjects with and without ADHD were recruited via advertisements in the greater Boston area. Subjects with ADHD were also ascertained from referrals to psychiatric clinics at the Massachusetts General Hospital (MGH). After receiving thorough explanations of all study procedures, subjects provided written informed consent under procedures approved by the Institutional Review Board of Massachusetts General Hospital. All procedures were therefore performed in accordance with the ethical standards established by the Declaration of Helsinki in 1964. Subjects were compensated up to $100 for participation.
Based on our previous work, we considered a subject to have ADHD if the subject met full DSM-IV criteria for the disorder (N = 127) as well as subjects with late-onset ADHD [subjects meeting full DSM-IV criteria for ADHD except for the age at onset criterion (N = 79)]. We previously reported the demographic features of the study sample, demonstrating that full- and late-onset ADHD groups have similar clinical correlates, including patterns of Axis I comorbidity, personality traits, and neuropsychological deficits (Faraone et al. 2006b, 2007, 2009). Controls were subjects without full- or subthreshold ADHD (N = 123), where subthreshold ADHD was defined as a chronic history of three or more inattentive- and/or impulsive-hyperactive symptoms. Individuals with other mental health conditions other than full- or subthreshold ADHD were included in the control group. DESR symptom data were available from 206 adults in the ADHD group and 123 adults in the non-ADHD group.
Assessments
Subjects were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al. 1997) supplemented with modules from the Kiddie Schedule for Affective Disorder and Schizophrenia for School-Age Children-Epidemiologic Version (K-SADS-E) (Orvaschel 1994) to assess ADHD and other childhood disorders. We trained research assistants with undergraduate degrees to conduct direct interviews with subjects (Faraone et al. 2006d). When we asked questions about disorders typically recognized in childhood such as ADHD, oppositional defiant disorder, and conduct disorder, the subjects were first queried about childhood occurrence of K-SADS-E defined symptoms, and if they were present, they were asked about continuation of these symptoms into adulthood and the emergence of others.
The interviewers were blind to the subject’s ascertainment group and all prior assessments. The interviewers had undergraduate degrees in psychology and were extensively trained. First, they underwent several weeks of classroom style training, learning interview mechanics, diagnostic criteria, and coding algorithms. Then, they observed interviews by experienced raters and clinicians. They subsequently conducted at least six practice (non-study) interviews and at least three study interviews while being observed by senior interviewers. Trainees were not permitted to conduct interviews independently until they executed at least three interviews with perfect diagnostic agreement with an observing senior interviewer. Joseph Biederman, MD, supervised the interviewers. We computed kappa coefficients of agreement by having experienced, board-certified child and adult psychiatrists and licensed clinical psychologists diagnose subjects from audio-taped interviews. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was 0.98. Kappa coefficients for individual diagnoses included: ADHD (0.88), conduct disorder (1.0), major depression (1.0), mania (0.95), separation anxiety (1.0), agoraphobia (1.0), panic (0.95), substance use disorder (1.0), and tics/Tourette’s (0.89).
We considered a disorder positive if DSM-IV diagnostic criteria were unequivocally met on review of collected interview information. Interviewers assessed degree of impairment as minimal, moderate, or severe. We analyzed major depression only if the depressive episode was associated with severe impairment, in order to avoid false-positive diagnoses. This is consistent with prior research methods (Gershon et al. 1982; Leckman et al. 1982).
A committee of board-certified child and adult psychiatrists, who were blind to the subject’s ADHD status, referral source, and all other data, resolved diagnostic uncertainties. Diagnoses at review were considered positive when the committee determined that diagnostic criteria were met to a clinically meaningful degree. All diagnoses were recorded that an individual met criteria for, independent of whether they met criteria for other diagnoses. We estimated kappa coefficients of agreement for the clinician diagnosis review process. For these diagnoses, median reliability between individual clinicians and the review committee assigned diagnoses was 0.87. Kappa coefficients for individual diagnoses included: ADHD (1.0), conduct disorder (1.0), major depression (1.0), bipolar (0.78), separation anxiety (0.89), agoraphobia (0.80), panic (0.77), substance use disorder (1.0), and tics/Tourette’s (0.68).
We have previously published on rates of comorbidity in the study sample. Rates of lifetime comorbidity as defined by diagnostic assessment measures described above among adults with ADHD included: bipolar disorder (19 %), major depression (31 %), more than one anxiety disorder (35 %), alcohol dependence (28 %), substance dependence (22 %), smoking dependence (35 %), and antisocial personality (12 %) (Surman et al. 2010).
Deficient emotional self-regulation assessment
We used 8 items from the self-report Current Behavior Scale developed by Barkley (1997a, b) to assess DESR (Barkley 1997b). We chose those items designated by Barkley (personal communication) as measuring DESR. The scale asks subjects to describe their behavior in the prior 6 months. Responses to each item on this scale range from 0 (Never or Rarely) to 3 (Very Often). The DESR items are listed in Table 1.
Table 1.
Deficient emotional self-regulation (DESR) items and Cronbach’s alpha analyses
| Cronbach’s alpha if item deleted | Correlation between item and overall alpha | Correlation between item and other items | |
|---|---|---|---|
| 1. Quick to get angry or become upset | 0.8776 | 0.8760 | 0.8243 |
| 2. Easily frustrated | 0.8762 | 0.8849 | 0.8343 |
| 3. Overreact emotionally | 0.8812 | 0.8527 | 0.7928 |
| 4. Easily excited by activities going on around me | 0.9084 | 0.5951 | 0.4782 |
| 5. Lose my temper | 0.9052 | 0.6345 | 0.5226 |
| 6. Argue with others | 0.8961 | 0.7119 | 0.6315 |
| 7. Am touchy or easily annoyed by others | 0.8863 | 0.8079 | 0.7084 |
| 8. Am angry or resentful | 0.8890 | 0.7848 | 0.8243 |
Functional assessment
The Quality of Life, Enjoyment, and Satisfaction Questionnaire-Short Form (QLES-Q-SF) (Endicott et al. 1993) is an extensively used questionnaire that assesses the quality of life in several domains. Our research group previously described evidence for the validity of the QLES-Q-SF as a measure quality of life associated with ADHD (Mick et al. 2008).
The Social Adjustment Scale-Self-report (SAS-SR) (Weissman 1995) is an extensively applied 54-question scale that assesses adaptation to and satisfaction with adult social roles. The scale evaluates six major areas of functioning for a subject, over the past 2-week period: work (capturing function related to work as an employee, housewife, or student), social and leisure activities, relationship with extended family, intimate relationship, parental role, and role within the family unit. Within each role, questions assess performance at role tasks, friction with others, other aspects of interpersonal relationships, as well as feelings about and satisfaction with the role. T scores higher than 65 indicate significant impairment.
Statistical methods
The internal consistency reliability of the eight DESR items from the Barkley CBS scale was assessed using Cronbach’s alpha statistic. Associations between DESR symptoms and symptoms of ADHD were assessed with the Pearson’s correlation coefficient. All other analyses were conducted with Stata 10 using Poisson regression models with the DESR score as the dependent variable. All tests were two tailed, and statistical significance was defined at the 5 % level.
Results
Table 2 shows that the three comparison groups were significantly different in age and gender. Because subjects without ADHD or DESR were significantly younger than the two ADHD groups, subsequent analyses were corrected for group differences in age. There was a lower percentage of men in the ADHD group with DESR than in the ADHD group without DESR. There was no significant difference in age of onset between the two ADHD groups.
Table 2.
Demographic and DESR scale response features
| No ADHD No DESR (N = 119) Mean ± SD |
ADHD No DESR (N = 93) Mean ± SD |
ADHD With DESR (N = 113) Mean ± SD |
Test statistic | Omnibus p value | ||
|---|---|---|---|---|---|---|
| Age | 29.8 ± 8.8 | 35.3 ± 10.8a*** | 37.0 ± 10.7a*** | F(2, 332) = 16.28 | < 0.001 | |
| Age of onset | – | 6.5 ± 5.4 | 6.7 ± 4.5 | F(1, 202) = 0.11 | 0.74 | |
|
| ||||||
| N (%) | N (%) | N (%) | ||||
|
| ||||||
| Number of males | 55 (46) | 55 (59) | 50 (44)b* |
|
0.07 | |
|
| ||||||
| Mean ± SD | Mean ± SD | Mean ± SD | ||||
|
| ||||||
| DESR scale total score | 2.7 ± 2.2 | 6.2 ± 2.5 | 14.7 ± 4.1 | |||
|
| ||||||
| Minimum, maximum | Minimum, maximum | Minimum, maximum | ||||
|
| ||||||
| DESR scale total score | 0, 9 | 0, 9 | 10, 24 | |||
For pairwise comparisons:
versus No ADHD, No DESR;
versus ADHD, No DESR
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Deficient emotional self-regulation
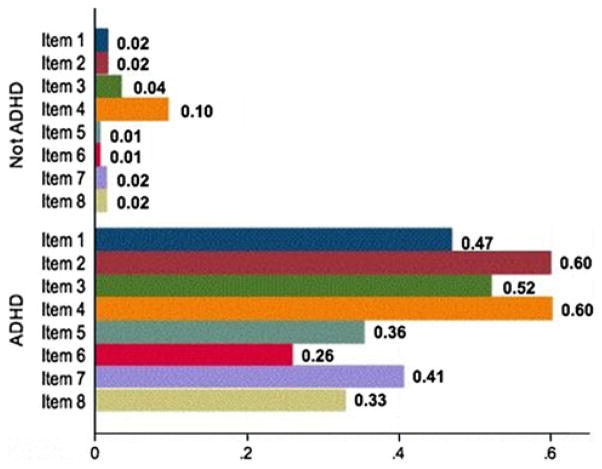
Cronbach’s alpha is a standard psychometric method for assessing the internal consistency of a set of items, that is, the degree to which items measure a unitary construct. If the items we chose to measure DESR measure a unitary construct, alpha should be significant and high (e.g., greater than 0.80). Cronbach’s alpha for the eight scale items was 0.90 in the entire study sample (ADHD and non-ADHD subjects) indicating high internal consistency for these items. We calculated total scores for each subject from answers to individual scale items. The mean total DESR scale score was significantly higher in ADHD subjects than in non-ADHD subjects (11.5 ± 5.48 vs. 2.68 ± 2.23; z = 23.51, p<0.001). Subjects with ADHD reported significantly more frequent occurrences of every item of DESR than non-ADHD subjects (p<0.001 for each item). As shown in Fig. 1, more ADHD than non-ADHD subjects reported that DESR items occurred “often” or “very often.” The portion of subjects with ADHD that endorsed DESR scale items at the rate of “very often” ranged between 9.2 % (for “argue with others”) and 31 % (for “easily frustrated”). In contrast, only two subjects (1.8 %) without ADHD reported an item at the rate of “very often”; both endorsed being very often “easily excited by activities going on around me.”
Fig. 1.
Proportion of subjects reporting DESR items occurring often or very often
Among the ADHD subjects, the DESR score was associated with severity of current ADHD symptoms with a correlation of 0.70 (p<0.001). We defined an extreme DESR total score as the score that exceeded the 95th percentile of DESR scores among controls. Scores of 9 and above exceeded this level, which occurred in 54.9 % of subjects with ADHD and 3.3 % of subjects without ADHD.
DESR and psychiatric comorbidity
In the overall sample (ADHD and non-ADHD subjects), all past (lifetime) comorbid conditions were associated with higher total DESR scale scores. When we included past comorbid disorders and ADHD as predictors of DESR in the same regression model, we found a significant effect of both ADHD and all lifetime comorbid disorders, except bipolar disorder, substance abuse, and alcohol dependence. In the total sample (ADHD and non-ADHD), all current comorbid conditions except alcohol abuse and substance dependence were associated with higher total DESR scores. When current comorbid disorders and ADHD were both included as predictors of DESR in the same regression model, we found a significant effect of both ADHD and all current comorbid disorders, except for bipolar disorder, alcohol abuse, substance abuse, and substance dependence.
As shown in Fig. 2 and Table 3, among the ADHD probands, individuals with higher numbers of current comorbid conditions tended to have higher DESR scores.
Fig. 2.
Mean DESR score at each level of current comorbid disorders
Table 3.
DESR score in ADHD subjects at each level of comorbidity
| Number of current comorbid mental health conditions | Mean | SD |
|---|---|---|
| 0 | 5.9 | 4.7 |
| 1 | 8.9 | 6.0 |
| 2 | 11.2 | 6.2 |
| 3 | 15.2 | 5.7 |
| 4 | 16.7 | 5.2 |
| 5 | 16.6 | 4.8 |
| 6 | 18.7 | 2.1 |
Among ADHD probands, past oppositional defiant disorder (ODD) was significantly associated (p<0.01) with extreme (>95th percentile of control subjects) DESR scores, and current ODD was not; 71.4 % of ADHD probands with current ODD, and 69.4 % of ADHD probands with past ODD had extreme DESR scores. However, high percentages of ADHD probands not meeting criteria for ODD had extreme DESR scores (53.0 % for those with no current ODD, and 47.0 % for those with no past ODD).
DESR and quality of life impairment
Among the ADHD subjects, individuals with extreme DESR symptoms had lower quality of life reports on all 16 individual items from the QLES-Q-SF, except vision and medication (see Table 4), and a lower proportion of the maximum possible total score of the QLES-Q-SF items. Adults with ADHD with extreme DESR also reported significantly worse social adjustment as reflected in significantly higher mean T scores on individual SAS functional domains except for work and parenting, and higher total scale mean T score (see Table 5).
Table 4.
Quality of Life, Enjoyment and Satisfaction Questionnaire-Short Form scores in adults with ADHD
| Extreme DESR Mean ± SD |
No DESR Mean ± SD |
p | |
|---|---|---|---|
| 1. Physical health | 3.6 ± 1.0 | 4.1 ± 0.8 | < 0.01 |
| 2. Mood | 2.9 ± 1.0 | 3.8 ± 0.9 | < 0.001 |
| 3. Work | 2.9 ± 1.1 | 3.6 ± 1.0 | < 0.05 |
| 4. Household activities | 2.8 ± 1.1 | 3.6 ± 0.9 | < 0.001 |
| 5. Social relationships | 3.0 ± 1.2 | 3.8 ± 0.9 | < 0.01 |
| 6. Family relationships | 3.2 ± 1.1 | 3.9 ± 0.9 | < 0.01 |
| 7. Leisure time activities | 2.9 ± 1.2 | 3.7 ± 0.9 | < 0.001 |
| 8. Ability to function in daily life | 3.2 ± 1.0 | 4.2 ± 0.8 | < 0.001 |
| 9. Sexual drive, interest, and/ or performance | 2.9 ± 1.3 | 3.8 ± 1.0 | < 0.001 |
| 10. Economic status | 2.5 ± 1.3 | 3.3 ± 0.9 | < 0.01 |
| 11. Living or housing situation | 3.2 ± 1.2 | 3.9 ± 0.9 | < 0.001 |
| 12. Ability to get around without feeling dizzy or falling | 4.4 ± 0.8 | 4.8 ± 0.5 | < 0.01 |
| 13. Vision in terms of ability to do work or hobbies | 3.9 ± 1.0 | 4.3 ± 0.7 | 0.1 |
| 14. Overall sense of well- being | 3.2 ± 1.0 | 4.1 ± 0.8 | < 0.001 |
| 15. Medication | 3.5 ± 0.9 | 4.0 ± 0.8 | 0.06 |
| 16. Overall life satisfaction | 2.9 ± 1.1 | 3.9 ± 0.8 | < 0.001 |
| Proportion maximum total score for items 1 through | 14 0.6 ± 0.1 | 0.8 ± 0.1 | < 0.001 |
Extreme DESR = total DESR scale score >95th percentile of control subjects
Table 5.
Social Adjustment Scale-Self-report mean T scores in adults with ADHD
| Functioning domain | Extreme DESR | No DESR | p |
|---|---|---|---|
| 1. Work | 69.3 | 56.1 | 0.05 |
| 2. Social/leisure | 62.7 | 51.3 | < 0.001 |
| 3. Extended family | 75.8 | 62.0 | < 0.01 |
| 4. Primary relationship | 65.1 | 54.1 | < 0.05 |
| 5. Parenting | 57.1 | 57.9 | 0.9 |
| 6. Family unit | 69.3 | 56.3 | < 0.01 |
| 7. Total SAS scale score | 72.0 | 56.1 | < 0.001 |
Extreme DESR = total DESR scale score >95th percentile of control subjects for ADHD probands only
ADHD subjects with extreme DESR were more likely to have never been married or to be divorced than ADHD subjects without extreme DESR (p<0.05). Rates of marriage and divorce in ADHD subjects were 22.5 and 20.7 %, respectively, for subjects with extreme DESR, and 32.6 and 9.0 %, respectively, for subjects without extreme DESR.
ADHD subjects with extreme DESR had higher risk for traffic accidents (z = 2.15, p<0.05) and being arrested (z = 2.31, p<0.05) than ADHD subjects without DESR. The ADHD subjects with extreme DESR and those without extreme DESR were similar in their history of traffic tickets (z = −0.62, p = 0.54), number of arrests (z = 1.71, p = 0.09), having been convicted (z = −0.15, p = 0.88), number of convictions (z = 1.49, p = 0.14), having been imprisoned (z = −0.62, p = 0.53), number of times in prison (z = 0.58, p = 0.56), and duration of time in prison (z = −0.69, p = 0.49).
Discussion
DESR as defined in this study was overrepresented in subjects with ADHD, as defined by early or late onset of DSM-IV symptoms, when compared with controls. In fact, 61 % of adults with ADHD reported extreme DESR of greater severity than 95 % of control subjects. DESR was associated with ADHD and with many of the comorbid psychiatric disorders seen among ADHD patients, but the association of ADHD and DESR was not entirely accounted for by either current or lifetime comorbid disorders. As Fig. 2 shows, we found a dose–response effect such that an increasing number of current comorbid disorders were associated with increasing DESR. DESR was also associated with significantly lower quality of life in all but the vision and medication domains evaluated by the QLES-Q-SF, significantly worse social adjustment in all but the work and parenting domains of the SAS-SR, reduced marital status, and higher risk for traffic accidents and arrests. These findings suggest that DESR may represent a burdensome source of morbidity in ADHD subjects that is partially independent of comorbid disorders and worthy of further investigation.
Our results are consistent with the work of Barkley et al. (2008) and with data from two clinical trial studies (Reimherr et al. 2005, 2007) showing that DESR is highly prevalent among ADHD adults. Because our community sample had high rates of comorbidity, it is well suited to identify the contribution of comorbidity to DESR in adults with ADHD. Although we have shown that comorbid disorders cannot fully account for symptoms of DESR among adults with ADHD, many of these disorders were associated with DESR. Similarly, DESR was correlated with mood or anxiety distress and measures of social impairment in the clinical trials that captured DESR data (Reimherr et al. 2005, 2007). These findings are consistent with the idea that DESR is an emotional expression of the ADHD patient’s difficulties with self-regulation. However, more work is needed to disentangle and differentiate the symptoms and functional impact of DESR from comorbid disorders.
We found DESR in adults with ADHD to be significantly associated with lower quality of life, worse social adjustment, and elevated traffic accidents and arrests, suggesting that DESR may be an important aspect of the clinical picture of ADHD that is important to identify and remedy. It is notable that the majority of items contributing to our measure of DESR refer to expressions of “temper.” DESR is also a feature of ODD, which is also prevalent among adults with ADHD. Because there is overlap between half of the items included in our definition of DESR and symptoms of DSM-IV ODD, we expected that the DESR scale would be sensitive to cases of current ODD. Although we found that ODD was associated with extreme DESR among adults with ADHD, ADHD adults who did not met criteria for ODD (59 % for those without current ODD, 53 % for those without past ODD) also had extreme DESR symptoms. In fact, among subjects without lifetime ODD, extreme DESR was more common among ADHD than control subjects. This suggests that DESR overlaps with ODD, but that identifying ODD in adults with ADHD is not sufficient to identify DESR.
DESR was strongly associated with lower quality of life as assessed by the QLES-Q-SF and SAS-SR, suggesting such symptoms are an important focus of clinical intervention. There is preliminary evidence that DESR may be responsive to treatment with atomoxetine (Reimherr et al. 2005) and OROS methylphenidate (Reimherr et al. 2007) pharmacotherapies administered to adults with ADHD, suggesting that appropriate interventions for DESR can be developed. Studies of psychosocial treatments should also determine whether such treatments reduce symptoms of DESR.
There are limitations to our study. Our sample was primarily Caucasian and, thus, may not generalize to other ethnic groups. In assessing the association of DESR with comorbidity, we did not account for subthreshold comorbidity conditions. However, we expect that current subthreshold conditions would have put subjects at high risk for lifetime comorbidity, for which we did account. Because similar language may describe emotional symptoms due to DESR and due to other mental health conditions, it is possible that emotional comorbidity is exaggerated by the presence of DESR in our population. We cannot identify from our data whether DESR should be considered as co-occurring with ADHD or a result of ADHD. Future research using a comparison group with mental health distress for comparison could further clarify the extent to which DESR is specifically associated with ADHD versus non-specifically associated with psychiatric illness. Identification of DESR might be enhanced in future studies with use of third-party reports of DESR symptoms. Although our analyses demonstrate internal consistency of the items that we chose as a measure of DESR and suggest that DESR as identified by these items has external validity because they are associated with greater impairment on measures of functioning, further study could clarify the validity of the 8-item scale we utilized to identify DESR. Finally, our statistical analyses are not adjusted for the number of analyses that we previously conducted on the data set for prior publications examining different issues.
Despite these considerations, we identified a robust association between DESR and ADHD as identified based on early or late onset of DSM-IV symptoms in a large sample of adults that appears to be partially independent of psychiatric comorbidity, and correlated with impaired quality of life. Our work is consistent with prior related work, suggesting that the differential diagnosis of impairing emotional expression includes DESR associated with ADHD, and that DESR is an important target for treatment in adults with ADHD.
Acknowledgments
Dr. Surman has received research support from Abbott, Alza, Cephalon, Eli Lilly, the Hilda and Preston Davis Foundation, McNeil, Merck, New River, National Institutes of Health, Organon, Pamlab, Pfizer, Shire, and Takeda; has been a speaker for Janssen-Ortho, McNeil, Novartis, and Shire; and has been a consultant/advisor for McNeil, Nutricia, Shire, Somaxon, and Takeda. Dr. Surman has also received honoraria from Reed Medical Education (a logistics collaborator for the MGH Psychiatry Academy). Commercial entities supporting the MGH Psychiatry Academy are listed on the Academy’s website www.mghcme.org. Dr. Surman receives royalties for books published by Penguin (FAST MINDS: How To Thrive If You Have ADHD (Or Think You Might)) and Springer (A Practical Guide To The Assessment And Management of ADHD in Adults). Dr. Joseph Biederman is currently receiving research support from the following sources: Elminda, Janssen, McNeil, and Shire. In 2012, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy and The Children’s Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded CME courses. In 2011, Dr. Joseph Biederman gave a single unpaid talk for Juste Pharmaceutical Spain, received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course, and received honoraria for presenting at international scientific conference on ADHD. He also received an honorarium from Cambridge University Press for a chapter publication. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Eli Lilly, Shire and AstraZeneca; these royalties are paid to the Department of Psychiatry at MGH. In 2010, Dr. Joseph Biederman received a speaker’s fee from a single talk given at Fundación Dr. Manuel Camelo A.C. in Monterrey Mexico. Dr. Biederman provided single consultations for Shionogi Pharma Inc. and Cipher Pharmaceuticals Inc.; the honoraria for these consultations were paid to the Department of Psychiatry at the MGH. Dr. Biederman received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, Astra-Zeneca, Boston University, Bristol Myers Squibb, Celltech, Cephalon, Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Glaxo, Gliatech, Hastings Center, Janssen, McNeil, Medice Pharmaceuticals (Germany), Merck, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth. Dr. Thomas Spencer has received research support from, has been a speaker for or on a speaker bureau or has been an Advisor or on an Advisory Board of the following sources: Alcobra, Shire Laboratories, Inc, Eli Lilly & Company, Glaxo-Smith Kline, Janssen Pharmaceutical, McNeil Pharmaceutical, Novartis Pharmaceuticals, Cephalon, Pfizer, the National Institute of Mental Health and the Department of Defense. Dr. Spencer receives research support from Royalties and Licensing fees on copyrighted ADHD scales through MGH Corporate Sponsored Research and Licensing. Dr. Spencer has a US Patent Application pending Provisional Number (61/233,686), through MGH corporate licensing, on a method to prevent stimulant abuse. In the past year, Dr. Faraone received consulting income and/or research support from Shire, Otsuka and Alcobra and research support from the National Institutes of Health (NIH). He is also on the Clinical Advisory Board for Akili Interactive Labs. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk About Your Child’s Mental Health and Oxford University Press: Schizophrenia: The Facts. Ms. Carolyn Miller and Ms. Katie McDermott report no competing interests. Shire Pharmaceuticals provided support for this analysis. This work was supported in part by grants to S. V. Faraone from the National Institute of Health (R01MH57934). We are grateful to Dr. Russell Barkley for generously allowing us to use his questionnaire from NIMH Grant 1R01MH054509-01A2.
Contributor Information
Craig B. H. Surman, Email: csurman@partners.org, Clinical and Research Program in Pediatric Psychopharmacology and Adult ADHD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, Boston, MA, USA
Joseph Biederman, Clinical and Research Program in Pediatric Psychopharmacology and Adult ADHD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, Boston, MA, USA.
Thomas Spencer, Email: sfaraone@childpsychresearch.org, Clinical and Research Program in Pediatric Psychopharmacology and Adult ADHD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, Boston, MA, USA.
Carolyn A. Miller, Clinical and Research Program in Pediatric Psychopharmacology and Adult ADHD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, Boston, MA, USA
Katie M. McDermott, Clinical and Research Program in Pediatric Psychopharmacology and Adult ADHD, Pediatric Psychopharmacology Unit, Massachusetts General Hospital, Boston, MA, USA
Stephen V. Faraone, Department of Psychiatry and Behavioral Sciences, SUNY Upstate Medical University, 750 East Adams St, Syracuse, NY 13210, USA. Department of Neuroscience and Physiology, SUNY Upstate Medical University, Syracuse, NY, USA
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2. American Psychiatric Association; Washington: 1968. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. American Psychiatric Association; Washington, DC: 1987. Revised ed. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington: 2000. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997a;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley R. ADHD in adults: comorbidity and adaptive impairments. NIMH; 1997b. pp. Grant number1R01MH054509-01A2. [Google Scholar]
- Barkley RA. Attention deficit-hyperactivity disorder: a handbook for diagnosis and treatment. 3. Guilford Press; New York: 2006. [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: what the science says. Guilford Press; New York: 2008. [Google Scholar]
- Biederman J, Faraone SV. Attention deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Brown T. Brown attention deficit disorder scales. The Psychological Corporation; San Antonio: 1995. [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Conners’ adult ADHD rating scales (CAARS) Multi-Health Systems; North Tonawanda: 1999. [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- Faraone SV, Biederman J. What is the prevalence of adult ADHD? Results of a population screen of 966 adults. J Atten Disord. 2005;9:384–391. doi: 10.1177/1087054705281478. [DOI] [PubMed] [Google Scholar]
- Faraone S, Biederman J, Mick E. The age dependent decline of attention-deficit/hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006a;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Doyle A, Murray K, Petty C, Adamson JJ, Seidman L. Neuropsychological studies of late onset and subthreshold diagnoses of adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006b;60:1081–1087. doi: 10.1016/j.biopsych.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer TJ, Mick E, Murray K, Petty C, Adamson JJ, Monuteaux MC. Diagnosing adult attention deficit hyperactivity disorder: are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006c;163:1720–1729. doi: 10.1176/appi.ajp.163.10.1720. quiz 1859. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Wilens TE, Petty C, Antshel K, Spencer T, Biederman J. Substance use among ADHD adults: implications of late onset and subthreshold diagnoses. Am J Addict. 2007;16(Suppl 1):24–32. doi: 10.1080/10550490601082767. quiz 33–34. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Kunwar A, Adamson J, Biederman J. Personality traits among ADHD adults: implications of late-onset and subthreshold diagnoses. Psychol Med. 2009;39:685–693. doi: 10.1017/S0033291708003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Diagnostic and statistical manual of mental disorders. American Psychiatric Press; Washington: 1997. [Google Scholar]
- Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Targum SD, Nurnberger JI, Jr, Goldin LR, Bunney WE., Jr A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry. 1982;39:1157–1167. doi: 10.1001/archpsyc.1982.04290100031006. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the national comorbidity survey replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson D, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Mick E, Spencer T, Faraone SV, Biederman J, Zhang HF. Assessing the validity of the quality of life enjoyment and satisfaction questionnaire short form in adults with ADHD. J Atten Disord. 2008;11(4):504–509. doi: 10.1177/1087054707308468. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424– 1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for affective disorder and schizophrenia for school-age children epidemiologic version. Nova Southeastern University, Center for Psychological Studies; Ft. Lauderdale: 1994. [Google Scholar]
- Reimherr FW, Marchant BK, Strong RE, Hedges DW, Adler L, Spencer TJ, West SA, Soni P. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry. 2005;58:125–131. doi: 10.1016/j.biopsych.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Reimherr FW, Williams ED, Strong RE, Mestas R, Soni P, Marchant BK. A double-blind, placebo-controlled, crossover study of osmotic release oral system methylphenidate in adults with ADHD with assessment of oppositional and emotional dimensions of the disorder. J Clin Psychiatry. 2007;68:93–101. doi: 10.4088/jcp.v68n0113. [DOI] [PubMed] [Google Scholar]
- Rosler M, Retz W, Fischer R, Ose C, Alm B, Deckert J, Philipsen A, Herpertz S, Ammer R. Twenty-four-week treatment with extended release methylphenidate improves emotional symptoms in adult ADHD. World J Biol Psychiatry. 2010;11:709–718. doi: 10.3109/15622971003624197. [DOI] [PubMed] [Google Scholar]
- Surman CB, Biederman J, Spencer T, Yorks D, Miller CA, Petty CR, Faraone SV. Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. Am J Psychiatry. 2011;168:617–623. doi: 10.1176/appi.ajp.2010.10081172. [DOI] [PubMed] [Google Scholar]
- Surman CB, Monuteaux MC, Petty CR, Faraone SV, Spencer TJ, Chu NF, Biederman J. Representativeness of participants in a clinical trial for attention-deficit/hyperactivity disorder? Comparison with adults from a large observational study. J Clin Psychiatry. 2010;71:1612–1616. doi: 10.4088/JCP.09m05344pur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman M. Social adjustment scale. 1995. [Google Scholar]
- Wender PH. Attention-deficit hyperactivity disorder in adults. Oxford University Press; New York: 1995. [Google Scholar]