Abstract
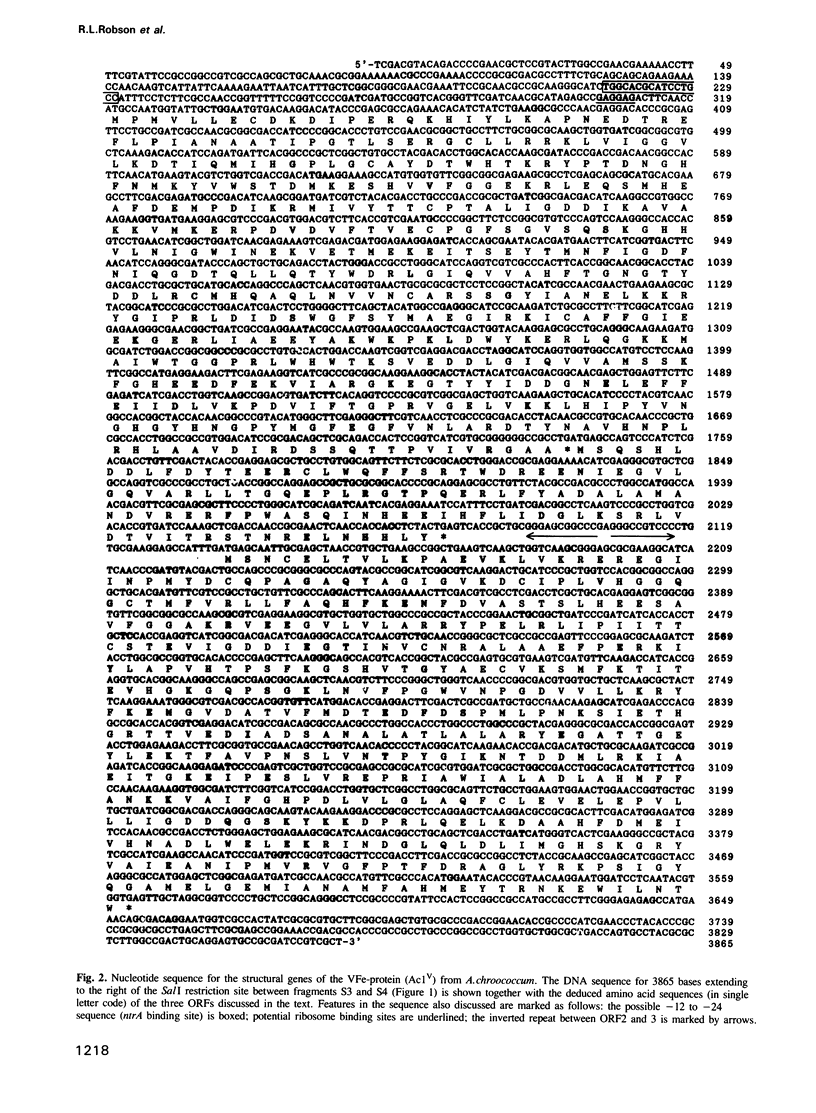
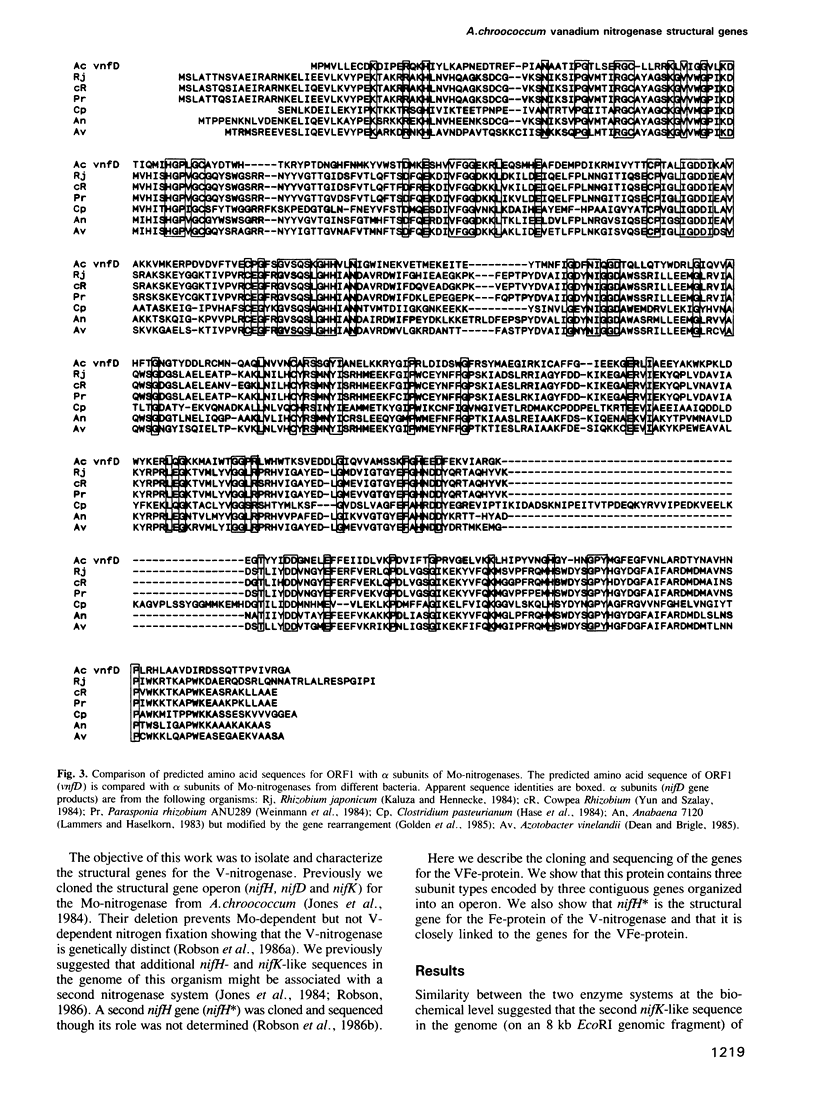
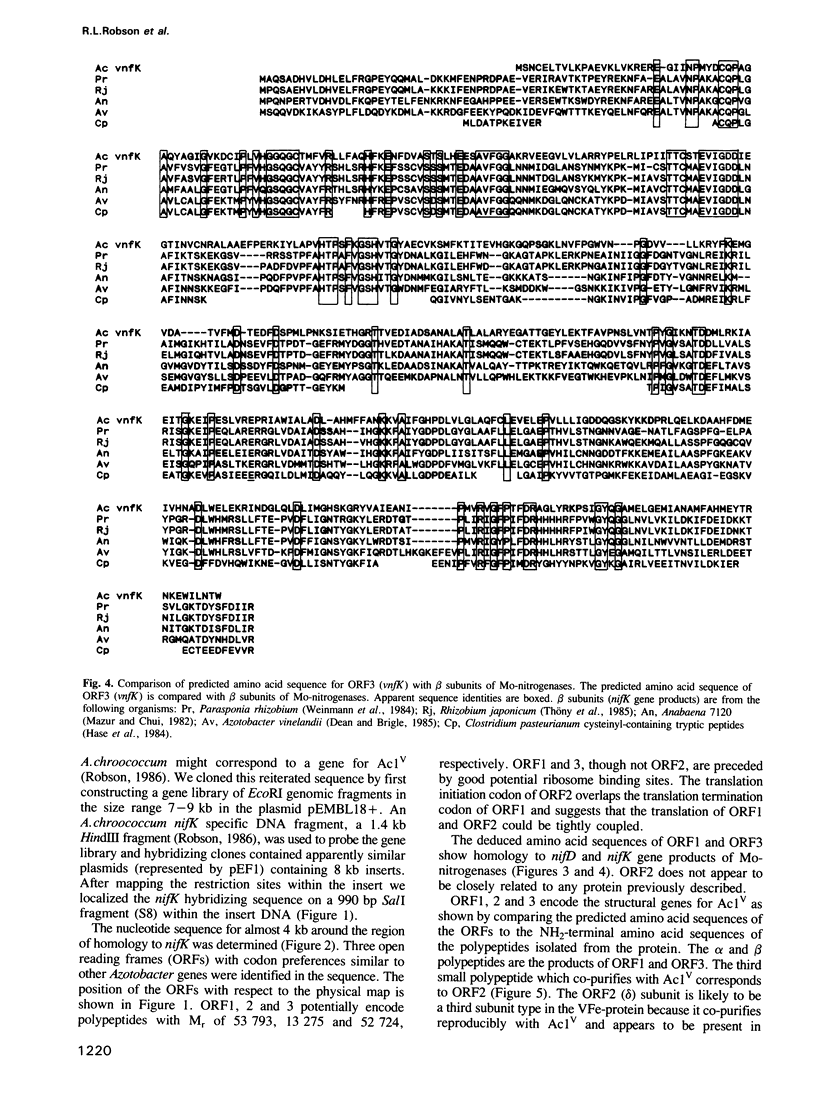
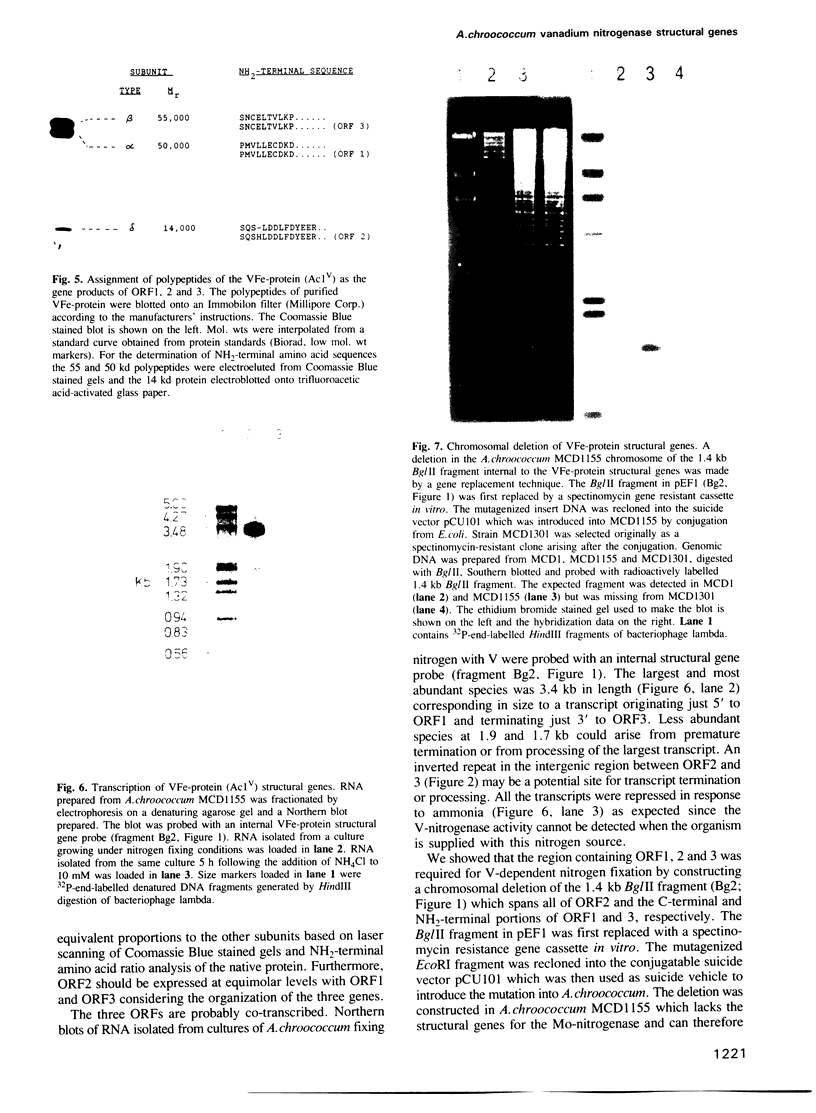
Structural genes for the VFe-protein (Ac1V) of the vanadium nitrogenase from Azotobacter chroococcum were cloned and sequenced. The VFe-protein contains three subunit types with Mr of 53,793 (alpha), 52,724 (beta) and 13,274 (delta). alpha and beta subunits show 18 and 15% sequence identity respectively, with alpha and beta subunits of the MoFe-protein of A.chroococcum molybdenum nitrogenase. The genes for the three subunits vnfD (alpha), vnfG (delta) and vnfK (beta) are contiguous and form an operon whose transcription is repressed in response to ammonia. The Fe-protein component of the V-nitrogenase (Ac2V) is the product of nifH* that we have previously cloned and sequenced. This gene was located 2.5 kb upstream of vnfD. A deletion in the vnfD, G and K gene cluster prevents V-dependent nitrogen fixation. A strain defective in both V-nitrogenase and Mo-nitrogenase structural genes showed no residual nitrogen fixing capacity arguing against the presence of a third nitrogen fixation system in this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chisnell J. R., Premakumar R., Bishop P. E. Purification of a second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J Bacteriol. 1988 Jan;170(1):27–33. doi: 10.1128/jb.170.1.27-33.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Brigle K. E. Azotobacter vinelandii nifD- and nifE-encoded polypeptides share structural homology. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5720–5723. doi: 10.1073/pnas.82.17.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Robson R. L., Richardson T. H., Miller R. W., Hawkins M. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J. 1987 May 15;244(1):197–207. doi: 10.1042/bj2440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Nakano T., Zumft W. G., Matsubara H. Structural homologies between the amino acid sequence of Clostridium pasteurianum MoFe protein and the DNA sequences of nifD and K genes of phylogenetically diverse bacteria. FEBS Lett. 1984 Jan 23;166(1):39–43. doi: 10.1016/0014-5793(84)80040-x. [DOI] [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. P., Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Woodley P., Robson R. Cloning and organisation of some genes for nitrogen fixation from Azotobacter chroococcum and their expression in Klebsiella pneumoniae. Mol Gen Genet. 1984;197(2):318–327. doi: 10.1007/BF00330980. [DOI] [PubMed] [Google Scholar]
- Krol A. J., Hontelez J. G., Roozendaal B., van Kammen A. On the operon structure of the nitrogenase genes of Rhizobium leguminosarum and Azotobacter vinelandii. Nucleic Acids Res. 1982 Jul 24;10(14):4147–4157. doi: 10.1093/nar/10.14.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers P. J., Haselkorn R. Sequence of the nifD gene coding for the alpha subunit of dinitrogenase from the cyanobacterium Anabaena. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4723–4727. doi: 10.1073/pnas.80.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau R. N., Mitchenall L. A., Robson R. L. Genetic evidence for an Azotobacter vinelandii nitrogenase lacking molybdenum and vanadium. J Bacteriol. 1989 Jan;171(1):124–129. doi: 10.1128/jb.171.1.124-129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Robson R. L. Characterization of an oxygen-stable nitrogenase complex isolated from Azotobacter chroococcum. Biochem J. 1979 Sep 1;181(3):569–575. doi: 10.1042/bj1810569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Chesshyre J. A., Wheeler C., Jones R., Woodley P. R., Postgate J. R. Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol. 1984 Jul;130(7):1603–1612. doi: 10.1099/00221287-130-7-1603. [DOI] [PubMed] [Google Scholar]
- Robson R., Woodley P., Jones R. Second gene (nifH*) coding for a nitrogenase iron protein in Azotobacter chroococcum is adjacent to a gene coding for a ferredoxin-like protein. EMBO J. 1986 Jun;5(6):1159–1163. doi: 10.1002/j.1460-2075.1986.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Eady R. R., Lowe D. J., Gormal C. The vanadium-iron protein of vanadium nitrogenase from Azotobacter chroococcum contains an iron-vanadium cofactor. Biochem J. 1988 Feb 15;250(1):299–302. doi: 10.1042/bj2500299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte V., Iyer V. N. Cloning of a plasmid region specifying the N transfer system of bacterial conjugation in Escherichia coli. Gene. 1983 Mar;21(3):227–236. doi: 10.1016/0378-1119(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Weinman J. J., Fellows F. F., Gresshoff P. M., Shine J., Scott K. F. Structural analysis of the genes encoding the molybdenum-iron protein of nitrogenase in the Parasponia rhizobium strain ANU289. Nucleic Acids Res. 1984 Nov 26;12(22):8329–8344. doi: 10.1093/nar/12.22.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Weininger M. S., Mortenson L. E., Rossmann M. G. Molecular symmetry of the MoFe protein of nitrogenase. Structural homology/nitrogen fixation/x-ray crystallography. J Biol Chem. 1982 Feb 10;257(3):1221–1223. [PubMed] [Google Scholar]
- Yates M. G., Planqué K. Nitrogenase from Azotobacter chroococcum. Purification and properties of the component proteins. Eur J Biochem. 1975 Dec 15;60(2):467–476. doi: 10.1111/j.1432-1033.1975.tb21025.x. [DOI] [PubMed] [Google Scholar]
- Yun A. C., Szalay A. A. Structural genes of dinitrogenase and dinitrogenase reductase are transcribed from two separate promoters in the broad host range cowpea Rhizobium strain IRc78. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7358–7362. doi: 10.1073/pnas.81.23.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]