Abstract
Purpose
i) to validate Blood Oxygenation Level Dependent (BOLD) breath hold cerebrovascular reactivity mapping (BH CVR) as an effective technique for potential detection of neurovascular uncoupling (NVU) in a cohort of patients with perirolandic low grade gliomas undergoing presurgical functional magnetic resonance imaging (fMRI) for sensorimotor mapping, and ii) to determine whether NVU potential, as assessed by BH CVR mapping, is prevalent in this tumor group.
Materials and Methods
We retrospectively evaluated 12 patients, with histological diagnosis of grade II glioma, who performed multiple motor tasks and a BH task. Sensorimotor activation maps and BH CVR maps were compared in two automatically defined regions of interest (ROIs), ipsilateral to the lesion (i.e., ipsilesional) and contralateral to the lesion (i.e., contralesional).
Results
Motor task mean T-value was significantly higher in the contralesional ROIs (6.00±1.74 vs 4.34±1.68, p=0.00004) as well as the BH mean T-value (4.74±2.30 vs 4.09±2.50, p=0.009). The number of active voxels was significantly higher in the contralesional ROIs (Z=2.99, p=0.03). Actual NVU prevalence was 75%.
Conclusion
Presurgical sensorimotor fMRI mapping can be affected by NVU-related false negative activation in low grade gliomas (76% of analyzed tasks).
Keywords: fMRI, low grade gliomas, cerebrovascular reactivity mapping, neurovascular uncoupling
Introduction
Presurgical mapping of the sensorimotor cortex in patients with focal lesions in spatial proximity to the sensorimotor areas has been one of the earliest clinical applications of BOLD fMRI (1). The preoperative localization of eloquent cortical areas by fMRI has been demonstrated to have an impact on patients' surgical management because it guides the selection of the safest trajectory to approach the lesion, the decision of whether to proceed with awake or asleep craniotomy, and it can also influence the efficiency, exposure and choice of technique for intraoperative mapping (2,3). Preoperative fMRI can also be used to minimize the risk of transient or permanent postsurgical neurological deficits as well as the risk of associated morbidity related to invasive electrocortical stimulation (ECS) (4).
For clinical applications of functional imaging, a critical limitation is that BOLD fMRI only detects hemodynamic changes accompanying neuronal activity rather than directly detecting the neural activity itself. The coupling between neuronal activity and the increase in blood volume, blood flow and oxygenation occurring in the adjacent vasculature has been reported to be disrupted in patients with brain tumors or other cerebral diseases, thereby making it difficult, if not impossible, to detect eloquent cortex with BOLD fMRI (5). This phenomenon, known as neurovascular uncoupling (NVU), has relevant clinical consequences from the neurosurgical planning standpoint because it can generate false negatives that can subsequently lead to the resection of cortex erroneously deemed as not critical for a particular function. In the literature cases have been reported where the findings of intraoperative mapping did not match the results of presurgical fMRI, and such discordance was often attributed to NVU (6). It has already been demonstrated how NVU can dramatically affect the reliability of the fMRI activation maps in glioblastomas due to tumor neo vascularity with altered structure and physiology (7).
However, little is known regarding the prevalence of NVU in patients with low grade gliomas and how it could be investigated because these lesions are not generally characterized by abnormal perfusion. The neurovascular coupling cascade linking neuronal firing to increase in blood volume, blood flow and blood oxygenation occurs through multiple steps involving neurotransmitters to astrocytes to chemical mediators and finally vascular smooth muscle (8). Low grade gliomas are slowly growing lesions that are known to infiltrate rather than destroy the cerebral cortex; therefore, it is possible that the neurovascular coupling cascade may be disrupted at any of the many involved levels. It is not possible to investigate NVU at the neuronal or astrocytic levels with currently available imaging techniques. However, the interruption of the neurovascular coupling cascade at any level will always be associated with an impaired cerebrovascular reactivity (CVR) that can be detected by MRI measuring the BOLD signal change due to a hypercapnia task. Maps of CVR might therefore be helpful in presurgical mapping because they determine areas of potential NVU in the brain where the BOLD response cannot be elicited. One purpose of this study was to validate BH CVR mapping as a viable tool for NVU potential detection through comparison of abnormalities on CVR maps to those seen on sensorimotor fMRI activation maps, since sensorimotor tasks involving bilateral movements are expected to yield robust activation bilaterally in the sensorimotor cortex (9). Another purpose was to determine whether NVU is prevalent in low grade gliomas. For these purposes, we studied a group of 12 patients with perirolandic low grade gliomas who underwent both BH CVR mapping and fMRI sensorimotor activation studies as part of a comprehensive presurgical fMRI examination. Furthermore, we correlated the imaging findings with the patients' clinical functional status to assess whether the imaging findings were truly indicative of NVU or simply NVU potential.
Materials and Methods
Patients
Data from twelve patients (5 females, age range=25-67 years, mean±stdev=43.5±13.1 years) who underwent presurgical fMRI mapping of the primary motor cortex at our institution were retrospectively analyzed in this study that was approved by our Institutional Review Board. Inclusion criteria were the presence of a de novo grade I or grade II glioma located in the perirolandic cortex and patient's ability to perform the prescribed tasks as assessed in a training session preceding the actual fMRI examination. In Table 1 age, sex, handedness, tumor location and histology are reported for each patient included in the study.
Table 1. clinical/demographic data for the patients included in the study.
| Sex | Age | Handedness | Tumor Location | Histology/Tumor Grade |
|---|---|---|---|---|
| M | 55 | R/2 | L frontal lobe | Oligodendroglioma 2 |
| F | 25 | R/0 | L frontal lobe | Oligodendroglioma 2 |
| M | 37 | R/0 | R frontal lobe | Astrocytoma 2 |
| F | 25 | R/0 | L frontal lobe | Oligoastrocytoma 2 |
| M | 46 | R/4 | L frontal lobe and insula | Oligodendroglioma 2 |
| M | 44 | R/0 | L posterior frontal lobe | Oligodendroglioma 2 |
| F | 67 | R/6 | R frontal perirolandic | Oligodendroglioma 2 |
| M | 40 | R/0 | R frontal | Oligodendroglioma 2 |
| M | 44 | R/1 | R frontal parietal | Oligodendroglioma 2 |
| F | 37 | R/3 | R hemispheric | Fibrillary Astrocytoma 2 |
| F | 55 | R/0 | R parietal | Oligoastrocytoma 2 |
| M | 59 | R/0 | R frontal | Oligodendroglioma 2 |
M: Male; F: Female; L: Left; R: Right. The number under the handedness score indicates the number of common tasks performed by the non-dominant limb according to the responses provided by each patient on the handedness questionnaire. The total number of questions was 22.
MR Imaging
Scanning was performed using standard clinical sequences on a 3.0 Tesla Siemens Trio MRI system (Siemens Medical Solutions, Erlangen, Germany) equipped with a 12 channel head matrix coil. Imaging protocol included a 3D T1 weighted imaging sequence (TR=2300 ms, TI=900 ms, TE=3.5 ms, 9° flip angle, 24-cm field of view, 256×256×176 acquisition matrix, slice thickness 1 mm) for structural imaging and multiple 2D GE-EPI T2* weighted BOLD sequences for functional imaging (TR=2000 ms, TE=30 ms, 90° flip angle, 24-cm field of view, 64×64×33 acquisition matrix, slice thickness 4 mm with 1 mm gap between slices) run while patients were performing a motor or a breath hold (BH) paradigm as described in more detail in the following subsection.
fMRI Paradigms
All patients performed one or more motor tasks and a BH task for CVR mapping. To map the hand representation areas a finger tapping task (30-second blocks of rest alternating with 30-second blocks of simultaneous bilateral self paced fingers to thumb opposition repeated 3 times, total duration 3 minutes) and a hand squeezing task (20 second blocks of rest followed by 20 second of self-paced opening and closing of the left hand in turn followed by 20 seconds of self-paced opening and closing of the right hand repeated 4 times, total duration 4 minutes) were used. To map the face representation area a 3 minute long tongue movement task was used, consisting of 30 second blocks of rest followed by 30 second blocks of repetitive vertical tongue movement repeated 3 times. The foot representation area was mapped with a paradigm where patients alternated 3 times 30 seconds of rest with 30 seconds of flexing and extending their ankles at a self-paced rate, total duration 3 minutes. In Table 2 the tasks performed by each patient are reported. The number and type of tasks varied from patient to patient and was dependent on the location and extent of the lesion in the perirolandic cortex. All of the motor tasks utilized tend to activate both the primary motor cortex and the corresponding regions of primary somatosensory cortex; thus, we refer to the activated regions as “sensorimotor activation.” Each patient performed also a BH task for CVR mapping including a normal breathing period of 40 seconds followed by a 4 second block of inspiration that immediately preceded a 16-second BH period (10). This cycle was repeated four times and at the end of the last BH period an additional normal breathing period of 20 seconds was added. Instructions for all tasks were visually cued. Each patient was accurately trained in a session outside the scanner to make sure that all tasks could be correctly performed. Patients' task performance in the scanner was observed through an external LCD monitor, that is part of the routine equipment used for clinical MRI examinations at our institution, located in the scanner console room.
Table 2.
Measurements of head motion maximum displacement, motor activation, BH CVR and BH baseline tSNR in the IL and CL ROI for each task performed by each patient included in the study. The number of active voxels was automatically calculated using the AMPLE algorithm.
| Patient # | Task | Head motion max displacement(mm) | Average task T-value | Average BH T-value | Average BH tSNR | Number of active motor voxels | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| IL | CL | IL | CL | IL | CL | IL | CL | |||
| 1 | FINGM | 1.07 | 3.28 | 5.93 | 1.13 | 1.80 | 89.71 | 92.04 | 17 | 43 |
| 1 | TM | 2.08 | 1.70 | 7.08 | -2.49 | 2.14 | 79.23 | 99.64 | 9 | 30 |
| 1 | HANDM | 1.10 | 3.99 | 5.71 | 0.07 | 1.74 | 117.75 | 93.04 | 36 | 47 |
| 2 | FINGM | 1.28 | 5.96 | 7.36 | 4.39 | 4.47 | 116.42 | 112.96 | 91 | 106 |
| 2 | TM | 0.94 | 2.65 | 3.49 | 3.99 | 4.29 | 138.84 | 129.25 | 82 | 59 |
| 3 | FINGM | 0.92 | 4.30 | 5.93 | 6.52 | 5.28 | 98.17 | 78.99 | 42 | 143 |
| 3 | FOOTM | 1.02 | 5.72 | 3.34 | 7.38 | 5.78 | 92.79 | 85.36 | 53 | 22 |
| 3 | TM | 1.36 | 2.24 | 5.97 | 5.74 | 4.26 | 97.77 | 77.58 | 36 | 89 |
| 4 | FOOTM | 1.39 | 4.58 | 5.51 | 3.25 | 2.41 | 106.83 | 76.43 | 133 | 224 |
| 4 | FOOTM | 0.66 | 3.17 | 2.33 | 3.25 | 2.41 | 106.83 | 76.43 | 28 | 9 |
| 4 | TM | 1.13 | 1.63 | 6.78 | 1.87 | 2.90 | 182.10 | 133.93 | 26 | 54 |
| 5 | FINGM | 0.66 | 4.74 | 6.50 | 4.53 | 4.45 | 142.87 | 120.81 | 102 | 72 |
| 5 | TM | 0.60 | 5.40 | 6.02 | 4.16 | 4.70 | 112.31 | 110.55 | 46 | 72 |
| 6 | FINGM | 0.28 | 6.17 | 7.13 | 5.87 | 6.65 | 183.51 | 144.76 | 92 | 124 |
| 6 | TM | 0.72 | 3.51 | 6.90 | 5.90 | 6.89 | 185.70 | 139.44 | 44 | 121 |
| 7 | FINGM | 0.36 | 7.01 | 9.23 | 4.01 | 4.38 | 186.22 | 145.06 | 134 | 259 |
| 7 | FOOTM | 0.78 | 6.00 | 6.72 | 5.08 | 5.44 | 169.76 | 153.37 | 150 | 185 |
| 7 | HANDM | 0.46 | 4.97 | 10.65 | 4.84 | 5.50 | 178.28 | 152.40 | 82 | 207 |
| 8 | FINGM | 0.66 | 2.67 | 5.58 | 0.88 | 1.96 | 114.12 | 107.93 | 36 | 69 |
| 8 | TM | 0.68 | 4.69 | 5.98 | 2.06 | 1.94 | 116.07 | 103.14 | 199 | 256 |
| 8 | FOOTM | 0.74 | 4.71 | 5.27 | 1.56 | 2.71 | 106.60 | 97.00 | 48 | 74 |
| 9 | FINGM | 0.41 | 1.25 | 6.44 | 1.55 | 3.75 | 161.51 | 118.83 | 2 | 30 |
| 9 | FOOTM | 1.31 | 2.64 | 6.24 | 2.75 | 3.41 | 169.23 | 133.45 | 19 | 53 |
| 9 | HANDM | 1.57 | 1.66 | 4.49 | 1.59 | 4.11 | 112.89 | 117.71 | 5 | 51 |
| 10 | FINGM | 0.40 | 5.96 | 6.10 | 7.56 | 9.40 | 103.39 | 110.85 | 38 | 54 |
| 10 | FOOTM | 0.82 | 3.53 | 5.35 | 9.35 | 10.10 | 86.75 | 80.77 | 47 | 38 |
| 10 | TM | 0.85 | 3.92 | 5.80 | 6.27 | 7.99 | 107.23 | 122.82 | 53 | 90 |
| 10 | HANDM | 0.99 | 4.78 | 3.68 | 6.57 | 8.36 | 89.17 | 96.75 | 60 | 41 |
| 11 | FOOTM | 1.62 | 6.91 | 6.25 | 5.48 | 5.41 | 178.10 | 146.65 | 402 | 350 |
| 11 | TM | 1.35 | 5.94 | 6.09 | 4.27 | 4.13 | 121.08 | 118.70 | 90 | 51 |
| 11 | HANDM | 1.48 | 5.93 | 4.71 | 3.36 | 2.60 | 133.12 | 135.08 | 43 | 50 |
| 12 | FINGM | 0.45 | 7.15 | 7.36 | 5.45 | 8.52 | 159.44 | 130.49 | 54 | 81 |
| 12 | TM | 0.41 | 4.50 | 5.98 | 6.92 | 6.53 | 138.84 | 128.73 | 127 | 133 |
| Average | 0.93 | 4.34 | 6.00 | 4.09 | 4.74 | 129.78 | 114.27 | 74 | 100 | |
| Std Dev | 0.43 | 1.68 | 1.57 | 2.50 | 2.30 | 34.13 | 23.69 | 75 | 80 | |
BH: breath hold; CVR: cerebrovascular reactivity; tSNR: temporal Signal to Noise Ratio; IL ipsilesional; CL contralesional; FINGM: finger tapping; HANDM: hand opening and closing; FOOTM: foot movement; TM: tongue movement;
Data Analysis
Preprocessing
Raw data were exported to an external Linux workstation. AFNI (11) and MRIcro software (http://www.mccauslandcenter.sc.edu/mricro/mricron/) was used for image processing. From the acquired BOLD EPI images 4D datasets were created containing the fMRI signal time series (TS) for each voxel. These TS were temporally interpolated to account for the different acquisition time of each slice in one brain volume. Then all the acquired volumes were spatially realigned to correct for head motion, coregistered to the structural images and spatially smoothed using a 4 mm isotropic FWHM Gaussian filter. The maximum spatial displacement from the volume taken as reference during motion correction calculation was reported as “Head motion maximum displacement,” an important quality control (QC) metric included in Table 2. For the BH task a temporal signal to noise ratio (baseline tSNR) metric of the TS first resting period (40 seconds) was calculated by detrending the signal, taking its baseline and dividing it by the standard deviation of the residual TS. For BH and sensorimotor tasks the entire preprocessed TS were fitted voxel wise with an expected signal TS obtained convolving each paradigm timing with a theoretical hemodynamic impulse response function (HRF). We used a standard gamma HRF for the motor tasks and we adopted the HRF proposed by Birn and colleagues to model the hemodynamic response to a BH task (12). From the fitted TS, T-value maps were created to express motor activation and BH CVR. For each motor task a ROI was generated centered on the maximum T-value in the sensorimotor cortex on the contralesional (i.e., contralateral to the tumor) hemisphere (CL ROI). The extent of the ROI included voxels inside the brain distant up to 3 cm from the maximum T-value (Figure 1). Each ROI was mirrored in the ipsilesional (i.e., ipsilateral to the tumor) hemisphere (IL ROI). These ROIs were defined taking advantage of expected symmetry of sensorimotor eloquent cortex (9,13) and were large enough to account for possible cortical reorganization that has been demonstrated to occur in presence of infiltrating slowly growing lesions, like grade II gliomas (14). The average T-value for each subject for the motor fMRI task was calculated in the IL and CL ROIs. Likewise for each subject the average T-value and the average baseline tSNR were calculated for the BH task in the same IL and CL ROIs generated for each motor task. Average motor and BH task T-values and baseline tSNR among the IL and CL ROIs were compared using a paired t-test. The ratio of the average T-value between the IL and CL ROIs for the motor (r-motor) and BH (r-CVR) tasks for each subject was also generated, and a correlation analysis between these two variables was performed.
Figure 1.
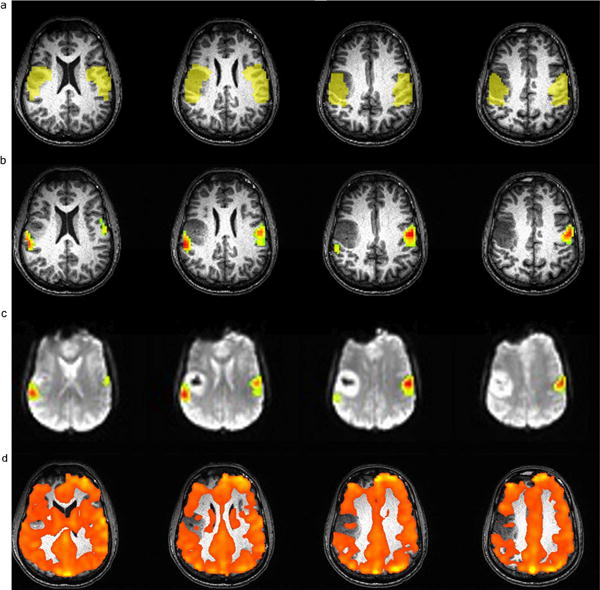
Example of IL and CL ROIs (1a), AMPLE (1 b and 1c) and BH CVR maps (1d) in a patient with a right frontal lobe grade II oligodendroglioma who performed a vertical tongue movement task. The spatial extent of the face representation area as mapped by fMRI in the ipsilesional ROI is markedly decreased compared to the contralesional homologue area. Decreased vascular reactivity was found also by the BOLD BH CVR maps (IL average T-value/CL average T-value=0.855). Figure 1c shows the same AMPLE map of Figure 1b overlaid on echo planar images.
AMPLE Analysis
Activation Mapping as Percentage of Local Excitation (AMPLE) motor maps were then calculated in each ROI, expressing the T-value as percentage of the maximum T-value in the considered ROI, and for each task voxels with T-values above 60% of the maximum were considered as active (15). The number of active voxels in the IL and CL ROIs were compared using a Wilcoxon test.
Assessment of NVU Potential vs Actual NVU
In order to distinguish between simply NVU potential and actual NVU in these 12 patients, comparison between the results of the above-described fMRI analysis and the patients' actual clinical functional status was performed, as tabulated in Table 3. While NVU potential may be demonstrated by ipsilesionally decreased sensorimotor task-related activation and corresponding ipsilesional decrease in regional CVR, as assessed by ipsilesionally decreased T- values compared to contralesional T-values, actual determination of NVU relies on these findings being present in the scenario of preserved clinical function attributable to the cortical region of interest. In other words, false negative activation related to actual NVU must be distinguished from true negative activation due to absence of viable eloquent cortex. One of the authors, who is a board-certified neuroradiologist with approximately 15 years of clinical functional neuroimaging experience, correlated the findings on the activation maps with findings in the patient's electronic medical record. These findings for each of the 12 patients are included in Table 3.
Table 3. Tabulation of exact gyral anatomic localization of regionally decreased CVR and corresponding decreased or absent expected sensorimotor activation as well as correlation with clinical functional status to determine whether findings are indicative of true NVU.
| Patient # | Gyrus displaying Ipsilesional decreased/absent motor activation corresponding to decreased CVR | Functional area Associated with Involved gyrus | Actual NVU(i.e., false Negative activation)? Yes/No | How clinical functional determination was made of false negative vs. true negative activation |
|---|---|---|---|---|
| 1 | L precentral gyrus | Face RA) of (PMC) | N | R facial droop |
| 2 | L precentral gyrus | Hand and more prominently face RA of PMC | Y | No motor deficit |
| 3 | R precentral gyrus | Face RA of PMC | Y | No face or other motor deficit |
| 4 | R precentral gyrus | Foot RA of PMC | Y | No left lower Extremity weakness |
| 5 | Left precentral gyrus | Hand RA of PMC | Y | No motor deficit despite asymmetrically decreased hand motor activation |
| 6 | R precentral gyrus | Face RA of PMC | Y | No face or other motor deficit |
| 7 | L superior frontal gyrus | L SMA | Y | No motor or language deficit |
| 8 | R postcentral gyrus | Hand RA of (PSC) | N | Mild left upper extremity sensory deficit |
| 9 | R superior frontal gyrus, superior aspect of R precentral gyrus | Foot RA of PMC, R SMA | N | Severe left lower extremity weakness |
| 10 | R postcentral gyrus | Face/tongue RA of PSC | Y | No sensory deficits |
| 11 | R superior frontal gyrus, superomedial aspect of right precentral gyrus | R SMA, R foot RA of PMC | Y | No motor or sensory deficits preoperatively |
| 12 | R precentral gyrus, R superior frontal gyrus | Foot RA of PMC and R SMA | Y | No motor deficit |
CVR: cerebrovascular reactivity; NVU: neurovascular uncoupling; L: Left; R: Right; RA: Representation area; PMC: Primary Motor Cortex; SMA: Supplementary Motor Area; PSC: Primary Somatosensory Cortex
Results
An example of analysis for an individual patient is provided in Figure 1.The automatically generated IL and CL ROIs (1a) with the corresponding AMPLE-generated fMRI activation maps overlaid on high resolution T1-weighted structural (1b) and T2*- weighted echo planar MR images (1c), as well as the BH CVR map (1d) are reported for a patient included in this study who performed a vertical tongue movement task.
In the third column of Table 2 the maximum amount of motion displacement is reported for each task and each patient, In every case this measurement was less than one voxel size in each direction, and therefore no data was discarded (16). The comparison of the sensorimotor activation maps and BH CVR maps at the group level in the IL and CL ROIs (Table 2, columns 4-7) demonstrated a significantly decreased mean T-value in the IL ROI compared to the CL ROI (t=-4.80, p=0.0004) for the motor tasks (referred to as ‘MT’ in Figure 2), and a similar result was obtained for the BH task (t=-2.79, p=0.0009), as illustrated in Figure 2. A strong correlation between r-CVR (i.e., the ratio of mean CVR T-value in the IL ROI to that of the CL ROI) and r-motor (i.e., ratio of mean sensorimotor activation T-value in the IL ROI to that of the CL ROI) was also found (R2=0.25, p=0.003, Figure 3). The baseline tSNR analysis (Table 2 column 8-9) for the BH task demonstrated statistically significantly higher tSNR in the IL compared to the CL ROIs (t=2.14, p=0.036). In particular, the IL mean was significantly higher than the CL mean (IL mean = 897±141 vs CL mean = 844±132, p=0.01) whereas the standard deviation was not different (IL stdev = 8.45±2.47 vs, CL stdev = 8.87±2.59, p=0.05). In 25 out of the 33 analyzed motor task data the number of active voxels calculated with the AMPLE method was lower in the IL ROI than in the CL ROI (Table 2 last two columns). According to the result of the Wilcoxon test this difference was statistically significant (Z=2.99, p=0.003).
Figure 2.
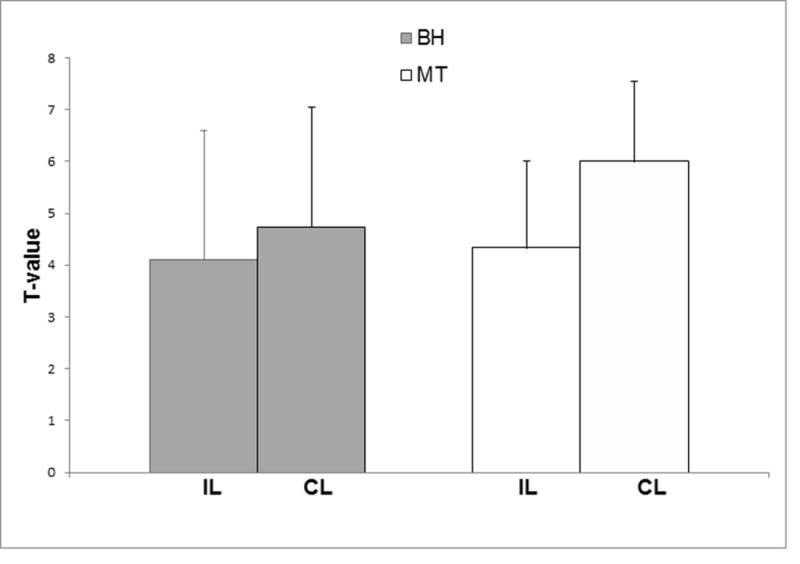
Bar plots illustrating decreased motor task (MT) and BH T-value in the IL ROI compared to CL ROI in the group of low grade glioma patients included in the study.
Figure 3.
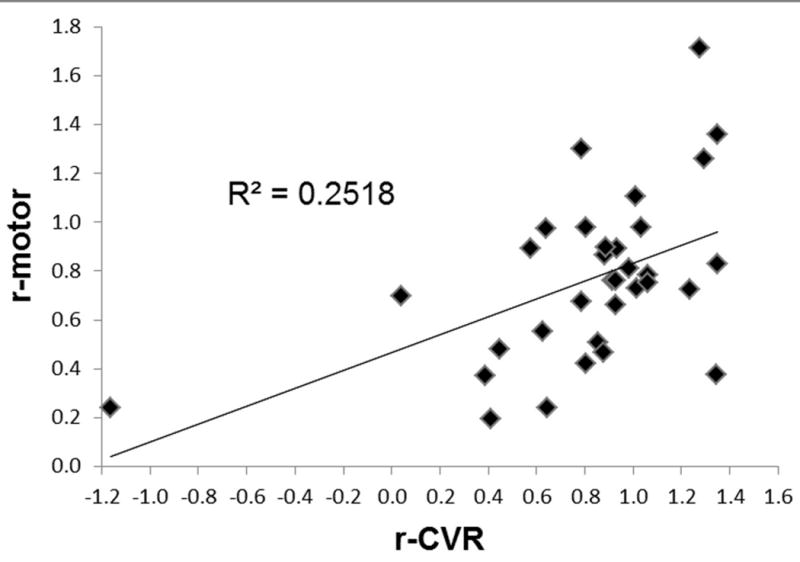
CL-normalized average sensorimotor fMRI activation T-value (r-motor) versus CL -normalized average BH signal T-value plotted for each of the 33 motor tasks analyzed in the study. Strong correlation is found between these two variables suggesting the ability of BH CVR mapping to determine areas of potential NVU.
Discussion
Presurgical fMRI localization of eloquent cortical and subcortical areas at risk of injury during the surgical removal of brain tumors or other resectable lesions can substantially impact surgical planning and affect immediate postoperative outcome (2,17).
However, concerns exist regarding the validity of the BOLD effect for the detection of eloquent cortex in the presence of altered vascular physiology associated with the cerebral mass lesions due to the phenomenon of neurovascular uncoupling (NVU)(18). The utility of the BH CVR method in detection of potential NVU relies on its ability to produce a selective hypercapnia-induced vascular challenge without eliciting simultaneous changes in CMRO2 (cerebral metabolism) since no neuronal activation is involved in the BH task, unlike sensorimotor, cognitive or visual fMRI tasks in which neuronal activation immediately precedes the microvascular response (19).
The findings of this study demonstrate impaired ipsilesional sensorimotor activation (in 26 out of 33 tasks) compared to homologous contralesional activation in a group of patients with a tissue diagnosis of low grade glioma. This result is demonstrated by corresponding ipsilesional decreased average T-values detected on both the fMRI motor activation maps and the BH CVR maps. The results of the baseline tSNR analysis demonstrated higher BOLD baseline signal in the ipsilesional ROI compared to the normal contralesional ROI. This could be explained by the inclusion of tumoral and peritumoral edema in the ipsilesional ROI since the BOLD signal is proportional to spin water density (20). Therefore, impaired BOLD sensorimotor fMRI activation and impaired regional CVR were not due to intrinsic lack of BOLD signal in the tumoral and peritumoral area (IL ROI) but rather to altered vascular physiology in LGG. Furthermore, the strong correlation between the CL-normalized signal amplitude ratios for the sensorimotor fMRI activation and the CVR signal changes indicates that BH CVR mapping allows accurate detection of NVU potential that may seriously compromise reliability of BOLD fMRI activation maps used for presurgical planning.
These results are in agreement with a previous study applying a BH task for CVR mapping in a group of 6 patients with low grade gliomas where absence of significant BOLD signal enhancement in the tumor bulk and peritumoral edema was found in each patient, but in this study the CVR mapping was not correlated with any task-related BOLD fMRI activation (21). In another study by Jiang and colleagues studying patients with grade II, III and IV gliomas, impaired activation was found in the grade III and IV patient group but not in the grade II patient group, and similar results were demonstrated for CVR; however, only 8 patients performing one task each were investigated, and the study was performed at lower magnetic field strength (1.5 T) (22). Furthermore, the authors applied a more liberal threshold (T<3.1), due the lower signal to noise ratio present compared to current state-of-the-art 3T functional imaging, that might have washed out the differences in BOLD signal enhancement between the ipsilesional and contralesional ROIs. We selectively chose to focus our analysis on low grade gliomas because it has already been demonstrated that BOLD fMRI activation is impaired in the vicinity of high grade gliomas. In particular, two studies demonstrated an association between increased cerebral blood flow and cerebral blood volume as detected by MR perfusion imaging and decreased activation in the tumoral and peritumoral area, and their conclusion was that NVU was probably due to tumor angiogenesis characterized by diminished autoregulatory capacity (7,22). In our study, we have shown that NVU is, indeed, highly prevalent in low grade glioma cases, just as it is known to be prevalent in higher grade glioma cases.
The observed reduction in regional BOLD response in this cohort of low grade gliomas necessitates a different explanation than that which has been advocated for high grade gliomas mainly because of the absence of increased perfusion in these lesions as reported in the literature (23). Glial tumors are infiltrating lesions that can compromise the neuronal contacts with surrounding microvasculature and astrocytes. Such infiltration of the astrocytes may result in physiologically abnormal vessels that are structurally intact and not increased in density compared to the contralateral homologous brain regions. Such physiologically abnormal vessels may be characterized by reduced reactivity as indicated by our BOLD CVR mapping where in 22 out of 33 analyzed ROI the average IL T-value was lower than the average CL T-value. In one case average CVR was negative in the IL ROI and this finding could be explained by the hypercapnia-induced steal effect that redistributes blood flow from tumor regions with unresponsive neovasculature to surrounding normal tissue, although this phenomenon is more common in more biologically aggressive grade III and IV lesions (24).
In a minority of cases we found more motor activity in the ipsilesional ROIs than in the contralesional ROIs. There are multiple possible explanations for these unexpected findings. In the majority of these cases (4 out of a total of 5 such cases) we detected IL motor activation T-values that were higher than CL motor activation T-values in addition to IL BH T-values that were similarly greater than CL BH T-values. One possible explanation for the findings in these cases is that the presence of NVU adjacent to the lesions may have been compensated by CVR increase in other areas in close spatial proximity to the lesions that were also included in the ROIs. In addition, areas of spurious activation or activation that is not specific to the performed task and associated robust CVR could have been included in the ROIs. In the fifth case (patient 10) IL hand motor activation T-values were higher than CL hand motor activation whereas IL BH T-value was less than the CL BH T-value. Therefore, it may be possible that the effort, strength and rate in task performance for this particular patient were not exactly the same between the two hands, although the patient had been instructed to try to use both hands equally during the task. It is thus advisable, both when applying this technique clinically in individual patients and for future investigations, to use ROIs of different sizes and to collect quantitative behavioral measurements during the fMRI data acquisition and include these variables in the data analysis.
To provide quantitative and objective measurements of activation we adopted a recently developed algorithm (AMPLE) that normalizes statistical T-value maps to the local peak activation amplitude within each functional brain region. This method has been demonstrated to provide highly reproducible maps when a standard threshold (60% of the maximum t-value in each analyzed ROI) is applied (25). We also defined a robust operator-independent method to select the ROI for investigating sensorimotor fMRI activation.
Some limitations are present in this study. First, qualitative CVR mapping was performed. CVR is defined as the change in blood flow per unit change in PCO2. By continuously monitoring the CO2 levels in the blood and controlling its changes while acquiring BOLD or Arterial Spin Labeling MRI data it is possible to obtain quantitative CVR measurements. Several techniques have been implemented and some commercial products are currently available that use face masks to deliver CO2 in a controlled quantitative manner alternating with delivery of normal air (26). However, the utilization of these systems in a routine clinical protocol could be problematic and it would require a long and dedicated training session for the patients in addition to the standard fMRI task training that already frequently takes between 30 and 60 minutes when an extensive battery of clinical paradigms is implemented, particularly in more neurologically-impaired patients. The premise of the methodology used in this study was expected bihemispheric symmetric activation and CVR. This may not be necessarily true since multiple studies have shown how in groups of healthy subjects handedness, type of tasks and task performance rate may introduce a degree of lateralization in motor activation (27-29). We did not have normal volunteer data to confirm these findings. However our study sample included a group of right handed patients but with lesions distributed almost evenly across the two hemispheres. Decreased motor activation has already been reported in regions ipsilateral to brain tumors (30) whereas differences in vascular reactivity in different areas of the brain have been found but not between the same homologous regions across the hemispheres (31). The particular methodology used in this study, although operator-independent, would not be applicable particularly to language or visual cortex whose pattern of activation is generally strongly lateralized and not as anatomically well-defined as for sensorimotor activation. However, given the results of this study, there is no reason why BH CVR should not be as effective for the detection of NVU potential in additional eloquent cortical regions of the brain, which could also be affected by NVU (32).
In conclusion, we demonstrated that NVU can adversely affect the results of presurgical BOLD fMRI activation maps in patients with low grade gliomas, similar to previously described findings in higher grade gliomas. NVU within and in the vicinity of these lesions is likely due to factors other than tumor angiogenesis, such as altered pH levels, abnormalities involving astrocytes, neurotransmitters and chemical mediators involved in regulating the vascular response. In addition to establishing that NVU is, indeed, prevalent in patients with grade II gliomas, we also demonstrated the utility of CVR mapping using a BH task for the assessment of NVU potential. The fact that none of the fMRI tasks showed complete absence of activation supports the clinical utilization of fMRI in presurgical planning because, despite 75% prevalence of NVU in our study, we were still able to localize, at least partially, eloquent motor cortex even in these cases. These results provide impetus for the development of calibrated fMRI methods to generate activation maps that take into account the decreased brain vascular reactivity in the vicinity of pathology such as brain tumors (33).
Acknowledgments
Funding Sources: Quantitative Imaging Biomarker Alliance of the Radiological Society of North America. Funded by The National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000050C (Subaward No. 19a).
Siemens Medical Solutions: JHU-2010-MR-87-01-36965-Pillai. Siemens played no role in the study design, data collection and analysis, or preparation of the manuscript
References
- 1.Mueller WM, Yetkin FZ, Hammeke TA, et al. Functional magnetic resonance imaging mapping of the motor cortex in patients with cerebral tumors. Neurosurgery. 1996;39:515–520. doi: 10.1097/00006123-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Petrella JR, Shah LM, Harris KM, et al. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006;240:793–802. doi: 10.1148/radiol.2403051153. [DOI] [PubMed] [Google Scholar]
- 3.Medina LS, Bernal B, Dunoyer C, et al. Seizure disorders: functional MR imaging for diagnostic evaluation and surgical treatment--prospective study. Radiology. 2005;236:247–253. doi: 10.1148/radiol.2361040690. [DOI] [PubMed] [Google Scholar]
- 4.Tieleman A, Deblaere K, Van Roost D, Van Damme O, Achten E. Preoperative fMRI in tumour surgery. Eur Radiol. 2009;19:2523–2534. doi: 10.1007/s00330-009-1429-z. [DOI] [PubMed] [Google Scholar]
- 5.Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 2000;21:1415–1422. [PMC free article] [PubMed] [Google Scholar]
- 6.Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M, Mark LP. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol. 2003;24:213–217. [PMC free article] [PubMed] [Google Scholar]
- 7.Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage. 2006;32:489–497. doi: 10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- 8.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kocak M. Proceedings of the American Society of Neuroradiology. Vancouver, Canada: 2002. Functional MR imaging of the motor homunculus: Toward optimizing paradigms for clinical scenarios. [Google Scholar]
- 10.Magon S, Basso G, Farace P, Ricciardi GK, Beltramello A, Sbarbati A. Reproducibility of BOLD signal change induced by breath holding. Neuroimage. 2009;45:702–712. doi: 10.1016/j.neuroimage.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 11.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 12.Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–272. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Pillai JJ. Insights into adult postlesional language cortical plasticity provided by cerebral blood oxygen level-dependent functional MR imaging. AJNR Am J Neuroradiol. 2010;31:990–996. doi: 10.3174/ajnr.A1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voyvodic JT. Activation mapping as a percentage of local excitation: fMRI stability within scans, between scans and across field strengths. Magn Reson Imaging. 2006;24:1249–1261. doi: 10.1016/j.mri.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 17.Wengenroth M, Blatow M, Guenther J, Akbar M, Tronnier VM, Stippich C. Diagnostic benefits of presurgical fMRI in patients with brain tumours in the primary sensorimotor cortex. Eur Radiol. 2011;21:1517–1525. doi: 10.1007/s00330-011-2067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol. 2000;21:1055–1063. [PMC free article] [PubMed] [Google Scholar]
- 19.Bandettini PA, Wong EC. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed. 1997;10:197–203. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<197::aid-nbm466>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2012;32:1188–1206. doi: 10.1038/jcbfm.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu YY, Chang CN, Jung SM, et al. Blood oxygenation level-dependent MRI of cerebral gliomas during breath holding. J Magn Reson Imaging. 2004;19:160–167. doi: 10.1002/jmri.10447. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Z, Krainik A, David O, et al. Impaired fMRI activation in patients with primary brain tumors. Neuroimage. 2010;52:538–548. doi: 10.1016/j.neuroimage.2010.04.194. [DOI] [PubMed] [Google Scholar]
- 23.Hakyemez B, Erdogan C, Ercan I, Ergin N, Uysal S, Atahan S. High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol. 2005;60:493–502. doi: 10.1016/j.crad.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Heyn C, Poublanc J, Crawley A, et al. Quantification of cerebrovascular reactivity by blood oxygen level-dependent MR imaging and correlation with conventional angiography in patients with Moyamoya disease. AJNR Am J Neuroradiol. 2010;31:862–867. doi: 10.3174/ajnr.A1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voyvodic JT, Petrella JR, Friedman AH. fMRI activation mapping as a percentage of local excitation: consistent presurgical motor maps without threshold adjustment. J Magn Reson Imaging. 2009;29:751–759. doi: 10.1002/jmri.21716. [DOI] [PubMed] [Google Scholar]
- 26.Vesely A, Sasano H, Volgyesi G, et al. MRI mapping of cerebrovascular reactivity using square wave changes in end-tidal PCO2. Magn Reson Med. 2001;45:1011–1013. doi: 10.1002/mrm.1134. [DOI] [PubMed] [Google Scholar]
- 27.Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci U S A. 1997;94:14015–14018. doi: 10.1073/pnas.94.25.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jancke L, Peters M, Schlaug G, Posse S, Steinmetz H, Muller-Gartner H. Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Brain Res Cogn Brain Res. 1998;6:279–284. doi: 10.1016/s0926-6410(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 29.Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. Eur J Neurol. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 30.Ludemann L, Forschler A, Grieger W, Zimmer C. BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J Magn Reson Imaging. 2006;23:435–443. doi: 10.1002/jmri.20530. [DOI] [PubMed] [Google Scholar]
- 31.Bright MG, Bulte DP, Jezzard P, Duyn JH. Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI. Neuroimage. 2009;48:166–175. doi: 10.1016/j.neuroimage.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pillai JJ, Zaca D. Comparison of BOLD Cerebrovascular Reactivity Mapping and DSC MR Perfusion Imaging for Prediction of Neurovascular Uncoupling Potential in Brain Tumors. Technol Cancer Res Treat. 2012;11:361–374. doi: 10.7785/tcrt.2012.500284. [DOI] [PubMed] [Google Scholar]
- 33.Zacà D, Nadar SR, Jovicich J, Pillai JJ. Cerebrovascular reactivity-based calibration of presurgical motor activation maps to improve detectability of the BOLD signal in patients with perirolandic brain tumors; Proceedings of the 21th Annual Meeting of ISMRM; Salt Lake City. 2013. p. 3554. [Google Scholar]


