Abstract
Objective
We assessed the neural correlates of adult ADHD in treatment-naïve participants, an approach necessary for identifying neural substrates unconfounded by medication effects.
Method
The sample consisted of 24 medication-naïve adults with Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV) diagnosed ADHD and 24 healthy controls, comparable on age, sex, handedness, reading achievement, IQ, and psychiatric comorbidity. All participants were assessed with structured diagnostic interviews. Magnetic resonance imaging (MRI)-based regional voxel-based morphometry (r-VBM) was used to assess volumetric differences in a priori defined brain regions of interest.
Results
VBM analysis revealed group differences in the hypothesized cortical and subcortical areas; however, only cerebellar volume reductions in ADHD retained significance (p < .05) after corrections for multiple comparisons.
Conclusion
These results support the notion that medication-naïve ADHD as expressed in adulthood, manifests subtle brain volume reductions from normal in the cerebellum, and possibly in other syndrome-congruent gray-matter structures. Larger samples are required to confirm these findings.
Keywords: ADHD, cerebellum, VBM, brain structure, treatment-naïve ADHD
Introduction
ADHD in adults is a childhood-onset, persistent, neurobiological disorder associated with high levels of morbidity and dysfunction estimated to afflict up to 5% of adults worldwide (American Psychiatric Association [APA], 1994; Kessler et al., 2006). Convergent data from neuroimaging, neuropsychological, and neurochemical studies have implicated abnormalities in neural systems dedicated to attention and executive function (EF) in these patients. Because much of the neuroimaging literature on ADHD derives from studies that included participants treated with psychostimulants and other medications (Seidman et al., 2011), it remains unclear whether the findings observed represent the effects of the disorder or those of its treatment. A key step needed to help resolve this critical issue is to study medication-naïve participants, who reach adulthood while still meeting diagnostic criteria for ADHD. This approach can help answer a fundamental scientific question as to what is the nature of brain abnormalities in the pure form of untreated adult ADHD reflecting the natural history of the neural underpinnings of this disorder. To the best of our knowledge, this issue has not been answered satisfactorily to-date in the neuroimaging literature of adults with ADHD.
A small number of previous pediatric studies using treatment-naïve populations showed an association of ADHD with brain alterations in the anterior cingulate cortex (ACC), cerebellar vermis and white matter (Bledsoe, Semrud-Clikeman, & Pliszka, 2009; Castellanos et al., 2002; Pliszka et al., 2006). For example,Bledsoe et al. (2009) showed reduced area in the posterior inferior cerebellar vermis in treatment-naïve ADHD children compared with treated and not treated controls. One study of adults reported brain dopamine transporter levels in drug-naïve adults with ADHD; however, brain structure was not reported (Volkow et al., 2007). To the best of our knowledge, there is only one small structural neuroimaging study that showed abnormalities in the ACC in medication-naïve adults with ADHD (Makris et al., 2010), a small pilot study focusing on the ACC conducted by our group. While limited, these magnetic resonance imaging (MRI) findings to date are consistent with current models in ADHD clinical research, and with basic neuroscience supporting the relevance of these brain structures to the phenomenology of ADHD symptoms and cognitive control (Bush, Luu, & Posner, 2000; Makris, Biederman, Monuteaux, & Seidman, 2009; Seidman, Valera, & Makris, 2005; Sonuga-Barke, 2003).
Recently, clinical neuroscientific thinking has increasingly moved toward a neural systems approach to understanding cognitive function in the brain (Makris et al., 2009). Where first-generation clinical neuroscience concerned itself primarily with localizing structural abnormalities and the function of individual structures, the development of new imaging and analysis techniques has made it possible to directly examine a range of properties of the neural substrates of normal and abnormal behavior. This is an essential development from the perspectives of psychiatry and classical neuropsychology, two fields that have historically emphasized systems level analyses to understand complex brain function and behavior. Within a neural systems formulation of ADHD, the set of gray structures hypothesized to be principally involved are the dorsolateral prefrontal cortex (DLPFC), ACC, orbitofrontal cortex (OFC), lateral parietotemporal cortex (IPL/TOP), and caudate nucleus and cerebellum (CBL) (Makris et al., 2009; Seidman et al., 2011; Seidman et al., 2005; Valera, Faraone, Murray, & Seidman, 2007).
The main aim of the present study was to investigate ADHD-associated regions of interest (ROIs), including frontal, lateral parietotemporal, striatal, and cerebellar abnormalities in a sample of medication-naïve adults with ADHD. Based on empirical data and accepted models of ADHD (Barkley, 1997; Makris et al., 2009; Sonuga-Barke, 2003), we hypothesized that treatment-naïve participants who reach adulthood while still meeting diagnostic criteria for ADHD show structural alterations in the ACC, DLPFC, OFC, IPL/TOP, caudate nucleus and CBL. We tested the above questions using MRI and regional voxel-based morphometry (r-VBM) analysis.
Method
Participants
Males and females between the ages of 18 and 59 years participated in the study. ADHD (n = 24) and control (n = 24) adults were group matched to be comparable on age, sex distribution, handedness, and IQ (Tables 1 and 2). With the exception of one participant who met criteria for ADHD-NOS (Attention Deficit / Hyperactivity Disorder: Not Otherwise Specified), all other participants with ADHD met full Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM-IV; APA, 1994) criteria for ADHD with childhood onset and persistence of symptoms onto adult life. While a majority of participants in this analysis had been included in a previously published VBM work by our group, the previous study did not address the treatment-naïve subsample separately (Seidman et al., 2011). Thus, this is our first report of the structural imaging dataset of medication-naïve adults, with and without ADHD, using all the treatment-naïve participants available.
Table 1.
Demographic and Clinical Characteristics in Controls and ADHD.
| Controls (n = 24) n (%) | ADHD (n = 24) n (%) | Test statistic | P value | |
|---|---|---|---|---|
| n (%) males | 62.50% | 62.50% | χ2(1) = 0.00 | 1.00 |
| Right handed | 95.83% | 79.17% | χ2(1) = 2.01 | .16 |
| M ± SD | M ± SD | |||
| Age (years) | 36.8 ± 12.1 | 36.8 ± 12.9 | t(48) = −0.01 | .99 |
| Hollingshead socioeconomic status | 1.54 ± 0.5 | 2.76 ± 1.1 | χ2(1) = 13 | .01 |
| IQ | 113.8 ± 13.6 | 113.5 ± 13.6 | t(46) = −0.06 | .95 |
| WRAT arithmetic | 106.5 ± 12.4 | 98.8 ± 10.1 | t(45) = 2.41 | .02 |
| WRAT reading | 106.1 ± 10.4 | 106.6 ± 9.2 | t(46) = −0.16 | .87 |
| Age of onset of ADHD (years) | — | 5.52 ± 2.2 | — | — |
| Number of ADHD symptoms | 1.15 ± 1.9 | 13.4 ± 3.1 | 4.09 | < .00 |
Note. WRAT = Wide Range Achievement Test.
Table 2.
Current Psychiatric Comorbidities in Controls and ADHD.
| Controls (n = 24) n (%) | ADHD (n = 24) n (%) | Test statistic | p value | |
|---|---|---|---|---|
| Major depressive disorder | 0 (0) | 2 (8) | Fisher’s exact | ns |
| Bipolar disorder | 0 (0) | 0 (0) | NA | NA |
| Psychoactive substance use disorder | 0 (0) | 0 (0) | NA | NA |
| Smoking | 0 (0) | 1 (4) | Fisher’s exact | ns |
| Multiple (≥2) anxiety disorders | 0 (0) | 0 (0) | NA | NA |
| Oppositional defiant disorder | 0 (0) | 1 (4) | Fisher’s exact | Ns |
| Antisocial personality disorder | 0 (0) | 0 (0) | NA | NA |
Exclusion criteria have been described previously (Seidman et al., 2011). These excluded participants with current mood, anxiety, other disruptive behavior, psychoactive substance use disorders, deafness, blindness, psychosis, neurological disorder, sensorimotor handicaps, inadequate command of the English language, or a Full Scale IQ estimate less than 80 as measured by the Wechsler Adult Intelligence Scale–3 (WAIS-3; Wechsler, 1997). Socioeconomic status (SES) was assessed with the Hollingshead scale (Hollingshead, 1975). We recruited ADHD patients from referrals to psychiatric clinics at the Massachusetts General Hospital (MGH) and advertisements in the greater Boston area, and control participants through advertisements in the same geographical area. After complete description of the study to the participants, written informed consent was obtained, and all participants received an honorarium for participating. The study was approved by the MGH Human Subjects institutional review board (IRB) committee.
Assessment Measures
Highly trained and well-supervised lay interviewers, blind to ascertainment status, interviewed all participants with the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1997) supplemented with modules from the Kiddie SADS–E (Orvaschel, 1994) to cover ADHD and other childhood disorders. All interviews were audiotaped for random quality control assessments.
Throughout the study, interviewers were supervised by a diagnostic committee of board certified child and adolescent psychiatrists and experienced licensed psychologists at weekly meetings. The interviewers’ data were reviewed by a diagnostic committee, so that a Best Estimate diagnosis could be made. The diagnostic committee was blind to the participant’s ascertainment group, all data collected from other family members, and all nondiagnostic (e.g., brain imaging) data. Diagnoses were made for two points in time: lifetime and past month prior to interview. As noted in previous work, we computed kappa coefficients of diagnostic agreement by having members of the committee diagnose participants from the audiotapes. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was .98. Kappa coefficients for individual diagnoses included ADHD (0.88), conduct disorder (1.0), major depression (1.0), mania (0.95), separation anxiety (1.0), agoraphobia (1.0), panic (.95), substance use disorder (1.0), and tics/Tourette’s (0.89).
A neuropsychological battery was also administered that included estimates of IQ and academic achievement, which are reported here. IQ was estimated from the Block Design and Vocabulary subtests of the WAIS-3 (Wechsler, 1997). Academic achievement was assessed with the Reading and Arithmetic tests of the Wide Range Achievement Test–Three (WRAT-III; Wilkinson, 1993). Learning disability was defined by a score less than or equal to 85 on the WRAT-III Reading and/or Arithmetic scaled scores (Seidman et al., 2006).
MRI Acquisition and Analyses
Whole-brain MR images were collected on a Siemens 1.5 Tesla scanner at the MGH Martinos Center (Charlestown, Massachusetts). As described in previous studies (Seidman et al., 2011), a sagittal localizer scan was performed for placement of slices, followed by a coronal T2-weighted sequence to rule out unexpected neuropathology. Two sagittal 3D MP-RAGE (T1-weighted, nonselective inversion-prepared spoiled gradient echo pulse) sequences were collected (TR/TE/T1/flip = 2.73 s/3.39 ms/1.0 s/7, bandwidth =190 Hz/pixel, sampling matrix = 256 × 192 pixels, FOV = 256 × 256 mm, effective slice thickness = 1.33 mm on a 170 mm slab of 128 partitions) and used for morphometric analyses conducted at the MGH Center for Morphometric Analysis (CMA).
Image Analysis
Structural scans were coded and cataloged for blind analysis using FSL-VBM 1.1, a voxel-based morphometry style analysis (Ashburner & Friston, 2000; Good et al., 2001) carried out with FSL tools (Smith et al., 2004) that we used previously (Seidman et al., 2011). First, all structural data were resampled to 2 × 2 × 2 mm3, then images were brain-extracted using BET (Smith, 2002). Next, tissue-type segmentation was carried out using FAST4 (Zhang, Brady, & Smith, 2001), resulting in gray-matter partial volume images. Utilizing CMA parcellations of those scans we created cortical and subcortical masks for each participant and separated each participant’s gray-matter partial volume into a cortical part and a subcortical part. Accordingly, we created an MNI152 cortical template and subcortical template, also based on CMA parcellations (Makris et al., 2009; Seidman et al., 2011).
For cortical gray-matter partial volume images, we aligned them to the MNI152 cortical template, using the affine registration tool FLIRT (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001) followed by nonlinear registration using FNIRT (Andersson, Jenkinson, & Smith, 2007a, 2007b). The resulting images were averaged to create a study-specific cortical template, to which the native cortical gray-matter images were then nonlinearly reregistered, by using FNIRT (Andersson et al., 2007a, 2007b). The registered cortical partial volume images were then modulated (to correct for local expansion or contraction), by dividing the Jacobian of the warp field. Similar procedures using the MNI subcortical template were applied to subcortical gray-matter partial volume images. The modulated segmented images (cortical and subcortical) were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm (full width at half-maximum = 7.05 mm).
Voxelwise general linear model (GLM) was applied using permutation-based nonparametric testing (5,000 permutations). Voxel-based thresholding, uncorrected and corrected for multiple comparisons, was adopted. The uncorrected significance level was set at p < .01 for preliminary identification of reductions or increases in gray-matter volume. The significance level with the familywise error (FWE) corrected was set at p < .05. Threshold-free cluster enhancement (Smith & Nichols, 2009) was used to control FWE. Based on previous studies of our group and others in ADHD (Makris et al., 2009; Makris et al., 2010; Seidman et al., 2011) we selected six a priori anatomical ROIs, namely, the ACC, DLPFC, OFC, IPL/TOP, caudate nucleus, and CBL.
Results
Demographic, Intellectual Functioning, and Clinical Characteristics
As Table 1 shows, medication-naïve adults with ADHD did not significantly differ from control participants on age, sex, handedness, IQ, or reading achievement. Both groups had above average IQ. The only significant differences observed were in social class, which was lower in ADHD participants (Biederman et al., 2008), and on the WRAT-III arithmetic test, which is commonly lower in ADHD and considered to be an effect of the disorder (Biederman et al., 2008; Seidman, Biederman, Monuteaux, Doyle, & Faraone, 2001; Seidman et al., 2006). As shown in Table 2, there were no significant differences between groups on rates of major depressive, anxiety, bipolar, psychoactive substance abuse, smoking, or antisocial disorders at the time of the structured interview, and rates were quite low in both groups.
VBM Measures
Voxel-by-voxel uncorrected t tests (at p < .01) revealed significant volumetric increases in some parts of DLPFC, OFC and IPL/TOP and decreases in dorsal ACC, CBL and caudate nucleus in individuals with ADHD compared with controls (Figure 1; Table 3): volume results at two different thresholds). With FWE correction (p < .05), the only difference between groups was for CBL volume, which was statistically smaller for ADHD adults relative to controls.
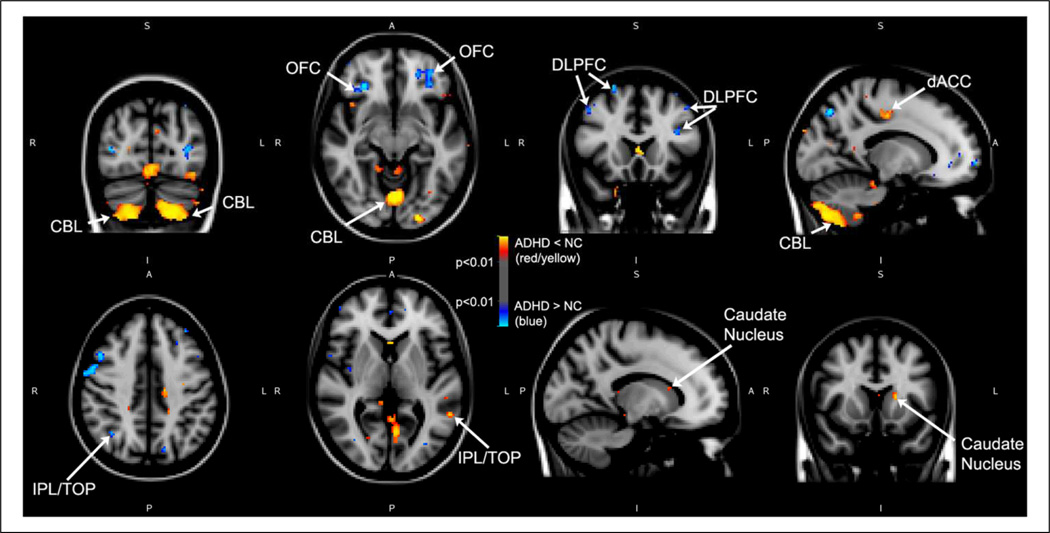
Figure 1.
Voxel-based morphometric differences between medication-naïve adults with ADHD and controls using FSL.
Note: Areas in blue represent larger volumes in medication-naïve participants with ADHD as compared with controls. Instead, red and yellow represent smaller volumes in medication-naïve participants with ADHD as compared with controls. p values are thresholded at p < .01 as shown on the color bar in the middle of the figure.
A = anterior; dACC = dorsal anterior cingulate cortex; CBL = cerebellum; DLPFC = dorsolateral prefrontal cortex; I = inferior; IPL/TOP = inferior parietal lobule/Temporo-occipito-parietal region; L = left; OFC = orbitofrontal cortex; NC = normal controls; P = posterior; R = right; S = superior.
Table 3.
Voxel-Based Morphometric Differences Between Adults With ADHD and Controls in our Selected ROIs.
| ROI–BA | Clusters of three voxels or larger at statistical threshold for control participant > ADHD (a) | ||
|---|---|---|---|
| Volume reduction in ADHD | MNI coordinates (x,y,z) | Uncorrected p = .01 | Corrected p = .05 |
| ACC–Left hemisphere | |||
| pmACC/BA24 | −14,−18,42 | 32 | |
| DLPFC–Left hemisphere | |||
| F3/BA46 | −56,30,−6 | 34 | |
| IPL/TOP–Left hemisphere | |||
| PO/BA40/S-II | −66,−24,30 | 52 | |
| SGa/BA40 | −66,−24,30 | (52) | |
| SGp/BA40 | −56,−50,4 | 23 | |
| PO/BA40/S-II | −54,−34,26 | 16 | |
| SGa/BA40 | −54,−34,26 | (16) | |
| Cerebellum–Left | |||
| VIIA_crusI-m | −56,−66,−34 | 51 | |
| VIIA_crusII-m | −42,−78,−42 | 25 | |
| VIIA_crusI-m | −42,−78,−42 | (25) | |
| VIIA_crusI-m | −40,−74,−14 | 295 | |
| IX-m | −26,−70,−62 | 1,399 | |
| VIIA_crusII-m | −26,−70,−62 | (1,399) | |
| VIIB-m | −26,−70,−62 | (1,399) | 139 |
| VIIIA-m | −26,−70,−62 | (1,399) | (139) |
| VIIIB-m | −26,−70,−62 | (1,399) | (139) |
| X-h | −26,−70,−62 | (1,399) | |
| IV-m | −18,−32,−36 | 29 | |
| IV-m | −14,−32,−22 | 19 | |
| VIIA_crusII-m | −4,−86,−24 | 16 | |
| VIIA_crusI-m | −4,−86,−24 | (16) | |
| VI-v | −2,−74,−14 | 825 | 19 |
| V-m | −2,−74,−14 | (825) | |
| Cerebellum–Right | |||
| VI-m | −2,−74,−14 | 825 | |
| VI-v | −2,−74,−14 | (825) | |
| X-h | 12,−36,−46 | 59 | |
| III-m | 14,−30,−22 | 62 | |
| IV-m | 14,−30,−22 | (62) | |
| IV-m | 20,−46,−6 | 62 | |
| IX-m | 30,−72,−62 | 1,692 | |
| VIIA_crusII-m | 30,−72,−62 | (1,692) | 618 |
| VIIB-m | 30,−72,−62 | (1,692) | (618) |
| VIIIA-m | 30,−72,−62 | (1,692) | (618) |
| VIIIB-m | 30,−72,−62 | (1,692) | |
| VIIB-m | 46,−52,−56 | 16 | |
| VIIIA-m | 46,−52,−56 | (16) | |
| VIIA_crusII-m | 50,−66,−46 | 32 | |
| VIIA_crusI-m | 50,−66,−46 | (32) | |
| VIIA_crusI-m | (56,−64,−34) | 66 | |
| Caudate nucleus–Left hemisphere | |||
| Caudate nucleus | −6,18,12 | 95 | |
| OFC–Left Hemisphere | |||
| OFC/BA47/12/VL | −56,30,−6 | 34 | |
| ROI–BA | Clusters of three voxels or larger at statistical threshold for ADHD > control participant (b) | ||
| Volume increase in ADHD | MNI coordinates (x,y,z) | Uncorrected p = .01 | Corrected p = .05 |
| DLPFC–Left hemisphere | |||
| F2/BA8 | −46,16,44 | 11 | |
| F2/BA9 | −46,16,44 | (11) | |
| F3/BA46 | −40,30,0 | 11 | |
| F2/BA9 | −38,20,24 | 33 | |
| F1/BA9/Med | 0,58,12 | 13 | |
| FP/BA9/Med | 0,58,12 | (13) | |
| DLPFC–Right hemisphere | |||
| F1/BA9/Med | 0,58,12 | 13 | |
| F1/BA8/Lat | 6,22,64 | 17 | |
| FP/BA9/Lat | 18,68,18 | 35 | |
| F1/BA8/Lat | 24,18,66 | 17 | |
| F2/BA9 | 48,14,44 | 88 | |
| F2/BA9 | 56,6,36 | 385 | |
| OFC–Left hemisphere | |||
| OFC (aFOG/pFOG)/BA11 | −18,32,−14 | 39 | |
| OFC–Right hemisphere | |||
| OFC (aFOG/pFOG)/BA11 | 12,30,−16 | 67 | |
| OFC (aFOG/pFOG)/BA11 | 30,32,−10 | 112 | |
| OFC/BA47/12/VL | 30,32,−10 | (112) | |
| IPL/TOP–Right hemisphere | |||
| AG/BA39 | 36,−58,42 | 10 | |
| Cerebellum–Right | |||
| VIIA_crusI-m | 30,−66,−28 | 190 | |
| VI-m | 30,−66,−28 | (190) | |
Note. FSL-voxel-based morphometry results comparing differences in gray-matter volume thresholded to a probability of p = .01 (uncorrected) and .05 (corrected). Local maxima are reported, including cluster size and anatomical region. Locations for statistical findings are reported in the standard Montreal Neurological Institute coordinate space (x, y, z). Some clusters border on more than one region—those clusters are reported more than once with cluster size in parentheses. ROIs are defined by the Center for Morphometric Analysis as in Caviness, Meyer, Makris, and Kennedy (1996) for the cortex and Makris et al. (2005) for the cerebellum. Cortex: ACC = anterior cingulate cortex; amACC = anterior middle cingulate cortex; pmACC = posterior middle cingulate cortex; DLPFC = dorsolateral prefrontal cortex; F1 = superior frontal gyrus; F2 = middle frontal gyrus; F3 = inferior frontal gyrus; FP = frontal pole; IPL/TOP = inferior parietal lobule/temporo-occipito-parietal region; AG = angular gyrus; PO = parietal operculum; SGa = supramarginal gyrus anterior; SGp = supramarginal gyrus posterior; S-II = somatosensory II; OFC = orbitofrontal cortex; aFOG = anterior fronto orbital gyrus; pFOG = posterior fronto orbital gyrus; VL = ventrolateral. Cerebellum: III-m = centralis; IV-m = culmen superior; V-m = culmen inferior; VI-m = simplex; VI-v = declive; VIIA_crusI-m = superior semilunar lobule; VIIA_crusII-m = inferior semilunar lobule; VIIB-m = paramedian/gracilis; VIIIA-m = biventer (pars copularis); VIIIB-m = biventer (pars paraflocculus dorsalis); IX-m = tonsil; X-m = flocculus; X-h = flocculus. Misc: BA = Brodmann’s area; CMA = Center for Morphometric Analysis; Lat = lateral; Med = medial; MNI = Montreal Neurological Institute; ROI = region of interest.
All results are in the direction of smaller volumes in the ADHD group than in control participants.
All results are in the direction of larger volumes in the ADHD group than in control participants.
Discussion
Our findings highlight significant structural volumetric alterations in the cerebellum for treatment-naïve participants, who reached adulthood while still meeting diagnostic criteria for ADHD. Differences in the DLPFC, dorsal ACC, IPL/TOP, OFC and caudate nucleus in medication-naïve adult ADHD participants relative to controls did not survive multiple comparison testing. Importantly, these structures are known to be key components of the neural systems responsible for attention, executive control and emotional regulation (Makris et al., 2009). To the best of our knowledge, this is the first documentation that medication-naïve ADHD affects the adult brain in the cerebellum, a syndrome-congruent brain region contributing to the regulation of EFs.
The results of this study indicate that medication-naïve participants affected by ADHD reach adult life with structural brain alterations. These findings are in agreement with results of other published studies in this field showing that the ACC, caudate nucleus, and CBL are volumetrically reduced in medication-naïve children with ADHD (Bledsoe et al., 2009; Semrud-Clikeman, Pliszka, Lancaster, & Liotti, 2006) and our previous VBM study that demonstrated a significantly smaller caudate nucleus volume in a larger but mixed treated and treatment-naïve sample. In this subsample study the caudate nucleus and the dorsal ACC were smaller than in controls at p < .01 uncorrected, which is consistent with prior work. These ROIs are key structural components of the anatomical neural systems subserving EF, attention, impulsivity, and emotional regulation. The DLPFC, ACC, and OFC are also important regulators of other cortical and subcortical brain regions as well, and their deficiencies appear to be consistent with the symptoms encountered in ADHD (cf. review article, Seidman et al., 2005). The mesh of connections between these frontal cortical centers qualifies them as critical networking nodes for the interface of drive, emotion, cognition, and motor function as well as for the modulation of cognitive control (Bush et al., 2000).
The observed morphometric phenotype characterized by alterations localized principally in the cerebellar hemispheres in medication-naïve adults with ADHD demonstrates that there is persistence of cerebral abnormalities. Moreover, there is an absence of resolution of an abnormal morphometric phenotype present earlier in childhood and adolescence, as has been shown in pediatric MRI studies (Castellanos et al., 2002). It is of interest that reductions in cerebellar volumes in previous work with children were shown to be present in ADHD children even after controlling for whole-brain volumes that had been significantly different (Castellanos et al., 2001). This observation combined with our current findings supports the conceptualization of the syndromatic continuity between pediatric and adult ADHD at the neural system level.
Although structural alterations in the cortical networks involved in EF, attention, and impulse control have been considered to be central to the symptoms of ADHD, the cerebellum may be another crucial structure accounting for ADHD’s phenomenology (Seidman et al., 2005; Valera et al., 2007; Valera et al., 2010). The notion of cerebellar involvement in ADHD has been proposed since the 1990s (Levinson, 1990) and since then volumetric changes have been documented in medicated children and adolescents (Berquin et al., 1998; Castellanos et al., 2001; Castellanos et al., 2002; Mostofsky, Reiss, Lockhart, & Denckla, 1998) as well as adults with ADHD, and in medication-naïve children with ADHD (Bledsoe et al., 2009). In the present study, the CBL showed the most statistically robust volume decreases in the brains of medication-naïve adult ADHD participants. Interestingly, these alterations were not diffusely scattered but localized in specific cerebellar regions (principally in the medial hemispheric zone, where 90% of the voxels showing statistically significant volumetric difference were localized) associated with motor (anterior lobe and lobule VIIIA/B), executive (lobules VI, Crus I, and VIIB), and emotional (lobules VI and Crus I as well as vermal and medial lobule VII) functions (Stoodley & Schmahmann, 2009; Strick, Dum, & Fiez, 2009).
The cerebellum is massively interconnected with the frontal, parietal, temporal, and occipital cerebral cortex (Strick et al., 2009) exerting an influence over nonmotor regions of the cerebrum and, therefore, plays an important role in human cognitive and emotional functions (Strick et al., 2009). Our findings of cerebellar volumetric alterations support the idea that cortico-subcortico-cerebellar loops (Strick et al., 2009) may be affected in ADHD and contribute to the symptomatology of this disorder (Castellanos et al., 2002; Makris et al., 2009). We suggest that the association of these structural brain abnormalities with the above behavioral alterations provide the anatomical-behavioral profile of an untreated adult participant who meets criteria for ADHD. Thus, untreated participants with ADHD attain adulthood with distinct brain abnormalities in their cerebellum.
Although the other ROIs hypothesized to be different in ADHD than in controls did not survive multiple comparison testing, to reject them out of hand could conceivably be making a Type II error. It is true, of course, that a voxel-by-voxel t-test approach assessing thousands of voxels with no correction for multiple comparisons and no cluster thresholding will by definition result in many regions looking significant at p < .01. However, we are reluctant to completely reject our hypotheses for these ROIs due to the partial support observed and the wealth of literature supporting this hypothesis. Functionally, convergent evidence from functional magnetic resonance imaging (fMRI; Bush et al., 2000) and evoked potential experimentation in humans suggest that DLPFC and ACC are associated with monitoring of conflict and modulation of cognitive control as well as modulation of allocation of attention in real time. Interactions between the DLPFC, OFC, IPL, amygdala, and brainstem centers such as the locus coeruleus or the ventral tegmental area, enable the ACC to integrate sensitive information in real time to monitor conflict associated with competitive cognitive tasks and, in concert with the DLPFC, to modulate cognitive control and produce balanced behavior (Bush et al., 2000). Furthermore, lateral OFC connections with lateral prefrontal and dorsal ACC (BA 24, 32) neurons are relevant in translating motivational information into action. Moreover, a pattern of deficits involving all of these frontal structures may cause a breakdown in monitoring of conflict as well as inefficient modulation of cognitive control and allocation of attention, which may result in the impulsivity, hyperactivity, and inattention characteristic of ADHD.
Our findings need to be viewed in the light of some methodological limitations. While VBM allows a large number of brains to be measured without the influence of raters, it suffers from its own intrinsic limitations, as do other automated procedures, particularly the problem of coregistration. Despite these limitations, the current methods of registration used in this study represent state of the art technology in this domain. We also performed a large number of statistical tests on six ROIs that vary in size, and thus we are vulnerable to making a Type I error. However, our main finding on the cerebellum is consistent with a priori hypotheses and with the bulk of the literature in children and adults with ADHD. These results were observed using samples of limited size, which constrained the ability to find significant differences at the FWE threshold. This could account for the lack of significance for some of the other ROIs. Moreover, generalizeability is limited until we conduct studies using larger samples.
Despite these considerations, in this sample of medication-naïve adults with ADHD we found volumetric alterations in critical cerebellar areas, which are key structures of the neural systems subserving EFs. Given their involvement in neural operations central to attention and EF, these syndrome-congruent MRI results in medication-naïve participants who reached adulthood while still meeting diagnostic criteria for ADHD, support the notion that ADHD is associated with cortical and functional alterations in brain regions that are critical for the regulation of EF across the life cycle.
Acknowledgments
We thank Sharmila Bandyopadhyay, Denise Boriel, Katherine Crum, Dr. Alysa Doyle, Dr. Ronna Fried, Jonathan Kaiser, Kalika Kelkar, Alexandra Lomedico, John Schlerf, Michael Schiller, Michael Vitulano, and Dr. Timothy Wilens for their contributions.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Financial Disclosures: Dr. Joseph Biederman is currently receiving research support from the following sources: APSARD, The Department of Defense, Elminda, Janssen, McNeil, Shire, and Vaya Pharma/Enzymotec. In 2013, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Shire and Sunovion; these royalties are paid to the Department of Psychiatry at MGH. In 2012, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy and The Children’s Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded CME courses. In 2011, Dr. Joseph Biederman gave a single unpaid talk for Juste Pharmaceutical Spain, received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course, and received honoraria for presenting at international scientific conference on ADHD. He also received an honorarium from Cambridge University Press for a chapter publication. Dr. Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Eli Lilly, , and AstraZeneca; these royalties are paid to the Department of Psychiatry at MGH. In 2010, Dr. Joseph Biederman received a speaker’s fee from a single talk given at Fundación Dr. Manuel Camelo A. C. in Monterrey Mexico. Dr. Biederman provided single consultations for Shionogi Pharma Inc. and Cipher Pharmaceuticals Inc.; the honoraria for these consultations were paid to the Department of Psychiatry at the MGH. Dr. Biederman received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Celltech, Cephalon, Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Glaxo, Gliatech, Hastings Center, Janssen, McNeil, Medice Pharmaceuticals (Germany), Merck, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth.
Dr. Valera has received travel support and/or honoraria from Galenea, Eli Lilly, Shire Pharmaceuticals, and divisions of Ortho-McNeil Janssen Pharmaceuticals (McNeil Pediatrics and Janssen Pharmaceuticals), Remedica Medical Education and Publishing, MGH Psychiatry Academy for a tuition-funded CME course and consulting, Reed Exhibitions. She also received money from the Society for Biological Psychiatry to help defray conference travel costs, reimbursements from Veritas Institute, and research support from the National Institutes of Health.
Dr. Thomas Spencer has received research support from, has been a speaker for or on a speaker bureau, or has been an Advisor or on an Advisory Board of the following sources: Shire Laboratories, Inc, Eli Lilly & Company, Glaxo-Smith Kline, Janssen Pharmaceutical, McNeil Pharmaceutical, Novartis Pharmaceuticals, Cephalon, Pfizer, the National Institute of Mental Health. Dr. Spencer receives research support from Royalties and Licensing fees on copyrighted ADHD scales through MGH Corporate Sponsored Research and Licensing. Dr. Spencer has a U.S. Patent Application pending (Provisional Number 61/233,686), through MGH corporate licensing, on a method to prevent stimulant abuse.
In the past year, Dr. Faraone received consulting income and research support from Shire and Alcobra and research support from the NIH. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by Shire, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health and Genetics of Mental Disorders and from Oxford University Press: Schizophrenia: The Facts.
Dr. Seidman currently receives research support from the NIH, the Commonwealth of Massachusetts, and the Sidney R. Baer Foundation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported, in part, by grants from NIDA 1R01DA027804-01, NIMH 1R21MH084041-01A1 (to N.M.); NIMH MH/HD 62152 and the Mental Illness and Neuroscience Discovery (MIND) Institute (to L.J.S.); National Research Service Award (NIMH F32 MH065040-01A1), Peter Livingston Fellowship through the Harvard Medical School Department of Psychiatry, the Clinical Research Training Program Fellowship in Biological and Social Psychiatry MH-16259, MH 071535 and R01 HD 067744 (to E.M.V.), NIMH MH 57934 (to S.V.F.); the National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award, the Pediatric Psychopharmacology Council Fund, and the Johnson and Johnson Center for the Study of Psychopathology (to J.B.); and The National Center for Research Resources (P41RR14075).
Biographies
Nikos Makris, MD, PhD, is an Associate Professor of Psychiatry and Neurology in the Harvard Departments of Psychiatry and Neurology at Massachusetts General Hospital. He is a systems neuroanatomist, imager and psychiatrist with a longstanding interest in understanding brain networks in neurodegeneration and psychiatric disorders such as ADHD, drug abuse and schizophrenia.
Lichen Liang, PhD, is a Postdoctoral Fellow at the Martinos Center for Biomedical Imaging at Massachusetts General Hospital.
Joseph Biederman, MD, is Chief of the Clinical and Research Programs in Pediatric Psychopharmacology and Adult ADHD at the Massachusetts General Hospital, Director of the Alan and Lorraine Bressler Clinical and Research Program for Autism Spectrum Disorders at the Massachusetts General Hospital, and Professor of Psychiatry at the Harvard Medical School. Dr. Biederman is Board Certified in General and Child Psychiatry.
Eve M. Valera, PhD, is currently an Assistant Professor of Psychiatry at Harvard Medical School and a Research Scientist at Massachusetts General Hospital. She is currently using structural and functional neuroimaging methodologies, including diffusion tensor imaging, to elucidate the neural substrate of Attention-Deficit/Hyperactivity Disorder (ADHD). A specific focus of interest is how cerebellar abnormalities and abnormalities in cortico-cerebellar connections may influence ADHD pathophysiology.
Ariel B. Brown, PhD, is a Postdoctoral Fellow at the Pediatric Psychopharmacology Clinic and the Martinos Center for Biomedical Imaging at Massachusetts General Hospital and Harvard Medical School
Carter Petty, MS, is a biostatistician. He received his master's degree in Statistics from Harvard University in 2002 and a bachelor's degree in Psychology from Stanford University in 1998.
Thomas J. Spencer, MD, is an Associate Professor of Psychiatry at Harvard Medical School and the Assistant Chief of the Pediatric Psychopharmacology Research Program at Massachusetts General Hospital.
Stephen V. Faraone, PhD, is a Professor in the Departments of Psychiatry and Neuroscience & Physiology at SUNY Upstate Medical University He is also Senior Scientific Advisor to the Research Program Pediatric Psychopharmacology at the Massachusetts General Hospital and a lecturer at Harvard Medical School. Dr. Faraone studies the nature and causes of mental disorders in childhood and has made contributions to research in psychiatric genetics, psychopharmacology, diagnostic issues and methodology.
Larry J. Seidman, PhD, is a Professor of Psychology in the Harvard Department of Psychiatry at Beth Israel Deaconess Medical Center. He is a neuropsychologist with a longstanding interest in understanding the neural substrates of neurodevelopmental disorders such as ADHD and schizophrenia.
Footnotes
Declaration of Conflicting Interests
Dr. Makris and the other authors have no disclosures.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear optimisation (FMRIB technical report No. TR07JA1) Oxford, UK: Oxford University Press; 2007a. [Google Scholar]
- Andersson J, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation (FMRIB technical report No. TR07JA2) Oxford, UK: Oxford University Press; 2007b. [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. NeuroImage. 2000;11(Pt. 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport JL, Castellanos FX. Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Kaiser R, Dolan CR, Schoenfeld S, Faraone SV. Educational and occupational underattainment in adults with attention-deficit/hyperactivity disorder: A controlled study. Journal of Clinical Psychiatry. 2008;69:1217–1222. doi: 10.4088/jcp.v69n0803. [DOI] [PubMed] [Google Scholar]
- Bledsoe J, Semrud-Clikeman M, Pliszka SR. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biological Psychiatry. 2009;65:620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, Rapoport JL. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Meyer J, Makris N, Kennedy DN. MRI-based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. Journal of Cognitive Neuroscience. 1996;8:566–587. doi: 10.1162/jocn.1996.8.6.566. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV Axis I disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson HN. The diagnostic value of cerebellar-vestibular tests in detecting learning disabilities, dyslexia, and attention deficit disorder. Perceptual and Motor Skills. 1990;71:67–82. doi: 10.2466/pms.1990.71.1.67. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Developmental Neuroscience. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, Schmahmann JD. MRI-based surface-assisted parcellation of human cerebellar cortex: An anatomically specified method with estimate of reliability. NeuroImage. 2005;25:1146–1160. doi: 10.1016/j.neuroimage.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Makris N, Seidman LJ, Valera EM, Biederman J, Monuteaux MC, Kennedy DN, Faraone SV. Anterior cingulate volumetric alterations in treatment-naive adults with ADHD: A pilot study. Journal of Attention Disorders. 2010;13:407–413. doi: 10.1177/1087054709351671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. Journal of Child Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for affective disorder and schizophrenia for school-age children epidemiologic version. 5th ed. Fort Lauderdale, FL: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, III, Xiong J, Liotti M. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. American Journal of Psychiatry. 2006;163:1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, Makris N. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biological Psychiatry. 2011;69:857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Monuteaux MC, Doyle AE, Faraone SV. Learning disabilities and executive dysfunction in boys with attention-deficit/hyperactivity disorder. Neuropsychology. 2001;15:544–556. doi: 10.1037//0894-4105.15.4.544. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Valera EM, Monuteaux MC, Doyle AE, Faraone SV. Neuropsychological functioning in girls with attention-deficit/hyperactivity disorder with and without learning disabilities. Neuropsychology. 2006;20:166–177. doi: 10.1037/0894-4105.20.2.166. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Pliszka SR, Lancaster J, Liotti M. Volumetric MRI differences in treatment-naive vs. chronically treated children with ADHD. Neurology. 2006;67:1023–1027. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neuroscience & Biobehavioral Reviews. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Seidman LJ. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Pradhan K. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. NeuroImage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale III [manual] 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wilkinson GS. WRAT3 Wide Range Achievement Test: Administration manual. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]