Abstract
Purpose
To compare and assess subregional, compartmental, and whole T1rho values of menisci in patients with doubtful-minimal [Kellgren-Lawrence (KL) grade 1–2] as compared to moderate-severe (KL3–4) osteoarthritis (OA) and healthy controls at 3T.
Materials and Methods
46 subjects were included in the study and subdivided into three subgroups: 16 healthy controls (4 females, 12 males; mean age = 34.4±10.2 years, age range 24–63 years), 20 patients with doubtful-minimal (KL1–2) OA (9 females, 11 males; mean age = 61.9±10.8 years, age range 40–80 years), and 10 patients with moderate-severe (KL3–4) OA (4 females, 6 males; mean age = 71.1±9.6 years, age range 58–89 years). All subjects were evaluated on a 3T MR scanner using a spin-lock-based 3D GRE sequence for T1rho mapping. Clinical proton density (PD)-weighted fast spin echoes (FSE) images in the sagittal (without fat saturation), axial, and coronal (fat-saturated) planes were acquired for cartilage Whole-Organ MR Imaging Score (WORMS) grading. Analysis of covariance (ANCOVA) was performed to determine whether there were any statistically significant differences between subregional, compartmental, and whole T1rho values of meniscus among healthy controls, OA patients with KL1–2 and with KL3–4.
Results
Lateral anterior (median±inter-quartile range: 26±3 ms) and medial posterior (29±6 ms) meniscus subregions in healthy controls had significantly lower T1rho values (p < 0.05) than the corresponding meniscus subregions in both KL1–2 (29±7 ms and 35±8 ms, respectively) and KL3–4 (30±12 ms and 40±13 ms, respectively) OA subjects. Significantly lower meniscus T1rho values (p < 0.05) were also identified in the medial compartment in healthy controls (28±5 ms) relative to both KL1–2 OA subjects and KL3–4 OA subjects (32±7 ms and 37±7 ms, respectively). The entire meniscus T1rho values in healthy controls (28±4 ms) were significantly lower than those of both KL1–2 and KL3–4 OA subjects (33±6 ms and 34±6 ms, respectively).
Conclusion
Significant elevations of T1rho values in specific regions of menisci in both KL1–2 and KL3–4 OA patients indicate that T1rho mapping may be sensitive to meniscus degeneration. The preliminary results suggest that damage in the medial posterior subregion and medial compartment of menisci may possibly be associated with osteoarthritis.
Keywords: Meniscus, T1rho mapping, Cartilage, Osteoarthritis, MRI
Introduction
Meniscus injury is one well-known risk factor in the pathogenesis of both post-traumatic and idiopathic osteoarthritis (OA) [1]. Knee OA is a multi-factorial disease, the origin and progression of which may be associated with any tissues of the joint and knee abnormalities such as cartilage, subchondral bone, synovium, capsule, anterior cruciate ligament (ACL), meniscus, and knee malalignment (1–5). Meniscus tear is associated with increased risk of radiographic OA and cartilage volume loss (1). T1rho values as well as T2 values of the meniscus have been found to correlate with clinical findings of OA, and can be used to differentiate healthy subjects from patients with mild or severe OA (2).
The earliest biochemical changes that occur in OA are molecular modifications within the cartilage matrix without obvious morphological defects (6, 7). When abnormal or repetitive mechanical loads exceed the adaptive capacity of normal articular cartilage, absolute overloading happens, which may result in biomaterial failure of the extracellular matrix (ECM) and/or chondrocyte injury (8). The loss of glycosaminoglycans (GAGs), the variation of water content, and molecular level changes in collagen are typical characteristics of early OA (9). It has been observed that the decrease of proteoglycan (PG) content is an initiating event in early OA; however, both the content and the type of collagen are not altered in early OA (8, 9).
Hyaline cartilage and menisci are mainly composed of water, collagen, and proteoglycans (PGs) (10, 11). Although hyaline cartilage and the meniscus mainly contain water, collagen, and PGs, the meniscus has a relatively lower PG concentration of 1%-2% (higher collagen content) compared to 5%-10% in that of hyaline cartilage (2). Generally, low GAG means higher T1rho values, and high GAG means lower T1rho values (3, 6, 7, 9); however, cartilage and menisci have different biochemical, structural, and biomechanical properties (2, 7, 10). Previous studies have reported that T1rho relaxation time is sensitive to early biochemical changes in cartilage, especially the PG content (7, 10). The menisci play a key role in distributing joint forces, load bearing, and enhancing joint stability within the knee joint. Meniscal tears alter joint biomechanics and correlate with an accelerated progression of cartilage degradation in knee OA compared to OA patients without meniscal tears (11, 12). The injury in meniscus diminishes its ability to disperse loads and absorb shock that can cause and exacerbate degenerative changes in the knee (1).
T1rho (or T1ρ- T1 relaxation time in the rotating frame)-weighted MR imaging has recently been proposed as an attractive alternative biomarker to existing conventional morphological MRI methods (7, 9, 10, 13–15). T1rho-weighted MR imaging was first described by Redfield (16), and related techniques have been used to investigate the slow motion interactions between macromolecular protons and bulk water (9, 14). T1rho mapping has been shown to be sensitive to changes in proteoglycan loss in cartilage (14). Recent work (1) has reported that UTE-T2* mapping is sensitive to subclinical meniscus degeneration. Other investigators evaluated meniscus degeneration using both T1rho and T2 mapping to compare OA patients in comparison with those of healthy controls (2). In this work, at 3T we measured and compared the subregional, compartmental, and global T1rho values of menisci in patients with doubtful to severe OA based on Kellgren-Lawrence (KL) grading compared to those of healthy controls. Based on the findings in the previously published studies (1–3), we hypothesized that in OA patients, T1rho values would be increased within specific focal regions such as medial central and posterior sub-regions of the meniscus, and that regional damage of menisci may possibly be associated with osteoarthritis.
Materials and Methods
Study population
All subjects provided written informed consent to participate in the study, which was approved by the local institutional review board (IRB). Inclusion criteria for all subjects were good health according to medical history, physical examination, and the absence of contraindications to MR imaging. For the healthy cohort, the inclusion criteria were: no history of chronic or frequent knee pain, absence of clinical symptoms and normal knee radiographs at the time of the MRI examination. For the cohort of OA patients, the inclusion criteria were the presence of clinical symptoms of knee OA (frequent or chronic knee pain and limited function as assessed by using medical history and physical examination) and radiographic evidence of OA required to be in the same knee. Exclusion criteria were inflammatory arthritis and knee OA secondary to some other causes (i.e., acute or chronic infection, previous surgery, or previous fracture).
46 subjects (n = 29 males and n = 17 females, ranging in age from 24 to 89 years, mean±SD = 54.3±18.2 years) (Table 1) with normal knees to severe OA based on radiographs [Kellgren-Lawrence (K-L) grading scale 0, 1, 2, 3, and 4 (16, 17)] were recruited. The radiographs were read by an experienced (8 years of experience) musculoskeletal radiologist ( _ _ ) who assigned a KL grade to each knee. The radiographic changes were classified as normal (0), doubtful (1), minimal (2), moderate (3), and severe (4) [17, 18]. The changes included marginal osteophytes, narrowing of joint space, sclerosis of subchondral bone, and altered bone contours (n = 6 as KL1, n = 14 as KL2, n = 6 as KL3, n = 4 as KL4). The subjects were then further divided into three subgroups as healthy controls (KL0, n = 16), KL1–2 (doubtful-minimal radiographic changes, n = 20) and KL3–4 (moderate-severe radiographic changes, n = 10), respectively. The subjects’ body height and weight were obtained in order to calculate the body mass index (BMI). Subjects with a BMI of greater than 24.9 kg/m2 were classified as overweight, and subjects with a BMI of greater than 29.9 kg/m2 were classified as obese (11).
Table 1.
Characteristics of the Study Population
| Volunteers group and characteristic |
Healthy controls | Doubtful-minimal OA (KL1–2) |
Moderate-severe OA (KL3–4) |
|---|---|---|---|
| All volunteers | |||
| No. of volunteers | 16 | 20 | 10 |
| Age (yrs) * | 34.4±10.2 | 61.9±10.8 | 71.1±9.6 |
| BMI (kg/m2) | 24.5±2.3 | 26.5±4.1 | 26.9±3.8 |
| Total WORMS ** | 0 | 13.5±12.7 | 32.8±11.9 |
| Female volunteers | |||
| No. of volunteers | 4 | 9 | 4 |
| Age (yrs) | 29.8±6.0 | 60.2±9.8 | 74.0±13.0 |
| Age range (yrs) | 24 – 38 | 40 – 74 | 58 – 89 |
| Male volunteers | |||
| No. of volunteers | 12 | 11 | 6 |
| Age (yrs) | 36.0±11.0 | 63.2±11.9 | 69.2±7.3 |
| Age range (yrs) | 25 – 63 | 46 – 80 | 60 – 80 |
Note: WORMS = Whole-Organ MR Imaging Score.
BMI = Body Mass Index.
There were significant differences (p < 0.05) in volunteers’ Age between healthy controls and doubtful-minimal (KL1–2) OA groups, healthy controls and moderate-severe (KL3–4) OA groups, and doubtful-minimal (KL1–2) OA and moderate-severe (KL3–4) OA groups, respectively.
There were significant differences (p < 0.05) in Total WORMS between healthy controls and doubtful-minimal (KL1–2) OA groups, healthy controls and moderate-severe (KL3–4) OA groups, and doubtful-minimal (KL1–2) OA and moderate-severe (KL3–4) OA groups, respectively.
Imaging hardware
All MRI experiments were performed on a 3.0T clinical MR scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany). An 18-cm diameter, 8-channel transmit-receive phased-array knee coil was employed for the imaging measurements.
Imaging protocol
Anteroposterior radiographs of the knee in the standing position in all patients with OA were obtained to determine the K-L grade.
The morphology of cartilage was assessed by acquiring routine clinical sequences which included sagittal proton density (PD)-weighted without fat saturation and axial and coronal PD-weighted fast spin echo (FSE) fat-saturated images used for Whole-Organ MR Imaging Score (WORMS) grading (19).
For 3D-T1rho Imaging, 3D T1rho-weighted images with parallel imaging (acceleration factor [AF] = 2) were acquired using the GRE sequence based on the spin-lock technique.
The acquisition parameters of all MR imaging sequence were listed in Table 2.
Table 2 .
MR Imaging Sequence Parameters
| Imaging Parameters | 2D FSE | 2D FSE | 2D FSE | 3D GRE |
|---|---|---|---|---|
| Weighting | PD | PD | PD | T1rho |
| Plane | Sagittal | Axial | Coronal | Sagittal |
| Fat sat | No | Yes | Yes | Yes |
| Matrix | 512 × 512 | 512 × 512 | 512 × 512 | 256 × 128 |
| No. of slices | 35 | 35 | 35 | 30 |
| FOV (mm) | 100 | 100 | 100 | 150 |
| Slice thickness (mm) | 2.5 | 2.5 | 2.5 | 3 |
| Flip angle (°) | 90 | 90 | 90 | 25 |
| TE (ms) | 19 | 19 | 19 | 2.04 |
| TR (ms) | 3030 | 3240 | 3240 | 175 |
| BW (Hz / pixel) | 255 | 255 | 255 | 260 |
| Interpolated in-plane spatial resolution (mm) | 0.19 × 0.19 | 0.19 × 0.19 | 0.19 × 0.19 | 0.59 × 0.59 |
| Echo train length | 5 | 5 | 5 | 1 |
| NEX | 1 | 1 | 1 | 1 |
| duration of each 900 pulse (us) | 1536 | 1536 | 1536 | 200 |
| Acceleration factor | _ | _ | _ | 2 |
| Spin-lock frequency (Hz) | _ | _ | _ | 300 |
| Time of spin-lock (TSL) [ms] | _ | _ | _ | 2/10/20/30 |
| Acquisition time (TA) | 2 minutes 4 seconds | 2 minutes 4 seconds | 2 minutes 5 seconds | 5 minutes 31 seconds / TSL |
MR images analysis and processing
The morphologic evaluation of cartilage was performed using 2D-sagittal PD-weighted FSE without fat saturation and axial and coronal PD-weighted FSE with fat saturation by two experienced musculoskeletal radiologists (_ _, 3 years of experience, and _ _, 15 years of experience, respectively). The radiologists were blinded to subjects’ clinical history, K-L scores, and T1rho relaxation data. Cartilage scoring was performed using the WORMS grading as defined in (19): 0 = normal thickness and signal; 1 = normal thickness but increased signal; 2.0 = partial thickness focal defect <1 cm in greatest width; 2.5 = full thickness focal defect <1 cm in greatest width; 3 = multiple areas of partial thickness (grade 2.0) defects intermixed with areas of normal thickness, or a grade 2.0 defect wider than 1 cm but <75% of the region; 4 = diffuse (≥75% of the region) partial thickness loss; 5 = multiple areas of full thickness loss (grade 2.5) or a grade 2.5 lesion wider than 1 cm but <75% of the region; 6 = diffuse (≥75% of the region) full-thickness loss.
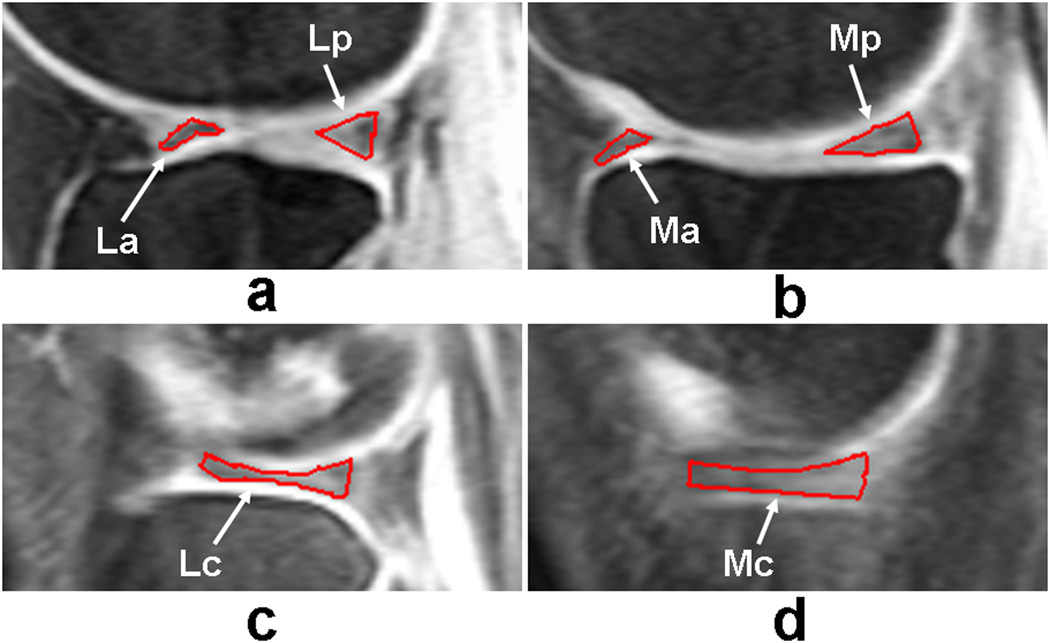
For quantitative T1rho assessment of meniscus, all the MR images were analyzed based on the subregional, compartmental, and whole meniscus. Six subregions, two compartmental regions, and whole meniscus were defined for meniscus assessment: lateral anterior (La), lateral central (Lc), lateral posterior (Lp), medial anterior (Ma), medial central (Mc), medial posterior (Mp), lateral side meniscus (L), medial side meniscus (M), and entire meniscus (Entire) as defined in Fig. 1 (19, 20).
Fig. 1.
Representative sagittal fat-saturated T1rho-weighted 3D gradient-echo images with time of spin-lock (TSL) = 2 ms illustrating how the regions of interest (ROIs) of meniscus subregions in the lateral [Fig. 1(a, c)] and medial [Fig. 1(b, d)] compartments were defined, obtained from a doubtful (KL1) OA patient. White arrowheads mark the location of the meniscus subregions.
T1rho-weighted images with shortest spin-lock length (TSL = 2 ms) were used for the segmentation of menisci in both subregions and whole regions. Regions of interest (ROIs) as above defined were segmented manually (by _ _) for each slice for all the subjects. These segmentations were used to draw ROIs for each MR image with different TSL values (7, 9). The in-house developed routines in MATLAB (version 7.1; The Mathworks, Natick, MA, USA) and C++ (version 6.0, Microsoft Corp., USA) were used for offline processing of the acquired MR images. T1rho maps were computed with custom-built MATLAB and C++ routines using the signal expression as shown in Equation 1 (7).
| (1) |
Here x is the pixel coordinate, α is the flip angle, TR is the repetition time, A(x) is the amplitude of the signal, and T1(x) is the spin-lattice relaxation time of the tissue to which the pixel belongs. This equation represents a decay when it is considered as a function of TSL. It is possible to estimate T1 ρ(x) by fitting the curve represented by the equation to the intensities of the pixel in images acquired with different TSL values. For each 3D dataset which consists of the 3D scans of the same subject with four different TSL values (four 3D data sets), each pixel in the ROIs was fitted to Equation 1 in least-squared (LS) sense (7). 3D-T1rho imaging technique has the advantage of whole knee joint coverage with same longitudinal steady state magnetization for each TR (7). Secondly, 3D T1rho-method requires shorter TSL durations due to small flip angle excitation compared to 2D-T1rho imaging method.
Although the K-L grading scale may be effective in defining the presence of and estimating the severity of articular cartilage degeneration within the femorotibial joint, in one previous study, it did not identify osteoarthritis in a large number of patients with the disease and had only a moderately strong correlation with the actual degree of articular cartilage degeneration within the femorotibial joint (18). Therefore, both K-L (based on radiographs) and WORMS (morphological MRI) grading scales were used in the clinical evaluation of cartilage in this study. For the WORMS grading of articular cartilage degeneration, the highest WORMS grade was used to represent the severity of osteoarthritis of the tibiofemoral joint (3, 19).
Statistical Methods
The inter-observer variability of cartilage WOMRS grading bewteen the two radiologists was performed using weighted Kappa statistics.
The regional T1rho values are summarized for each subject group as median ± inter-quartile range. The comparison subgroups (healthy controls, KL = 0; doubtful-minimal OA, KL = 1–2; moderate-severe OA, KL = 3–4) were not matched in terms of gender, age or BMI. Consequently, subgroup comparisons were adjusted for these potential confounding factors. Due to near perfect confounding of the comparison subgroups with WORMS score (e.g., all controls had WORMS0–1; 9 of 10 moderate-severe OA patients had WORMS5–6), the comparisons could not be meaningfully adjusted for WORMS. Since the outcome (T1rho) were not observed to follow a normal distribution and the sample sizes were not sufficient for an asymptotic justification of the normality assumption, nonparametric methods were used to compare the subject subgroups in terms of T1rho. Specifically, exact Mann-Whitney tests were used to compare the subgroups in terms of T1rho without covariate adjustment and analysis of covariance (ANCOVA) based on ranks was used to make the comparisons while adjusting for age, gender, and BMI. All comparisons were stratified by compartment. For the ANCOVA, the T1rho values recorded for a given compartment were first converted to ranks and the ranks were used as the dependent variable. The ANCOVA model for each compartment included age and BMI as numeric covariates, gender as a nominal covariate and subject subgroups as the between-subjects nominal factor of interest. All statistical tests were conducted at the two-sided 5% comparison-wise significance level and tabulated p values are marked with an asterisk when significant at the Bonferroni-corrected significance level of 0.05/27, where 27 is the total number of tests conducted using SAS 9.3 (SAS Institute, Cary, NC).
Results
The mean BMI of the subjects included in this study was 25.9±3.6 kg/m2. The BMI was within the normal range in 11 subjects (52%); 11 subjects were overweight (28%) and 8, obese (20%). There were no significant differences (p > 0.05) in BMI among the three subgroups of healthy controls, doubtful-minimal (KL1–2) and moderate-severe (KL3–4) OA patients (Table 1).
16 subjects were clinically evaluated as WORMS0–1 (normal), 10 as WORMS2–4 (early degeneration), and 20 as WORMS5–6 (advanced degeneration). The weighted Kappa statistic to assess the agreement between the two radiologists of the cartilage WORMS grading was 0.93 and a 95% confidence interval for this Kappa coefficient extended from 0.81 to 1.0. These results imply near perfect agreement for the inter-observer reliability.
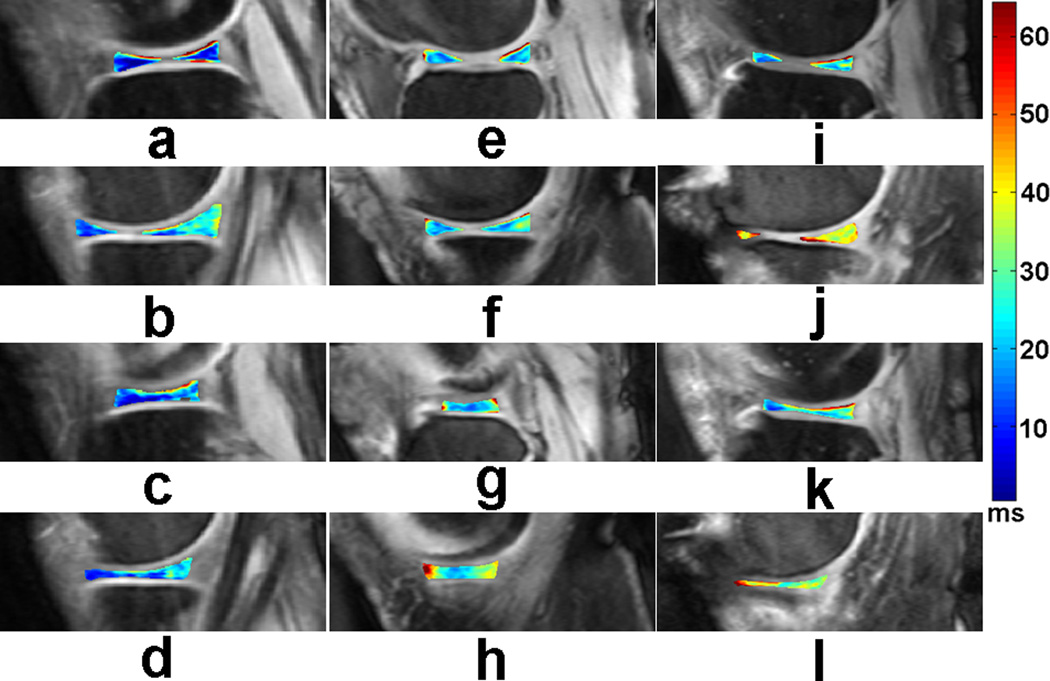
Representative T1rho maps of menisci in the lateral (a, c, e, g, i, k) and medial (b, d, f, h, j, l) compartments, obtained from a healthy control (a, b, c, d), a minimal (KL2) OA patient (e, f, g, h), and a severe (KL4) OA patient (i, j, k, l), respectively are displayed in Fig. 2.
Fig. 2.
Representative T1rho maps of menisci in the lateral (a, c, e, g, i, k) and medial (b, d, f, h, j, l) compartments, obtained from a healthy control (a, b, c, d), a minimal (KL2) OA patient (e, f, g, h), and a severe (KL4) OA patient (i, j, k, l), respectively. The color bar on the right shows the range of T1rho values.
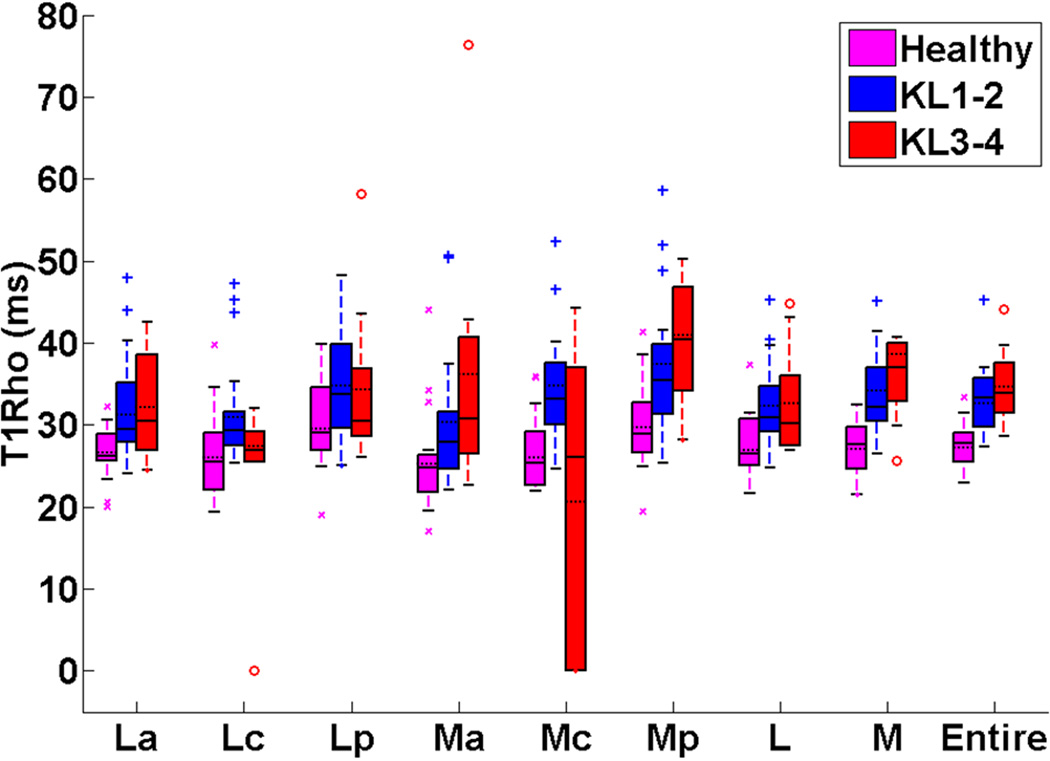
Fig. 3 showed the box and whisker plots of meniscus T1rho values in the different meniscus subregions and for the whole meniscus among healthy controls, doubtful-minimal (KL1–2) OA patients, and moderate-severe (KL3–4) OA patients. The T1rho values for subregions of the meniscus and the whole meniscus (La, Lc, Lp, Ma, Mc, Mp, L, M, entire) for healthy controls, doubtful-minimal (KL1–2) OA patients, and moderate-severe (KL3–4) OA patients were summarized in Table. 3.
Fig. 3.
Box and whisker plots of meniscus T1rho values (median±interquartile range) in the different meniscus subregions and for the whole meniscus among healthy controls, doubtful-minimal (KL1–2) OA patients, and moderate-severe (KL3–4) OA patients. The box and whisker plot shows the five statistics (minimum, first quartiles, median, third quartiles, and maximum). The horizontal dashed lines on the boxes show the corresponding mean values.
Table 3.
T1rho values in ms (median±interquartile range) of meniscus among healthy controls, doubtful-minimal (KL1–2) OA, and moderate-severe (KL3–4) OA.
| Subregional / Compartmental / Whole |
Control (KL=0) | KL1–2 | KL3–4 |
|---|---|---|---|
| La | 26±4 | 29±9 | 30±12 |
| Lc | 25±7 | 29±4 | 27±11 |
| Lp | 29±8 | 34±11 | 30±10 |
| Ma | 25±5 | 28±8 | 31±15 |
| Mc | 25±7 | 33±8 | 26±37 |
| Mp | 29±6 | 35±9 | 40±13 |
| L | 26±6 | 31±6 | 30±10 |
| M | 28±6 | 32±7 | 37±8 |
| Entire | 28±4 | 33±6 | 34±7 |
As listed in Tables 3 and 4, when compared based on ANCOVA analysis, lateral anterior (26±4 ms) and medial posterior (29±6 ms) meniscus subregions in healthy controls had significantly lower T1rho values (p < 0.03) than the corresponding meniscus subregions in both KL1–2 (29±9 ms and 35±9 ms, respectively) and KL3–4 (30±12 ms and 40±13 ms, respectively) OA subjects. Significantly higher T1rho values of lateral central meniscus subregion (Lc) [p = 0.046] were identified in KL1–2 OA patients (29±4 ms) than those of KL3–4 OA patients (27±11 ms). Healthy controls (25±7 ms and 26±6 ms, respectively) had significantly lower T1rho values than the KL1–2 OA patients (33±8 ms and 31±6 ms, respectively) medial central meniscus subregion (Mc; p < 0.01) and in the lateral compartment (L; p = 0.012). Meniscus in medial compartment (M) had significantly lower T1rho values among healthy controls (28±6 ms) than among both the KL1–2 (32±7 ms) (p = 0.0047) and KL3–4 (37±8 ms) OA (p = 0.0038) subjects. The entire meniscus T1rho values in healthy controls (28±4 ms) were significantly lower (p < 0.02) than those of both KL1–2 (33±6 ms) and KL3–4 (34±7 ms) OA subjects.
Table 4.
p values from ANCOVA to compare subject groups in terms of T1rho adjusted for age, gender, and BMI. p values are shown in italic bold when significant at the 5% comparison-wise significance level and denoted by an asterisk when also significant at the Bonferroni-adjusted significance level of 0.05/27, where 27 is the total number of tests conducted.
| Subregional / Compartmental / Whole |
Subject groups compared | ||
|---|---|---|---|
| Control vs. KL1–2 | Control vs. KL3–4 | KL1–2 vs. KL3–4 | |
| La | 0.0199 | 0.0239 | 0.4297 |
| Lc | 0.4124 | 0.6241 | 0.0457 |
| Lp | 0.1038 | 0.2365 | 0.8506 |
| Ma | 0.3732 | 0.2550 | 0.4248 |
| Mc | 0.0014 * | 0.1180 | 0.0552 |
| Mp | 0.0152 | 0.0046 | 0.0759 |
| L | 0.0195 | 0.0572 | 0.9329 |
| M | 0.0047 | 0.0038 | 0.1981 |
| Entire | 0.0009 * | 0.0011 * | 0.2132 |
Note: vs. - versus
Likewise, when compared based on Mann-Whitney tests (Tables 3 and 5), lateral anterior (26±4 ms), medial anterior (25±5 ms), and medial posterior (29±6 ms) meniscus subregions in healthy controls had significantly lower T1rho values (p < 0.02) than the corresponding meniscus subregions in both KL1–2 (29±9 ms, 28±8 ms, and 35±9 ms, respectively) and KL3–4 (30±12 ms, 31±15 ms, and 40±13 ms, respectively) OA subjects. Healthy controls (25±7 ms, 25±7 ms, and 26±6 ms, respectively) had significantly lower T1rho values than the KL1–2 OA patients (29±4 ms, 33±8 ms, and 31±6, respectively) in the lateral central meniscus subregion (Lc; p = 0.011), and the medial central (Mc; p < 0.01) meniscus subregion and in the lateral compartment (L; p < 0.01). The meniscus in the medial compartment in healthy controls (28±6 ms) had significantly lower T1rho values (p < 0.01) than the corresponding meniscus compartment on the medial side in both KL1–2 (32±7 ms) and KL3–4 (37±8 ms) OA subjects. The entire meniscus T1rho values in healthy controls (28±4 ms) were significantly lower (p < 0.01) than those of both KL1–2 (33±6 ms) and KL3–4 (34±7) OA subjects.
Table 5.
p values from exact Mann-Whitney tests to compare subject groups in terms of T1rho without covariate adjustment. p values are shown in italic bold when significant at the 5% comparison-wise significance level and denoted by an asterisk when also significant at the Bonferroni-adjusted significance level of 0.05/27, where 27 is the total number of tests conducted.
| Subregional / Compartmental / Whole |
Subject groups compared | ||
|---|---|---|---|
| Control vs. KL1–2 | Control vs. KL3–4 | KL1-2 vs. KL3–4 | |
| La | 0.0152 | 0.0166 | 0.8782 |
| Lc | 0.0106 | 0.7266 | 0.0598 |
| Lp | 0.0918 | 0.3629 | 0.4795 |
| Ma | 0.0225 | 0.0141 | 0.2441 |
| Mc | 0.0003 * | 0.9320 | 0.1185 |
| Mp | 0.0037 | 0.0002 * | 0.1395 |
| L | 0.0049 | 0.0500 | 0.7105 |
| M | 0.0004 * | < 0.0001 * | 0.2508 |
| Entire | 0.0001 * | < 0.0001 * | 0.5304 |
Note: vs. - versus
Discussion
In this work, subregional, compartmental, and whole 3T T1rho values of menisci in patients with doubtful-minimal (KL1–2) and moderate-severe (KL3–4) OA were evaluated and compared together with those of healthy controls.
T1rho mapping technique has been recently proposed as an attractive potential biomarker to evaluate the biochemical changes in the cartilage matrix non-invasively (7). This technique has been used to investigate the slow motion interactions between the macromolecular protons and bulk water protons, as well as chemical exchange between protons in the surrounding environment (10). T1rho-weighted MR imaging method has obvious advantages of easily being translated to a clinical setting without any hardware modifications and requiring no exogenous contrast agent in comparison with other existing methods (7, 21–24).
The meniscus T1rho values showed relatively obvious differences between doubtful-minimal (KL1–2) and moderate-severe (KL3–4) OA predominantly in the lateral central subregion (Lc) as shown in Fig 3 and Table 4. Of note, we found that T1rho values of meniscus were significantly higher in the lateral anterior subregion, medial posterior subregion, medial-compartment meniscus, and entire meniscus of both KL1–2 and KL3–4 OA patients than those of healthy controls. An interesting phenomenon in our results was that most significant differences are distributed predominantly between healthy controls and KL1–2 OA patients, less between healthy controls and KL3–4 OA patients, and least between KL1–2 and KL3–4 OA patients. These findings may imply that T1rho MR imaging of meniscus may be a feasible potential alternative of biomarker in especially associating OA progression with nearby meniscal injuries. Regional meniscus deterioration may result in progressive degeneration of both whole femorotibial cartilage and the nearby menisci. Another result was that although there were significant differences in T1rho values of lateral central meniscus subregion (Lc) between KL1–2 and KL3–4 OA patients, T1rho value of Lc meniscus subregion in KL1–2 OA patients was significantly higher than that of KL3–4 OA patients. This may possibly be due to a more progressive loss of lateral central weight-bearing meniscus resulting in higher T1rho values in patients at early stage of OA (KL1–2) compared to those in patients at advanced degenerative stage of OA (KL3–4), in which loss of lateral central weight-bearing meniscus may be relatively not distinct (3, 25, 26).
On the other hand, in KL3–4 OA patients most of the menisci is degenerated severely, however, in early OA patients with KL1–2, menisci may possibly be still intact morphologically but compromised biochemically and structurally, and this degeneration of menisci is definitely not uniform due to the complicated weight-bearing pattern between cartilage and the adjacent menisci (9). This may be one of the reasons why T1rho values of meniscus are higher in individual specific subregions in KL1–2 OA patients than those of KL3–4 OA patients. OA is a multifactorial disease (4, 5). The pathogenesis of OA remains not clear. Much work is warranted to further explore the association between OA and the menisci degeneration.
This study has limitations in terms of measurement error coming from the manually-drawn ROIs among the different sub-regions in the anterior horn, central (body segment), and posterior horn meniscus. Compared to those of human cartilage, meniscus has even smaller measurable regions of anterior, central, and posterior subregions in both lateral and medial compartments. Given these measurement limitations in T1rho values, further investigation is required. Additional limitations of this study are that there may be errors in K-L and WORMS grading of cartilage, which is highly subjective, and that group comparisons in terms of T1rho values are confounded with differences in terms of WORMS scores since this factor could not be adjusted statistically. The age mismatch among healthy controls, KL1–2 OA patients, and KL2–3 OA subjects (34.4±10.2 yrs vs. 61.9±10.8 yrs vs. 71.1±9.6 yrs) is also a deficiency of the current study which possibly affects the significance of the comparison results. A larger sample size may be one possible solution to some of these problems.
Conclusion
This study demonstrates that there are some statistically significant differences between T1rho values of meniscus subregions in the lateral and medial compartments in subjects with doubtful to severe OA. Our preliminary results suggest that there were statistically significant differences in meniscus T1rho values in medial compartment between healthy controls and KL1–2 OA subjects, healthy controls and KL3–4 OA subjects. The entire meniscus T1rho values in healthy controls were significantly lower than those of both KL1–2 and KL3–4 OA subjects. These preliminary findings indicate that T1rho values of meniscus are higher in medial posterior subregion and medial compartment and regional damage of menisci may be associated with osteoarthritis. Future work is necessary to determine the clinical significance of the association between meniscal degeneration and OA.
Acknowledgements
The authors would like to acknowledge the support by research grants RO1 AR053133, RO1 AR056260, and RO1 AR060238 A2 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH). Ding Xia, M.Sc, from Center for Biomedical Imaging, Department of Radiology, New York University Langone Medical Center, is thanked for technical support.
Footnotes
The first/corresponding author and all co-authors have no conflict of interest to disclose.
References
- 1.Williams A, Qian Y, Golla S, Chu CR. UTE-T2* mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthr Cartilage. 2012;20(6):486–494. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauscher I, Stahl R, Cheng J, et al. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Chang G, Xu J, et al. T1rho MRI of menisci and cartilage in patients with osteoarthritis at 3T. Eur J Radiol. 2012;81:2329–2336. doi: 10.1016/j.ejrad.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–336. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 5.Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56(4):1204–1211. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 6.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 7.Pakin SK, Xu J, Schweitzer ME, Regatte RR. Rapid 3D-T1rho mapping of the knee joint at 3.0T with parallel imaging. Magn Reson Med. 2006;56:563–571. doi: 10.1002/mrm.20982. [DOI] [PubMed] [Google Scholar]
- 8.Dijkgraaf LC, de Bont LG, Boering G, et al. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Benjamin Ma C, Link TM, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3T MRI. Osteoarthr Cartilage. 2007;15(7):789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosher TJ, Zhang Z, Reddy R, et al. Knee Articular Cartilage Damage in Osteoarthritis: Analysis of MR Image Biomarker Reproducibility in ACRIN-PA 4001 Multicenter Trial. Radiology. 2011;258(3):832–842. doi: 10.1148/radiol.10101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich KM, Shepard T, de Oliveira VS, et al. T2 Measurements of Cartilage in Osteoarthritis Patients With Meniscal Tears. Am J Roentgenol. 2009;193(5):W411–W415. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange AK, Fiatarone Singh MA, Smith RM, et al. Degenerative meniscus tears and mobility impairment in women with knee osteoarthritis. Osteoarthr Cartilage. 2007;15(6):701–708. doi: 10.1016/j.joca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Regatte RR, Akella SV, Borthakur A, Kneeland JB, Reddy R. In vivo proton MR three-dimensional T1rho mapping of human articular cartilage: initial experience. Radiology. 2003;229(1):269–274. doi: 10.1148/radiol.2291021041. [DOI] [PubMed] [Google Scholar]
- 14.Regatte RR, Akella SV, Borthakur A, Reddy R. Proton spin-lock ratio imaging for quantitation of glycosaminoglycans in articular cartilage. J Magn Reson Imaging. 2003;17(1):114–121. doi: 10.1002/jmri.10228. [DOI] [PubMed] [Google Scholar]
- 15.Akella SV, Regatte RR, Gougoutas AJ, et al. Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med. 2001;46(3):419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 16.Redfield AG. Nuclear Magnetic Resonance Saturation and Rotary Saturation in Solids. Phys Rev. 1955;98:1787–1809. [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kijowski R, Blankenbaker D, Stanton P, Fine J, Smet AD. Arthroscopic Validation of Radiographic Grading Scales of Osteoarthritis of the Tibiofemoral Joint. Am J Roentgenol. 2006;187:794–799. doi: 10.2214/AJR.05.1123. [DOI] [PubMed] [Google Scholar]
- 19.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartilage. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Eckstein F, Ateshian G, Burgkart R, et al. Proposal for a nomenclature for Magnetic Resonance Imaging based measures of articular cartilage in osteoarthritis. Osteoarthr Cartilage. 2006;14:974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 22.McKenzie CA, Williams A, Prasad PV, Burstein D. Three-dimensional delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) at 1.5T and 3.0T. J Magn Reson Imaging. 2006;24:928–933. doi: 10.1002/jmri.20689. [DOI] [PubMed] [Google Scholar]
- 23.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Kimelman T, Vu A, Storey P, McKenzie C, Burstein D, Prasad P. Three-dimensional T1 mapping for dGEMRIC at 3.0 T using the Look Locker method. Invest Radiol. 2006;41:198–203. doi: 10.1097/01.rli.0000195842.49255.ea. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein F, Wirth W, Hunter DJ, et al. Magnitude and regional distribution of cartilage loss associated with grades of joint space narrowing in radiographic osteoarthritis e data from the Osteoarthritis Initiative (OAI) Osteoarthr Cartilage. 2010;18(6):760–768. doi: 10.1016/j.joca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirth W, Hellio Le Graverand MP, Wyman BT, et al. Regional Analysis of Femorotibial Cartilage Loss in a Subsample from the Osteoarthritis Initiative Progression Subcohort. Osteoarthr Cartilage. 2009;17(3):291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]