Abstract
Chromosomal translocations that juxtapose the androgen-sensitive TMPRSS2 gene promoter to the oncogenic ETS-family transcription factor ERG result in excessive ERG overexpression in approximately 50% of prostate cancer (PCa) patients. Although numerous studies have investigated ERG-downstream genes, such studies have not attempted to examine miRNAs, which however are emerging to be important regulators of cancer. Through bioinformatics analysis of ChIP-Seq ERG data and miRNA expression profiling data we nominated miR-200c as a direct target of ERG. Experimentation of PCa cells with ERG overexpression or knockdown demonstrated that ERG directly repressed miR-200c expression by physically binding to the ETS motif within its promoter. Consequently, miR-200c was down-regulated in ERG-positive PCa and miR-200c target gene expression was restored. In addition, the expression pattern of miR-200c target genes predicted ERG status in clinical PCa specimens. Furthermore, miR-200c was found important in modulating ZEB1 up-regulation by ERG. Most importantly, miR-200c reconstitution fully reversed ERG-induced epithelial-to-mesenchymal transition, cell migration and invasion. Therefore, our study report miR-200c as a first miRNA target of ERG and a critical inhibitor of PCa cell motility. Therapeutic delivery of miR-200c may provide personalized treatment for patients with the molecular subtype of PCa that harbors TMPRSS2-ERG gene fusions.
Introduction
Prostate Cancer (PCa) is the most frequently diagnosed non-skin cancer and a leading cause of cancer-related deaths in American men.1 While organ-confined tumors are largely treatable, metastatic diseases are inevitably lethal. During the initiation and progression of prostate cancer, many genetic mutations and deregulation occur and accumulate. Among these, chromosomal translocations that juxtapose the androgen-sensitive promoter of the TMPRSS2 (transmembrane protease, serine 2) gene to the coding region of the oncogenic ETS (erythroblast transformation-specific) family transcription factor ERG (v-ets avian erythroblastosis virus E26 oncogene homolog), termed TMPRSS2-ERG gene fusions, have been found in 40–80% of PCa.2–5 In addition to PCa, recurrent gene fusions involving the ERG gene have also been previously reported in Ewing’s sarcoma and acute myeloid leukemia.6,7 ERG has been shown to induce multiple oncogenic processes, out of which the most commonly reported are its induction of epithelial-to-mesenchymal transition (EMT) and increase of cell motility.8–10 Numerous studies have in the past few years examined the molecular mechanisms and downstream mediators of these oncogenic roles of ERG. Such studies have yielded highly significant findings showing ERG regulation of pathways that are highly important in PCa, including androgen receptor (AR) pathway,11,12 Wnt/TCF signal transduction,13–15 and polycomb group proteins and cell self-renewal.11,16 While it is clear that these molecular pathways are important mediators of ERG-induced oncogenesis in PCa, very few studies have attempted to examine how ERG might regulate microRNAs, which are increasingly recognized as potent regulators of gene expression and cellular processes.
A microRNA (miRNA) is a small non-coding RNA that is usually 18–22 nucleotides long. They are expressed endogenously in cells and, to date, more than 2000 unique mature miRNAs have been found in human cells. The miRNAs negatively regulate gene expression through mRNA degradation or translational repression via binding to the 3′UTR of target genes.17 Since miRNAs can target and repress a large set of genes, small changes in miRNA levels can have major effects on cellular processes and diseases including cancer.18,19 The expression levels of miRNAs are thus tightly regulated. Global miRNA profiling in human cancer patient samples has identified a large set of miRNAs that are differentially expressed in cancer.20,21 These miRNAs are de-regulated often through mechanisms such as promoter methylation, genomic deletion, histone modifications, and upstream protein alteration.20,22,23 In particular, several miRNAs such as miR-34, miR-145, and miR-31 have been shown to be down-regulated in PCa patients. They regulate important factors such as c-Myc, stem-cell markers, and AR, thereby controlling PCa progression.24–26 There are about 30 such miRNAs that have been explored in PCa to determine their downstream genes and how they contribute to PCa initiation, progression, and metastasis.27
As miRNAs play important roles in gene regulation and they are often dys-regulated in cancer, it is plausible that some miRNAs may be targets of ERG and their loss may convey some of the ERG-induced prostate tumorigenesis. Surprisingly, although many studies have investigated the downstream genes of ERG, very few studies have examined the miRNAs that are regulated by ERG. Up to date, there are only two studies that have examined correlation between ERG and miRNAs in PCa. In one study, Hart et al. showed that miR-145 inhibits ERG expression by directly targeting its 3′UTR. Loss of miR-145 may provide a TMPRSS2-ERG gene fusion-independent means to ERG up-regulation in PCa.28 In the other study, through analysis of PCa samples, Gordanpour et al. found that miR-221 is down-regulated in patients with tumors bearing TMPRSS2-ERG gene fusions.29 However, no mechanistic studies were carried out to determine whether and how ERG regulates miR-221 expression. To fill in this gap, in this study we carried out comprehensive bioinformatics analysis to identify miRNAs that are downstream of ERG and nominate miR-200c as a robust and important ERG-regulated miRNA.
The miR-200c is a member of the miR-200 family of miRNAs that also include miR-200a, miR-200b, miR-141, and miR-429. MicroRNA expression profiling has revealed miR-200c to be down-regulated in metastatic vs. primary tumors.30 The loss of miR-200c is linked to poor differentiation and stem cell-like cancer cells31 and is regulated by DNA methylation, oncogene activation, or loss of tumor suppressor genes such as p53.32–35 Functional studies have clearly demonstrated essential roles of miR-200c in suppressing EMT and inhibiting metastasis of various cancer types.36,37 Mechanistically, this is mediated by reciprocal repression of miR-200c and ZEB1, a critical mediator and activator of EMT.33,38 Reconstitution or expression of miR-200c has thus been attempted, which was shown to inhibit EMT, reverse drug resistance, and sensitize cancer cells to treatments.37,39,40 Despite this well-established importance of miR-200c in various cancer types, only very few studies have explored miR-200c in PCa. Specifically, one study has demonstrated miR-200c as a mediator of EZH2 and BMI1, which are oncogenes highly up-regulated in metastatic PCa, suggesting that miR-200c may also play important functions in PCa.41
In this study, by integrating ERG ChIP-Seq data with miRNA expression data profiling PCa cells with or without ERG dys-regulation, we nominated miR-200c as an ERG target miRNA. Using gene-specific primers, we demonstrated that ERG directly repressed miR-200c expression by binding to the ETS motif within its promoter. We further showed that this regulation is relevant in human PCa through analysis of gene expression in clinical specimens. Moreover, using functional assays we illustrated an important role of miR-200c in mediating ERG-induced EMT and cell motility through regulation of ZEB1 expression.
Results
microRNA expression profiling nominated miR-200c as a downstream target of ERG
In previous studies, we have carried out ERG ChIP-Seq in PCa cells and have discovered many ERG-bound target genes.11 As miRNAs are increasingly recognized as important regulators of gene expression and cellular processes, we examined whether ERG protein binds to the cis-regulatory elements of miRNAs. Bioinformatics analyses were carried out to scan ERG binding events within 5kb upstream of all miRNA genes reported. ERG ChIP-Seq data derived from the ERG-positive VCaP cells and the LNCaP cells with stable ectopic ERG overexpression (LNCaP+ERG) were utilized. Our analysis revealed a total of 140 and 115 miRNAs that harbor at least one ERG binding event within their regulatory regions in the VCaP and LNCaP+ERG cells, respectively (Supplementary Table S1 and S2). These data suggested that ERG might be able to transcriptionally regulate the expression of miRNAs through binding to their cis-regulatory elements. Next, we attempted to identify these ERG-regulated miRNAs.
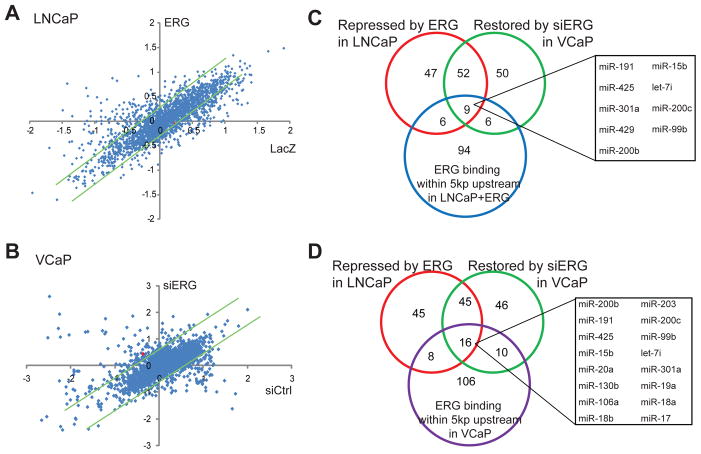
We utilized the Exiqon miRNA microarrays to obtain global expression profiles of miRNA in control cells and cells with ERG dys-regulation. We first analyzed the effect of ectopic ERG overexpression in LNCaP cells, which do not express endogenous ERG. Through miRNA profiling we found that while a majority of the miRNAs was not differentially expressed, the expression of some miRNAs was altered by ERG (Figure 1A). The fold of alteration was generally low, which is consistent with previous notion that miRNAs are tightly regulated and that small changes in miRNAs may yield significant downstream effects. After removing the miRNAs that were expressed at undetectably low levels, we obtained a list of 104 and 18 miRNAs that were respectively repressed and induced following ERG overexpression in LNCaP cells (Supplementary Table S3).
Figure 1. miRNA expression profiling nominated miR-200c as a target of ERG.
A. Scatter plot of miRNAs that were differentially regulated by ERG overexpression in LNCaP cells. LNCaP control and ERG-expressing stable cells were analyzed for miRNA expression using Exiqon miRNA microarrays. In the scatter plot, X-axis represents log2-transformed normalized expression values in LacZ-treated sample while Y-axis the ERG-treated sample. The dot representing miR-200c is shown in red. The green trend lines indicate 1.2-fold differences.
B. Scatter plot of miRNAs that were differentially expressed by ERG knockdown in VCaP cells. VCaP control and ERG-knockdown cells were analyzed for miRNA expression using Exiqon miRNA microarrays. The green trend lines indicate 1.5-fold differences.
C. An overlapping set of miRNAs that were directly repressed by ERG in LNCaP cells. The miRNAs that were repressed by ectopic ERG overexpression in LNCaP or restored upon ERG knockdown in VCaP were identified through miRNA microarray experiments. ERG ChIP-Seq data was obtained from LNCaP cells with stable ERG overexpression.
D. An overlapping set of miRNAs that were directly repressed by ERG in VCaP cells. ERG-regulated miRNAs were examined for ERG occupancy at the promoter regions in VCaP cells, which were analyzed by ERG ChIP-seq.
As the effect of ectopic ERG overexpression may be less physiological, we on the other hand carried out ERG knockdown using RNA interference in the ERG-positive VCaP cells. Using miRNA profiling we identified 117 and 40 miRNAs that were respectively up-regulated or down-regulated following ERG knockdown (Figure 1B; Supplementary Table S4). Remarkably, there was approximately 50% overlap between miRNAs that were repressed by ERG in LNCaP cells and those that were restored by ERG knockdown in VCaP cells (Figure 1C–D). Similarly, about 50% of miRNAs that were induced by ERG in LNCaP cells were repressed by ERG knockdown in VCaP cells (Figure S1). These overlapping miRNAs represent a robust set of candidate ERG target miRNAs that warrant further characterization. In this study, we chose to focus on ERG-repressed miRNAs as they might provide unique opportunities for miRNA delivery or reconstitution as therapeutics for the molecular subtype of PCa that harbor TMPRSS2-ERG gene fusions.
To identify miRNAs that are directly regulated by ERG, we integrated the ERG-regulated miRNA gene expression data with ERG-bound miRNAs identified using ChIP-Seq data (Supplementary Table S1–2). Out of the 61 robust ERG-repressed miRNAs, 16 and 9 of them were directly bound by ERG protein in VCaP and LNCaP+ERG cells, respectively (Figure 1C–D). Out of these, 8 of them overlapped. Out of these 8 miRNAs, miR-200c has been previously shown to play important tumor suppressive roles by inhibiting cell motility and EMT.36,38 Repression of miR-200c could lead to increased cell motility and EMT, a function of ERG that has been extensively demonstrated.8,10 In addition, even though miR-200c functional roles and downstream targets have been widely studied in various caner types, studies of miR-200c in PCa have been very limited. Therefore, we decided to focus this study on characterizing miR-200c as an ERG-regulated miRNA in PCa.
ERG directly regulates miR-200c expression
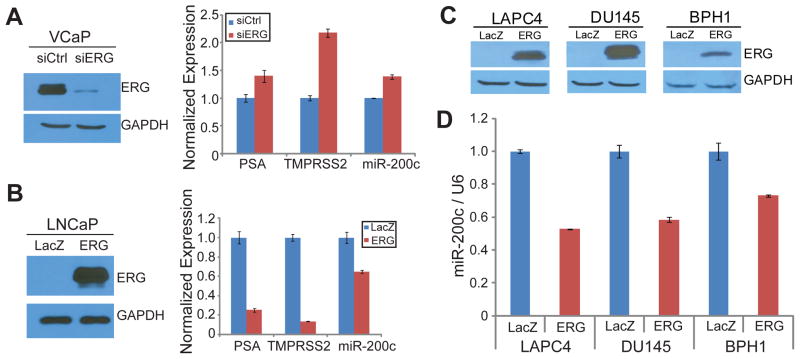
To validate our computational results nominating miR-200c as a direct target of ERG, we utilized miRNA TaqMan Assay to verify ERG regulation of miR-200c expression using gene-specific primers. We first carried out ERG knockdown via RNA interference (Figure 2A). Western blot analysis confirmed that ERG protein was reduced more than 80%. QRT-PCR analysis demonstrated that miR-200c expression was significantly up-regulated following ERG knockdown, and so did previously reported ERG-repressed genes such as PSA and TMPRSS2.11 On the other hand, we carried out ERG overexpression in LNCaP cells, which was confirmed by western blot analysis (Figure 2B). Concordantly, qRT-PCR analysis revealed that ERG inhibited the expression of miR-200c as well as known ERG-repressed target genes such as PSA and TMPRSS2. To further verify this in additional prostate cell models, we overexpressed ectopic ERG in a panel of PCa cell lines including LAPC4, DU145 and BPH1. First, we verified ERG overexpression using immunoblot analysis (Figure 2C). Then, miR-200c level was tested using TaqMan miRNA assay, and we found that miR-200c expression was drastically decreased by ERG in all of these cell lines (Figure 2D). These results demonstrated that miR-200c is a robust target of ERG-mediated transcriptional repression. Next, we asked whether this regulation is mediated by direct ERG binding to miR-200c cis-regulatory elements.
Figure 2. miR-200c expression is inhibited by ERG.
A. ERG knockdown in VCaP restored miR-200c expression. VCaP cells were transfected with either siLUC or siERG. Western blot confirmed ERG knockdown and qRT-PCR of PSA and TMPRSS2 which have been previously shown to be inhibited by ERG were tested. miR-200c was tested by TaqMan miRNA assay normalized to U6 internal control. Error bars indicate n=3, mean ±SEM, p<0.05
B. Ectopic ERG expression in LNCaP inhibited miR-200c expression. LNCaP cells were infected with either LacZ or ERG adenovirus. Western blot confirmed ERG overexpression and qRT-PCR of PSA and TMPRSS2 and TaqMan miRNA assay for miR-200c were tested. Error bars indicate n=3, mean ±SEM, p<0.05
C–D. miR-200c expression was repressed by ERG in prostate cancer cells. LAPC4, DU145 and BPH1 cells were infected with either LacZ or ERG adenovirus. (C) Western blot confirmed ERG overexpression and (D) miR-200c level was tested by miRNA TaqMan assay. Error bars indicate n=3, mean ±SEM, p<0.05.
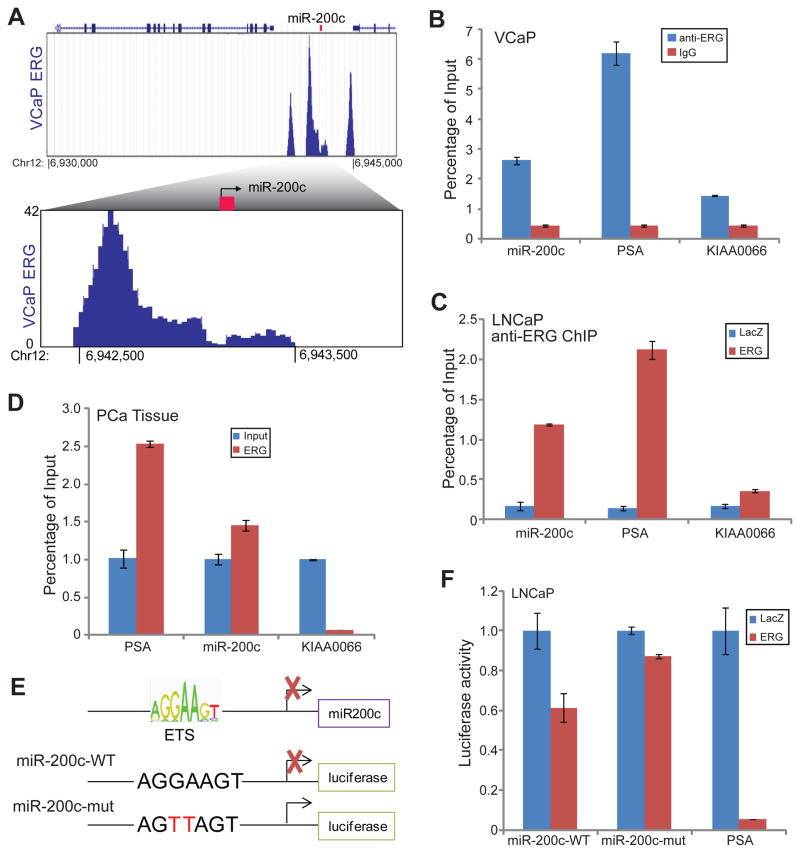
ChIP-Seq data revealed several strong ERG binding events at genomic regions flanking the miR-200c gene in VCaP cells (Figure 3A). In particular, there is a strong ERG binding peak at 1kb upstream to the transcription start site (TSS) of the gene. Being consistent with these, ChIP-Seq revealed similar ERG binding events in LNCaP+ERG cells (Supplementary Figure 3A). To further validate this, we carried out ChIP-PCR utilizing primers that specifically flank the ERG binding event at the miR-200c promoter. ERG and IgG ChIP in VCaP cells confirmed significantly enriched ERG binding compared to IgG on the miR-200c promoter as well as the PSA enhancer, which has been previously shown to be occupied by ERG.11 In addition, using ChIP-PCR we demonstrated that ectopic ERG overexpression in LNCaP cells resulted in a remarkable increase of ERG occupancy on the miR-200c promoter and PSA enhancer (Figure 3C). By contrast, ERG binding on KIAA0066, a negative control gene, was only marginally increased. To determine whether this holds true in clinical PCa specimens, we carried out ERG ChIP in several human prostate cancer tissues that harbor the TMPRSS2-ERG gene fusion. Due to the limited amount of tissue and thus ChIP-enriched DNA, linear amplification of the DNA was carried out along with equal amount of input DNA. ChIP-PCR experiment showed that miR-200c promoter and PSA enhancer were significantly enriched in the ERG-pull down DNA relative to the input (Figure 3D). Therefore, ERG directly occupied miR-200c promoter in both PCa cell lines as well as human PCa tissues.
Figure 3. ERG directly regulates miR-200c expression.
A. VCaP ChIP-Seq revealed strong ERG occupancy near the miR-200c gene promoter. ERG ChIP-Seq data were obtained from VCaP cells. Three main ERG binding peaks were shown around the region. In the bottom panel, the ERG ChIP-Seq peak near the miR-200c promoter was further zoomed in.
B. ChIP-qPCR confirmed ERG binding at the miR-200c promoter in VCaP cells. ChIP-qPCR verified ChIP-seq that miR-200c promoter is enriched in anti-ERG compared to IgG. PSA is a positive control gene that is enriched and KIAA0066 is a negative control gene that is not enriched by anti-ERG antibody. Error bars indicate n=3, mean ±SEM, p<0.05.
C. ChIP-qPCR confirmed ectopic ERG binding at the miR-200c promoter in the LNCaP+ERG cells. LNCaP ERG overexpression ERG ChIP-qPCR verified that miR-200c promoter is enriched in LNCaP+ERG compared to LacZ control. PSA is a positive control gene that is enriched and KIAA0066 is a negative control gene that is not enriched by anti-ERG antibody. Error bars indicate n=3, mean ±SEM, p<0.05.
D. The miR-200c promoter was bound by ERG in clinical PCa specimens. ERG ChIP was carried out in PCa tissues that express high levels of ERG. Equal amount of ChIP-enriched and input DNA were amplified through ligation-mediated PCR and subjected to qPCR analysis.54 PSA is a positive control gene that is enriched and KIAA0066 is a negative control gene that is not enriched by anti-ERG antibody. Error bars indicate n=3, mean ±SEM, p<0.05. Shown here is representative data from 3 PCa specimens.
E. Schematic of the ETS motif with the miR-200c promoter and the luciferase reporter constructs. An ETS motif was found around 500bp upstream of miR-200c gene. A 1kb DNA fragment flanking this region was cloned into a luciferase reporter vector to generate the miR-200c wild-type (WT) construct. In the miR-200c mutant construct, the same fragment with 2 nucleotide mutations within the ETS motif (shown in red) was cloned into the luciferase reporter vector.
F. ERG inhibited wild-type but not mutant miR-200c promoter activity. Luciferase reporter assays were carried out in LNCaP cells with or without ectopic ERG overexpression. PSA promoter/enhancer luciferase construct was used as a positive control. Results were normalized to Renilla internal control. Error bars indicate n=3, mean ±SEM, p<0.05.
Next, we attempted to address whether this ERG occupancy at the cis-regulatory element of miR-200c can indeed lead to regulation of the promoter activity. Examination of the sequence of miR-200c upstream promoter revealed a highly conserved ETS motif at the center of the ERG binding peak that is 1kb upstream of the miR-200c promoter (Figure 3E). We thus cloned a 1kb DNA fragment that flanks this ERG binding event into a luciferase reporter vector. Next, we performed luciferase reporter assays to determine how ERG regulates the activity of this promoter. Through serial dilutions of ERG adenovirus, we showed that increasing amount of ectopic ERG overexpression in LNCaP cells resulted in a dose-dependent gradual decrease of miR-200c promoter activity (Supplementary Figure 3B). This was further verified in another independent PCa cell line 22Rv1, wherein luciferase assay showed that ERG drastically inhibited the activities of both miR-200c promoter and the PSA promoter/enhancer (Supplementary Figure 3C). To determine whether this repression is indeed mediated by the ETS motif, we generated a mutant miR-200c promoter construct that has 2 core nucleotides with the motif mutated. Luciferase reporter assay illustrated that while ERG overexpression in LNCaP cells significantly inhibited miR-200c wild-type (WT) promoter and PSA promoter/enhancer activities, the activity of the miR-200c mutant promoter was only marginally repressed (Figure 3F). Taken together, our data demonstrated that ERG represses miR-200c through direct binding and regulation of its cis-regulatory element.
The miR-200c target genes predict ERG status in prostate cancer
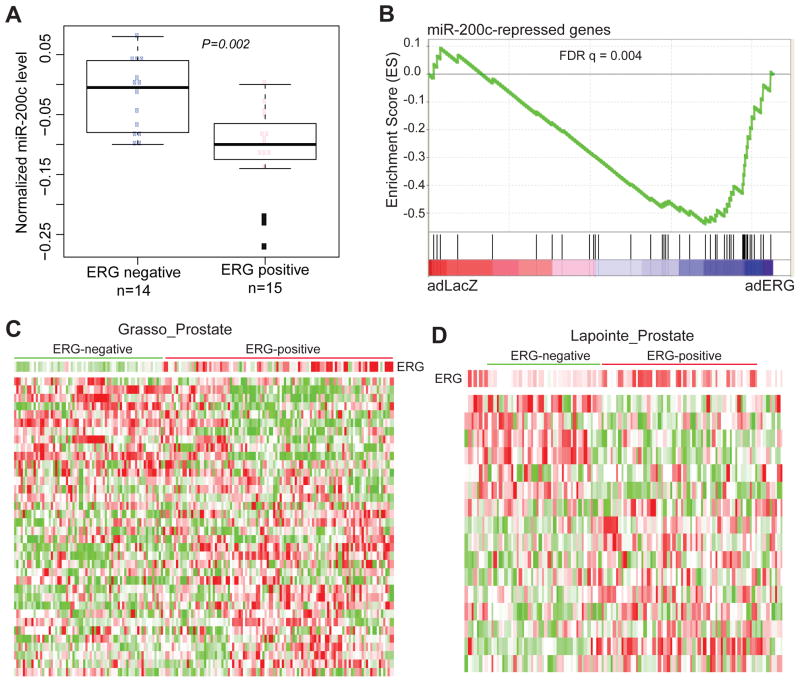
We next took our study one step further and examined whether ERG regulation of miR-200c is physiologically relevant in clinical specimens. Since ERG inhibits miR-200c expression, we expect to see miR-200c down-regulation in TMPRSS2-ERG fusion-positive human PCa. We analyzed a previously published microarray dataset that profiled both mRNA and miRNA expression in matched set of samples 42. We limited our analysis to localized prostate cancer as previous studies have extensively shown miR-200c down-regulation in metastatic diseases, while TMPRSS2-ERG gene fusions, by contrast, are present in similar percentages in localized and metastatic PCa. Based on the level of ERG transcript, we stratified the PCa specimens into 2 categories: ERG-positive and ERG-negative (Supplementary Figure S3). Our analysis revealed that miR-200c was expressed at significantly lower levels in the ERG-positive PCa tissues relative to the ERG-negative ones (Figure 4A).
Figure 4. The expression of miR-200c target genes stratifies ERG status in prostate cancer.
A. ERG-positive patients have lower miR-200c expression compared to ERG-negative patients. Using GSE21032, we categorized localized prostate tumors into ERG-positive and ERG-negative based on ERG expression level in these samples (Supplementary Figure S3). The level of miR-200c expression was plotted using box plot.
B. The miR-200c-repressed genes were enriched for up-regulation upon ERG overexpression. GSEA was carried out to examine the expression of a set of miR-200c-repressed genes in a microarray dataset that profiled LNCaP cells with control or ERG overexpression.
C–D. The miR-200c-repressed genes stratified prostate tumors into ERG-negative and ERG-positive clusters. The expression levels of the miR-200c-repressed genes were obtained from publically available microarray datasets that profiled a large set of human PCa samples. Clustering analysis was carried out to stratify the samples based on the expression pattern of the miR-200c-repressed genes. The resulted “ERG-negative” and “ERG-positive” PCa groups have significantly different expression levels of ERG, with P=8.2E-13 and P=0.00015 respectively for (C) GSE35988 and (D) GSE3933.
As there are very few microarray dataset that have profiled mRNA and miRNA in matched clinical specimens, our analysis of ERG and miR-200c in human samples were largely limited. To address this, we attempted to obtain a set of signature genes that are downstream to miR-200c, which can then be analyzed in more independent datasets. We thus conducted expression microarray profiling of LNCaP cells with control or miR-200c overexpression. Microarray analysis revealed a total of 37 and 56 genes that were respectively induced and repressed by miR-200c for at least 2 fold. We chose the miR-200c-repressed genes for further analysis as they are more likely to represent direct effects of miR-200c expression. We expected the expression of these genes to be up-regulated by ERG, since ERG inhibits miR-200c. To test this, we carried out Gene Set Enrichment Analysis (GSEA) of this gene set in a microarray dataset where we profiled LNCaP cells with control or ERG overexpression. GSEA analysis revealed that miR-200c-repressed genes were significantly enriched for up-regulation in ERG-overexpressing LNCaP cells (Figure 4B). These genes thus comprise a miR-200c signature that is responsive to ERG expression.
To determine whether these miR-200c signature genes are responsive to ERG regulation in clinical specimens, we asked whether their expression as a whole can accurately predict ERG expression level in human PCa. Utilizing publically available microarray datasets that have profiled large number of human PCa tissues we obtained the expression profiles of the miR-200c signature genes. Samples were then clustered based on the expression patterns of these genes. Remarkably, we found the miR-200c signature genes were able to separate PCa into ERG-negative and ERG-positive categories in two independent PCa datasets (Figure 4C–D).25,43 ERG expression levels were significantly different (P=8.2E-13 and P=0.00015 for Grasso et al. and Lapointe et al., respectively) between the “ERG-negative” and “ERG-positive” samples stratified by the miR-200c signature genes. These results supported the clinical relevance of our findings in human PCa.
ERG induces ZEB1 expression through modulating miR-200c level
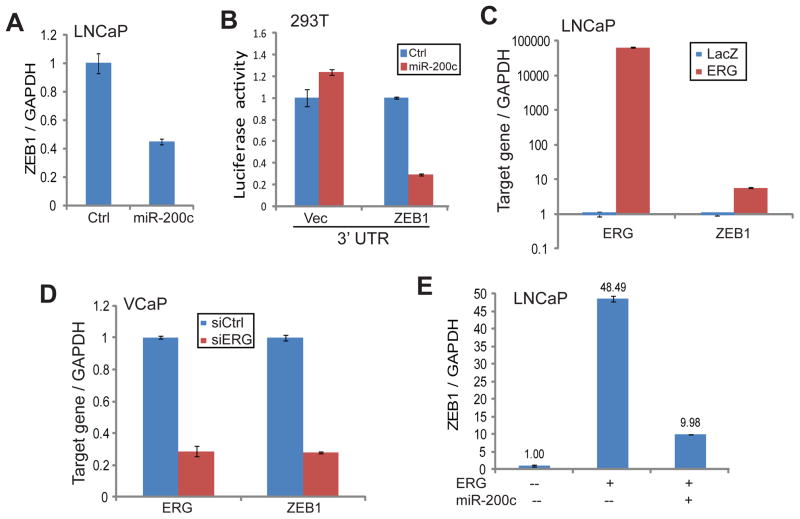
Previous studies have extensively shown miR-200c as a critical suppressor of EMT and thus an inhibitor of cell motility and tumor metastasis.36,37,44–46 This activity is in direct contrast to that of ERG, which induces EMT and cell motility. Studies of other cancer types have also revealed that this functionality of miR-200c is at least in part mediated by its reciprocal repression of the ZEB1 gene.33,36,38 Whether or not this miR-200c-ZEB1 axis holds true in PCa and whether this axis mediates ERG-induced cell motility have not been studied. QRT-PCR analysis confirmed that ZEB1 gene transcript was markedly repressed in LNCaP cells with miR-200c overexpression compared to the control cells (Figure 5A). On the other hand, miR-200c inhibition using miRNA knockdown constructs significantly restored ZEB1 expression (Supplementary Figure 4A). To determine whether this repression is due to miR-200c directly targeting the 3′ UTR of the ZEB1 gene, we cloned the 3′ UTR of the ZEB1 gene into a luciferase reporter vector. Luciferase reporter assay confirmed that, upon miR-200c overexpression, ZEB1 3′UTR luciferase activity was greatly diminished (Figure 5B). Our data thus confirmed that ZEB1 is a target of miR-200c in PCa.
Figure 5. ERG induces ZEB1 expression through modulating miR-200c level.
A. Ectopic miR-200c expression inhibited ZEB1 expression. LNCaP cells were infected with either control or miR-200c retrovirus. QRT-PCR was utilized to analyze gene expression with GAPDH as an internal control. Error bars indicate n=3, mean ±SEM, p<0.05.
B. miR-200c expression suppressed ZEB1 3′UTR luciferase activity. The 293T cells were transfected with control or luciferase vector containing the 3′UTR of ZEB1 gene. Luciferase signal was normalized to Renilla internal control. Error bars indicate n=3, mean ±SEM, p<0.05.
C. Ectopic ERG overexpression induced ZEB1 expression. LNCaP cells were infected with either LacZ or ERG adenovirus and analyzed by qRT-PCR. Error bars indicate n=3, mean ±SEM, p<0.05.
D. ERG knockdown down-regulated ZEB1 expression. VCaP cells were transfected with control siRNA (siCtrl) or siRNA target ERG (siERG) and gene expression was analyzed by qRT-PCR. Error bars indicate n=3, mean ±SEM, p<0.05.
E. miR-200c expression blocked ERG induction of ZEB1. LNCaP cells were infected with LacZ control or ERG-expressing adenovirus along with pQCXIH control or miR-200c-expressing retrovirus. Gene expression was assayed using qRT-PCR and normalized to GAPDH. Error bars indicate n=3, mean ±SEM, p<0.05.
Since ERG inhibits miR-200c expression, it may lead to ZEB1 induction. Indeed, qRT-PCR analysis showed that ZEB1 transcript was significantly up-regulated in LNCaP cells following ERG overexpression (Figure 5C). Being consistent with this, ERG knockdown in VCaP cells, on the other hand, remarkably down-regulated ZEB1 expression (Figure 5D). To further demonstrate that miR-200c is the mediator of ERG induction of ZEB1, we asked whether miR-200c expression is able to attenuate the increase of ZEB1 following ERG overexpression. QRT-PCR analysis first confirmed that ZEB1 is drastically increased in LNCaP cells with adenoviral overexpression of ectopic ERG (Figure 5E; Supplementary Figure S4B). Reconstitution of miR-200c in these cells indeed greatly suppressed ZEB1 level, thereby reversing its induction by ERG. Our data thus support miR-200c as an important mediator of ERG-induced ZEB1 expression. As ZEB1 is an essential regulator of EMT and cell motility, we next investigated how miR-200c relates to ERG-induced oncogenic functions.
miR-200c mediates ERG-induced cell motility
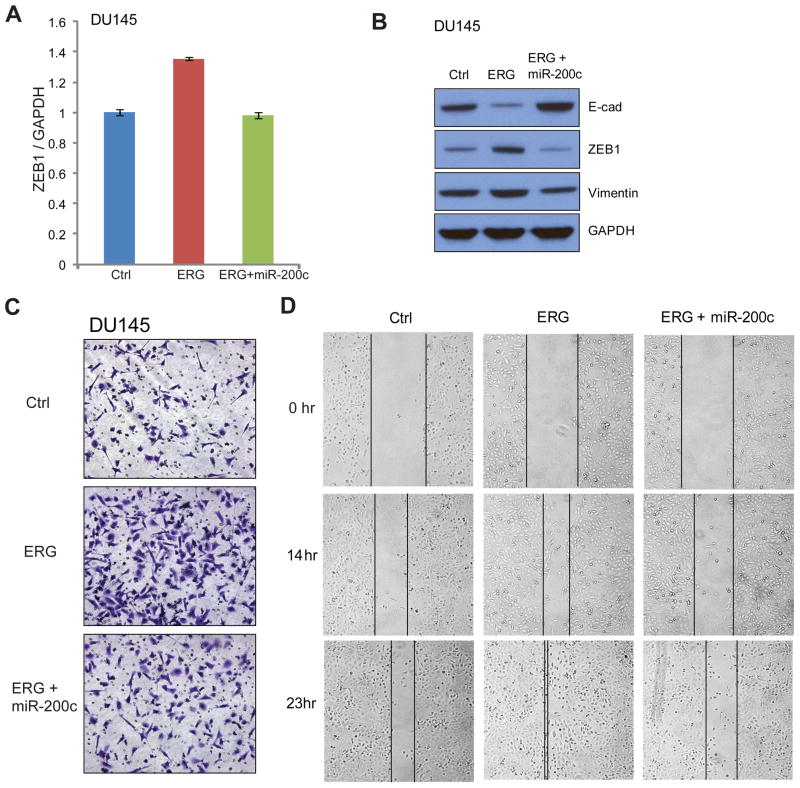
ERG has been extensively shown to induce tumorigenesis through increasing cell invasion and migration with a mesenchymal phenotype.8,11,12,47,48 To study the role of miR-200c in regulating these functions, we examined the ERG-miR-200c-ZEB1 axis in the DU145 PCa cell line that is readily feasible for cell migration and cell invasion assays. DU145 cells with stable ERG overexpression were generated (Supplementary Figure 5A). Concordant with the results shown in Figure 5, ERG overexpression significantly induced ZEB1 expression, while miR-200c reconstitution in these cells blocked this increase (Figure 6A). In addition, western blot analysis showed that ERG inhibited the expression of epithelial marker E-cadherin but induced mesenchymal markers ZEB1 and Vimentin (Figure 6B). Most importantly, these changes were markedly reversed by miR-200c overexpression in the DU145+ERG stable cells. Therefore, miR-200c suppresses ERG-induced PCa cell transformation.
Figure 6. miR-200c mediates ERG-induced cell motility.
A. miR-200c expression blocked ERG-induced expression of ZEB1 in DU145 cells. DU145 cells were infected with control or ERG-expressing lentivirus to generate stable cell lines. ERG-expressing stable cell lines were further infected by miR-200c expressing lentivirus. ZEB1 expression was analyzed by qRT-PCR and normalized to GAPDH. Error bars indicate n=3, mean ±SEM, p<0.05.
B. Stable ectopic ERG overexpression in DU145 induced mesenchymal markers but repressed epithelial marker, which was reverted by miR-200c expression.
C. Stable ectopic ERG overexpression in DU145 induced cell invasion, which was reverted by miR-200c expression. Cell invasion was carried out using Boyden chamber assay. Briefly, DU145 cells were plated in the top chamber above matrigel and incubated for 16 hrs to invade through the membrane. The invaded cells were then stained and photographed. Experiments were repeated at least 3 times and one representative result is shown.
D. Ectopic ERG overexpression increased DU145 cell migration, which was reverted by miR-200c expression. Cells were plated at a same density then wound was made by scratching. Images were taken at 0, 14, and 23 hrs. Experiments were repeated at least 3 times and one representative result is shown.
As mesenchymal transition is causatively related to increased cell motility, we next examined whether miR-200c affects ERG-induced cell motility. Boyden chamber assay was utilized to monitor cell invasion, while wound healing assay was carried out to determine cell migration. Being consistent with previous reports, ERG overexpression alone greatly induced DU145 cell invasion and migration (Figure 6C–D). Remarkably, miR-200c reconstitution fully blocked ERG-induced cell invasion (Figure 6C, Supplementary Figure 5B). Moreover, wound healing assay demonstrated that at 14 hrs, ERG overexpression alone closed about 50% of the wound, yet cells with co-expression of ERG and miR-200c showed very minimal closure. By 23 hrs after the wound was created, cells with ERG overexpression alone have fully closed the wound while cells with ERG and miR-200c co-expression only closed about 40% of the wound, a level that was even less than the control cells (Figure 6D, Supplementary Figure 5C). Therefore, our data demonstrated that miR-200c reconstitution is able to fully invert PCa cell transformation and cell motility caused by ERG.
Discussion
Since the initial discovery of its involvement in recurrent gene fusions in 40–80% of PCa, the ERG gene has been extensively studied in terms of its tumorigenic function.2 The most prominent role of ERG that has been consistently shown in numerous studies is its ability to increase cell migration and invasion via abrogating prostate epithelial differentiation and inducing EMT and motility-associated genes such as MMPs.8,11,49,50 Many target genes and molecular pathways have been implicated in mediating the oncogenic roles of ERG, including AR, Wnt signaling, NF-kB and PARP1.11,14,51,52 However, studies of miRNAs in the context of this gene fusion are very limited. Only one study has described one miRNA, miR-221, to be down-regulated in the fusion-positive PCa, while potential regulatory mechanisms accountable for this association were not investigated.29 As miRNAs are increasingly shown to be vital regulators of cellular behavior as well as diseases including cancer, it is highly important to identify and characterize miRNAs that are regulated by ERG. In this study, we utilized bioinformatics approaches to integrate ERG ChIP-Seq data with miRNA profiling data to identify a robust set of miRNAs whose cis-regulatory elements were occupied by ERG and that were differentially expressed upon ERG dys-regulation in PCa cells (Supplementary Table S1–4 and Figure 1). Although in this study we chose to focus on miR-200c, many of these miRNAs may similarly play significant roles in ERG-mediated cellular processes and PCa progression, which can be important areas for future studies.
The miR-200c gene is one of the best studied miRNAs. It was found to be down-regulated in various metastatic types of solid tumors when compared with primary tumors.30 Extensive research has shown miR-200c to play critical roles in inhibiting EMT, cell motility and tumor metastasis of various cancer types such as breast, lung and colon cancers.33–36. Surprisingly, less than a handful of papers have explored miR-200c in PCa to some degree, linking miR-200c to docetaxel resistance, polycomb group protein regulation, and Notch signaling.41,45,46 Nevertheless, the regulation and function of miR-200c in PCa remains largely to be examined. Our study is the first to demonstrate that miR-200c is a direct target of ERG and is repressed in ERG fusion-positive PCa. In addition, we showed that miR-200c loss mediates ERG-induced EMT and cell motility. Our data suggest that therapeutic delivery of miR-200c may provide targeted treatment of patients with ERG fusion-positive PCa.
In the present study, we showed that ZEB1 is a target gene of miR-200c in PCa, being consistent with observations previously reported in other cancer types.33,38 In addition, we showed that miR-200c reverted ERG-induced oncogenesis by repressing ZEB1. However, miR-200c might affect the expression of many downstream molecules in addition to ZEB1. Considering the essential tumor suppressive roles of miR-200c in PCa as demonstrated in our study, it warrantees further investigation of miR-200c downstream genes or pathways in PCa. It will be of great interest for future studies to determine the expression profile of miR-200c during different stages of PCa progression and in various molecular subtypes and investigate how miR-200c may regulate other important disease pathways of PCa, such as AR signaling and Wnt pathways. In addition, it will be highly impactful to examine how miR-200c reconstitution alters these pathways and thus affects drug response and treatments of PCa. These will be important lines for future studies.
In summary, this study identified many miRNAs that are potentially regulated by TMPRSS2-ERG gene fusions, thereby offering a new research paradigm that may provide novel mechanisms to ERG-induced prostate tumorigenesis. In addition, these miRNAs may be in general involved in PCa progression and thus warrantee future investigation. As increasing evidence has suggested that circulating miRNAs may be useful biomarkers for clinical diseases, future studies of these miRNAs may potentially provide noninvasive diagnostic or prognostic tools. This study characterized the first ERG-target miRNA gene miR-200c and suggested that miR-200c reconstitution may provide targeted therapies for patients with TMPRSS2-ERG gene fusion-positive PCa.
Materials and Methods
Human tissue specimens
Prostate cancer tissues were collected by the Northwestern University Prostate Cancer Specialized Program of Research Excellence Tissue Core (SPORE). Tissue samples were collected with informed consent of the patients and prior Institutional Review Board approval.
Cell Lines
Prostate cancer cell lines LNCaP, VCaP, DU145, and 22Rv1 were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in either RPMI1640 or DMEM with 10% FBS. BPH1 cells were provided by Dr. Jonathan Licht (Northwestern University) and grown in RPMI1640 supplemented with 10% FBS. LAPC4 cells were provided by Dr. C. Shad Thaxton (Northwestern University) and grown in IMEM supplemented with 10% FBS and 1nM R1881. DU145 ERG stable cell were created by infecting ERG lentivirus followed by one week of puromycin selection.
Plasmids, small interfering RNA, and microRNA
For ERG stable overexpression, ERG gene was amplified by PCR from VCaP genomic DNA and cloned into pLenti CMV/TO Puro DEST (670-1) vector (Addgene). For miRNA overexpression, human precursor-miR200c was amplified by PCR from HEK293T genomic DNA and cloned into pQCXIH vector, provided by Dr. Zhao Bin (University of California, San Diego). For miRNA knockdown, two oligonucleotide sequences containing mature miR-200c were synthesized and cloned into miRZIP vector, provided by Dr. Jonathan Licht (Northwestern University) For luciferase assay constructs, miR-200c promoter with ERG binding region was amplified by PCR from HEK293T genomic DNA and cloned into pGL4.1[luc2] luciferase reporter vector (Promega, Madison, WI). For miR-200c promoter with ETS mutation was generated by QuiKChange II Site-Directed Mutagenesis Kits with manufacture’s protocol using wild type pGL4.1[luc2]-miR-200c promoter (Agilent Technologies, Santa Clara, CA). For 3′UTR luciferase reporter construct, ZEB1 3′UTR was amplified by PCR from HEK293T genomic DNA and cloned into pMIR-REPORT luciferase vector, provided by Dr. Arul Chinnaiyan (University of Michigan, Ann Arbor). For RNA interference, control luciferase GL2 duplex siRNA (D-00110-01-20), and ERG siRNA (D-003886-01) were used from Dharmacon (Lafayette, CO). Plasmid and siRNA transfections were performed using lipofectamin 2000 (Invitrogen, Carlsbed, CA).
MicroRNA profiling and bioinformatics analysis
Total RNA was extracted using TRIzol with manufacture’s protocol (Invitrogen). MicroRNA profiling and data extraction were performed using miRCURY™ LNA array (Exiqon) after confirmation of RNA integrity. Enrichment of gene sets was analyzed using the Gene Set Enrichment Analysis (GSEA) tool (Broad Institute) as previously described.53 Expression of miR-200c signature genes in publically available dataset was queried using Oracle database.25,43 Clustering of clinical specimens was carried out using Cluster software and visualized using TreeView.
Western Blots and antibodies
Protein extraction and western blot analysis were performed using a standard protocol. Antibodies used were as follows: anti-GAPDH (ab9385, Abcam, Cambridge, MA), anti-ERG (963A, CPRD for western blot), anti-ERG (sc-354x, Santa Cruz, Santa Cruz, CA for ChIP), anti-E-cadherin (3195P, cell signaling, Danvers, MA), anti-ZEB1 (3396P, cell signaling, Danvers, MA), and anti-vimentin (3932P, cell signaling, Danvers, MA).
ChIP, ChIP-qPCR, quantitative qRT-PCR, TaqMan miRNA assay
ChIP was carried out as previously described.54 Quantitative PCR was performed in Applied Biosystems StepOne Plus Real Time PCR system using GoTaq qPCR Master Mix 2X (Promega, Madison, WI) for genes and TaqMan microRNA assays for miRNAs (Applied Biosystems). All primers were designed using primer 3 and synthesized by Integrated DNA Technologies (Coralville, IA) (Supplementary Table S5).
Functional assays
Luciferase assay was performed as previously described.55 In brief, for promoter luciferase assay, pGL4.1[luc2]-miR200c or pGL4.1[luc2]-miR200c mutant were transfected with Renilla internal control. After 6hrs, either LacZ or ERG adenovirus was added, then incubated for 48hrs before assays were performed. For 3′UTR luciferase assay, pMIR-REPORT or pMIR-REPORT-ZEB1 was co-transfected with either control or miR-200c overexpression vector and Renilla internal control. Then, cells were incubated for 48hrs before assays were performed. Wound healing and invasion assays were performed as previously described using DU145 control and ERG stable cell line infected with either empty vector or miR-200c expressing retrovirus.55 For wound healing assay, images were taken after 0hr, 14hrs and 23hrs after wound was made using Olympus CKX41. For invasion assay, cells were stained with crystal violet for image and invaded cells were counted using imageJ.
Supplementary Material
Acknowledgments
We thank Jianjun Yu for helpful discussions. This work was supported by funding from the NIH R01CA172384 (to J.Y.), the Research Scholar Award RSG-12-085-01 (to J.Y.) from the American Cancer Society, and the NRSA pre-doctoral fellowship T32 CA080621 (to J.K.).
Footnotes
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interest were disclosed
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 3.Clark J, Merson S, Jhavar S, Flohr P, Edwards S, Foster CS, et al. Diversity of TMPRSS2-ERG fusion transcripts in the human prostate. Oncogene. 2007;26:2667–2673. doi: 10.1038/sj.onc.1210070. [DOI] [PubMed] [Google Scholar]
- 4.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu T, Trojanowska M, Watson DK. Ets proteins in biological control and cancer. J Cell Biochem. 2004;91:896–903. doi: 10.1002/jcb.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Cai Y, Yu W, Ren C, Spencer DM, Ittmann M. Pleiotropic biological activities of alternatively spliced TMPRSS2/ERG fusion gene transcripts. Cancer Res. 2008;68:8516–8524. doi: 10.1158/0008-5472.CAN-08-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carver BS, Tran J, Chen Z, Carracedo-Perez A, Alimonti A, Nardella C, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457:E1. doi: 10.1038/nature07738. discussion E2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai C, Wang H, He HH, Chen S, He L, Ma F, et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J Clin Invest. 2013;123:1109–1122. doi: 10.1172/JCI66666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker-Santos DD, Guo Y, Ghaffari M, Vickers ED, Lehman M, Altamirano-Dimas M, et al. Integrin-linked kinase as a target for ERG-mediated invasive properties in prostate cancer models. Carcinogenesis. 2012;33:2558–2567. doi: 10.1093/carcin/bgs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 15.Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI, et al. TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res. 2006;66:10242–10246. doi: 10.1158/0008-5472.CAN-06-1986. [DOI] [PubMed] [Google Scholar]
- 16.Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, et al. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3. 1 in prostate cancer. PLoS One. 2010;5:e10547. doi: 10.1371/journal.pone.0010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 18.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 19.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14:844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 21.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujambio A, Calin GA, Villanueva A, Ropero S, Sanchez-Cespedes M, Blanco D, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci U S A. 2008;105:13556–13561. doi: 10.1073/pnas.0803055105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi XB, Tepper CG, White RW. MicroRNAs and prostate cancer. J Cell Mol Med. 2008;12:1456–1465. doi: 10.1111/j.1582-4934.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamura S, Saini S, Majid S, Hirata H, Ueno K, Deng G, et al. MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS One. 2012;7:e29722. doi: 10.1371/journal.pone.0029722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012 doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep. 2012;28:1831–1837. doi: 10.3892/or.2012.2015. [DOI] [PubMed] [Google Scholar]
- 27.Fang YX, Gao WQ. Roles of microRNAs during prostatic tumorigenesis and tumor progression. Oncogene. 2013 doi: 10.1038/onc.2013.54. [DOI] [PubMed] [Google Scholar]
- 28.Hart M, Wach S, Nolte E, Szczyrba J, Menon R, Taubert H, et al. The proto-oncogene ERG is a target of microRNA miR-145 in prostate cancer. FEBS J. 2013;280:2105–2116. doi: 10.1111/febs.12236. [DOI] [PubMed] [Google Scholar]
- 29.Gordanpour A, Stanimirovic A, Nam RK, Moreno CS, Sherman C, Sugar L, et al. miR-221 Is down-regulated in TMPRSS2:ERG fusion-positive prostate cancer. Anticancer Res. 2011;31:403–410. [PMC free article] [PubMed] [Google Scholar]
- 30.Baffa R, Fassan M, Volinia S, O’Hara B, Liu CG, Palazzo JP, et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol. 2009;219:214–221. doi: 10.1002/path.2586. [DOI] [PubMed] [Google Scholar]
- 31.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vrba L, Jensen TJ, Garbe JC, Heimark RL, Cress AE, Dickinson S, et al. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS One. 2010;5:e8697. doi: 10.1371/journal.pone.0008697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45:777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 37.Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 39.Cittelly DM, Dimitrova I, Howe EN, Cochrane DR, Jean A, Spoelstra NS, et al. Restoration of miR-200c to ovarian cancer reduces tumor burden and increases sensitivity to paclitaxel. Mol Cancer Ther. 2012;11:2556–2565. doi: 10.1158/1535-7163.MCT-12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopp F, Oak PS, Wagner E, Roidl A. miR-200c sensitizes breast cancer cells to doxorubicin treatment by decreasing TrkB and Bmi1 expression. PLoS One. 2012;7:e50469. doi: 10.1371/journal.pone.0050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Q, Mani RS, Ateeq B, Dhanasekaran SM, Asangani IA, Prensner JR, et al. Coordinated regulation of polycomb group complexes through microRNAs in cancer. Cancer Cell. 2011;20:187–199. doi: 10.1016/j.ccr.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu M, Yu J, Taylor JM, Chinnaiyan AM, Qin ZS. On the detection and refinement of transcription factor binding sites using ChIP-Seq data. Nucleic Acids Res. 2010;38:2154–2167. doi: 10.1093/nar/gkp1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tellez CS, Juri DE, Do K, Bernauer AM, Thomas CL, Damiani LA, et al. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 2011;71:3087–3097. doi: 10.1158/0008-5472.CAN-10-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallejo DM, Caparros E, Dominguez M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 2011;30:756–769. doi: 10.1038/emboj.2010.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, et al. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol. 2012;181:2188–2201. doi: 10.1016/j.ajpath.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, True LD, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci U S A. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leshem O, Madar S, Kogan-Sakin I, Kamer I, Goldstein I, Brosh R, et al. TMPRSS2/ERG promotes epithelial to mesenchymal transition through the ZEB1/ZEB2 axis in a prostate cancer model. PLoS One. 2011;6:e21650. doi: 10.1371/journal.pone.0021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 50.Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, Furusato B, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–5353. doi: 10.1038/onc.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Cai Y, Shao LJ, Siddiqui J, Palanisamy N, Li R, et al. Activation of NF-{kappa}B by TMPRSS2/ERG Fusion Isoforms through Toll-Like Receptor-4. Cancer Res. 2011;71:1325–1333. doi: 10.1158/0008-5472.CAN-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao JC, Yu J, Runkle C, Wu L, Hu M, Wu D, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22:322–331. doi: 10.1101/gr.131508.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 2007;67:10657–10663. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–431. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.