Abstract
We and others have reported the successful conversion of human fibroblasts into functional induced neuronal (iN) cells; however the reprogramming efficiencies were very low. Robust reprogramming methods must be developed before iN cells can be used for translational applications such as disease modeling or transplantation-based therapies. Here, we describe a novel approach in which we significantly enhance iN cell conversion efficiency of human fibroblast cells by reprogramming under hypoxic conditions (5% O2). Fibroblasts were derived under high (21%) or low (5%) oxygen conditions and reprogrammed into iN cells using a combination of the four transcription factors BRN2, ASCL1, MYT1L and NEUROD1. An increase in Map2 immunostaining was only observed when fibroblasts experienced an acute drop in the O2 tension upon infection. Interestingly, cells derived and reprogrammed under hypoxic conditions did not produce more iN cells. Approximately 100% of patched cells fire action potentials in low O2 relative to 50% under high O2 growth conditions, confirming the beneficial aspect of reprogramming under low O2. Further characterization showed no significant difference in the intrinsic properties of iN cells reprogrammed in either condition. Surprisingly, the acute drop in oxygen tension did not affect cell proliferation or cell survival and is not synergistic with blockade of GSK3 beta and Smad-mediated pathways. Our results show that lowering the O2 tension at initiation of reprogramming is a simple and efficient manner to enhance the production of iN cells which will facilitate their use for basic discovery and regenerative medicine.
Keywords: reprogramming, induced neuronal cells, hypoxia, low oxygen
INTRODUCTION
The prospect to derive functional neurons directly from human fibroblasts has major translational implications for the evolving field of regenerative medicine and potentially very broad applications for pathogenetic studies of various neurodegenerative and neurodevelopmental diseases (Vierbuchen and Wernig, 2011). Various somatic, non-neuronal cells from mice and humans have now been reprogrammed into induced neuronal (iN) cells by several groups (Ambasudhan et al., 2011; Kim et al., 2011; Marro et al., 2011; Pang et al., 2011; Qiang et al., 2011; Son et al., 2011; Vierbuchen et al., 2010). Unfortunately, to date the reported efficiencies of iN cell conversion in human fibroblasts have been low. So far, only one study reported a significant improvement of the reprogramming process by the addition of four small molecules modulating GSK3β and the Smad pathways (Ladewig et al., 2012), but the effect of microenviromental changes on iN cell reprogramming has never been tested. Atmospheric O2 content of ~21% is considerably higher than the physiological levels in tissues (Panchision, 2009) particularly the brain which has levels ranging from 0.55% to 8% O2 (Erecińska and Silver, 2001). These levels are finely tuned and if altered could lead to irreversible damages in brain functions (Masamoto and Tanishita, 2009). The in vitro beneficial effects of low O2 tensions similar to physiological levels on cell survival, proliferation and differentiation in neural precursor cells has been previously reported ((Review Zhang et al., 2011)). Furthermore, mild hypoxic conditions can increase the generation efficiency of iPSCs from human somatic cells (Yoshida et al., 2009). These studies led us to hypothesize that culturing cells in O2 levels that resemble physiological conditions would be beneficial for the newly converted neurons and potentially increase the iN cell reprogramming efficiency. Here, we report the significant enhancement of human iN cell conversion when cells are derived in high but reprogrammed in low oxygen conditions that is independent of viability and cell proliferation, and cannot be further improved by previously beneficial GSK3β and Smad pathway interference.
MATERIAL AND METHODS
Cell Culture
Human primary fibroblast (HPF) were established from dissociated foreskin tissue derived from 1–3-day-old newborns and plated in 2 plates with MEF media (DMEM high glucose (Invitrogen), 10% calf serum, sodium pyruvate (Invitrogen), non-essential amino acids (Invitrogen), penicillin/streptomycin (Invitrogen) and β-mercaptoethanol). One plate was placed in an incubator set at 5% O2 and the other at normal atmospheric conditions. Primary fibroblast cells used in the experiments were passaged at least two times after derivation and were not used after passage five. To maintain the iN cell cultures, cells were grown in N3B27 medium (DMEM/F12, N2 supplement, B27 supplement, insulin (5 μg ml−1) and penicillin/streptomycin) (Invitrogen). The media was changed every 3–4 days.
Viral Infection
Lentiviral production and fibroblast infections were performed as described previously (Vierbuchen et al., 2010). Briefly, HPFs were plated and infected with concentrated lentiviral particles and polybrene (8 μg μl−1) in fresh MEF medium. Viral medium was removed after 16–24 h and replaced with N3B27 medium containing doxycycline (Dox) (2 μg ml−1). The media was changed every 3–4 days.
Small Molecule Experimental Conditions
Conditions for the small molecule experiments were done as described in (Ladewig et al., 2012) with slight modifications. The day after infection viral containing media was changed to MEF media containing Dox (2 μg ml−1). After two days the media was changed to N3B27 containing Dox (2 μg ml−1), SB-431542 (10 μM, Tocris), noggin (100 ng ml−1, R&D), and LDN-193189 (0.5 μM, Tocris) and/or CHIR99021 (2 μM, Cayman). This media was changed every 3–4 days for two weeks. At two weeks the media was changed to N3B27 with Dox (2 μg ml−1) until cell characterization at 23 days.
Immunofluorescence and Cell Quantification
Neuronal quantification was based off of Map2 positive cells which had a typical neuronal morphology i.e. rounded cell body with elongated thin neurites at least three times the size of the cell body. For immunofluorescence staining, cells were washed with PBS and then fixed with 4% paraformaldehyde (PFA) for 15–20 minutes at room temperature (RT). Cells were permeabilized and blocked in 0.1% Triton X-100 (Sigma), 5% calf serum in PBS for 30 minutes at RT. Primary and secondary antibodies were diluted in a solution of PBS containing 5% calf serum. Cells were placed in the primary antibodies over night at 4C, washed twice the next morning with PBS and then incubated with the secondary antibody for 30min. The cells were washed three more times in PBS after the secondary incubation. Antibodies: mouse anti-MAP2 (Sigma, 1:500), mouse anti-Tuj1 (Covance, 1:1000), mouse anti-NeuN (Millipore, 1:100), mouse anti-ASCL1 (Developmental Studies Hybridoma Bank (DSHB) 1:1,000), rabbit anti-Ki67 (Novocastra, 1:1000) mouse anti-Sox2 (Cell Signaling, 1:50), goat anti-DCX (Santa Cruz, 1:400 (c18)), mouse anti-NCAM (Santa Cruz 1:100 (ERIC 1)).
Electrophysiology
Patch-clamp recordings were performed at room temperature in external solution containing the followings (in mM): 140 NaCl, 10 glucose, 10 HEPES, 5 KCl, 1 MgCl2, and 2 CaCl2 (305 mOsm, pH adjusted to 7.3 with NaOH). The GFP-infected iN cells were visualized using an X-cite 120Q fluorescence lamp (Lumen Dynamics) and an Olympus BX51WI microscope equipped with a Rolera-XR camera (Qimaging). Whole-cell patches were established using MPC-200 manipulators (Sutter Instrument) and Multiclamp 700B amplifier (Molecular Devices) controlled by Clampex 10 Data Acquisition Software (Molecular Devices). Pipettes were pulled using PC-10 puller (Narishige) from borosilicate glass (OD: 1.5 mm, ID: 0.86 mm; Sutter Instrument) to a resistance of 3–4 MOhm and were filled with internal solution containing (in mM): 130 KMeSO3, 10 NaCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 0.16 CaCl2, 4 Na2ATP, 0.4 NaGTP, and 14 Tris-creatine phosphate (310 mOsm, pH adjusted to 7.3 with KOH). Cells were recorded in current-clamp mode for intrinsic firing properties (Vhold = −60 mV, adjusted with a small holding-current) and were switched to voltage-clamp mode (Vhold = −70 mV) for Na+ and K+ current measurements. Bar-graphs represent average values ± SE (error bars) and statistical comparisons between two conditions were made using unpaired, two-tailed t-test. Significant changes (asterisks) or non-significance (ns) were indicated for comparison between different conditions.
Neurite length
Neurite length tracing was performed using MetaMorph (Molecular Devices, LLC) with the pixel size set according to the manufacturer datasheet (QImaging, 6.45μm × 6.45μm). Twelve fields from each condition from each line were analyzed by the software. The number of MAP2 positive cells was counted manually and used to normalize the neurite length that the software computed.
RESULTS AND DISCUSSION
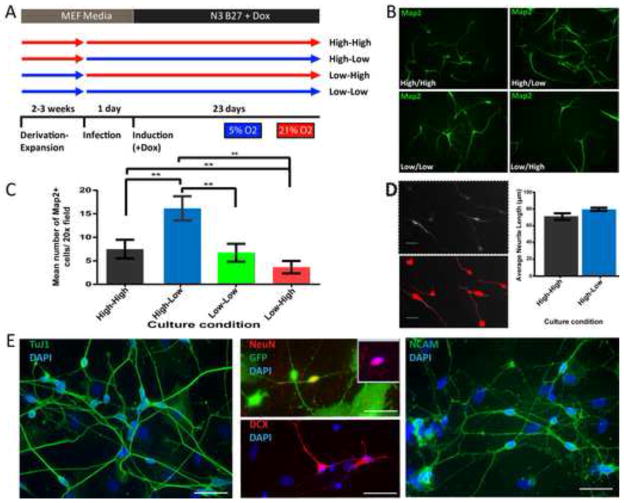
First, we wanted to test whether oxygen tension impacts the conversion of human postnatal fibroblasts (HPFs) to iN cells. Given that previous reports have shown that the derivation of human embryonic stem (ES) cells under hypoxic conditions aids in maintaining their long term pluripotency (Lengner et al., 2010) we also explored whether the derivation and expansion of HPFs in low O2 would affect their reprogramming capacity. To that end, we derived HPFs from four different foreskin samples and expanded them in either high (21%) or low (5%) O2 conditions and were maintained in these conditions until the time of reprogramming. Cells were virally transduced with EGFP and the four transcription factors Ascl1 (A), Brn2 (B), Myt1l (M) and NeuroD1 (N), that we showed previously were sufficient to reprogram human fibroblast into functional neurons (Pang et al., 2011). The day of infection cells derived in high O2 were either maintained in high O2 (High-High), or switched to low O2 (High-Low) conditions (Figure. 1A). The same was done with the cells derived in low O2 generating Low-Low and Low-High conditions (Figure. 1A). Twenty-three days after doxycycline (Dox) induction of the transgenes, reprogramming was assessed by staining for the neuronal marker Map2. We chose Map2 rather than the commonly used marker Tuj1 because the latter is already expressed in human fibroblasts (Pang et al., 2011). The quantification of Map2-positive cells revealed a significant 2.4-fold increase of Map2-positive cells with neuronal morphology in the High-Low condition when compared to the High-High condition (n = 3 independent experiments, P < 0.009, (Figure. 1B–C). Surprisingly, there was no significant difference in the number of Map2-positive cells between High-High and Low-Low conditions demonstrating that unlike derivation of ES cells (Lengner et al., 2010) (i) the derivation of fibroblasts in low O2 is not critical and (ii) not the absolute levels of O2 but the drop of oxygen tension at the beginning of reprogramming matter. Conversely, there was a significant reduction in the reprogramming capacity of HPFs derived in low O2 and reprogrammed in high O2 (n = 3 independent experiments, P < 0.05), suggesting that a relative increase in oxygen levels is detrimental for iN cell reprogramming. We also noticed that the HPF line-to-line reprogramming efficiency is quite variable, but the increase of Map2 positive cells in High-Low condition was observed in all cases, supporting our observation that reprogramming iN cells under slightly hypoxic conditions is beneficial and is not line specific (Figure. S1A). The cell line-dependent variability could explain the different reprogramming efficiencies reported (Ladewig et al., 2012; Yoo et al., 2011). We then confirmed the expression of several pan-neuronal markers in iN cells generated in High-Low oxygen conditions including Tuj1, Dcx, NeuN, and NCAM by day 23 (Figure. 1E). To begin to test whether there were differences between the Map2-positive neuronal like cells from the High-High condition and those in the High-Low condition we took a simplistic approach and measured neurite length of Map2-positive cells in both conditions. Despite reaching significance in one line, overall we were unable to detect a significant increase in the average neurite length of these cells (Figure. 1D, S1B), suggesting that there are no major morphological difference between the cells reprogrammed in the High-High and High-Low conditions.
Figure 1. Acute drop of oxygen tension improves human iN cell reprogramming.
(A) Cartoon representation of experimental design. (B) Map2 staining (green) showing representative field of views from all four experimental conditions, High-High, High-low, Low-Low and Low-High. (C) Quantification of Map2 positive cells from three HPF lines. Bar graphs represent average number of Map2 positive cells in 20x field with normal neuronal morphology. Counts from each HPF line are based on twenty 20x fields from two replicate wells. The High-Low condition showed significantly (n = 3, P < 0.005) higher counts of Map2+ cells, when compared to the High-High condition. (D) Neurite length was quantified using MetaMorph (Molecular Devices, LLC) software. Analysis was done on three different HPF lines. (Bar scale=20μm) (E) Immunoflurescent staining of pan-neuronal markers TuJ1, DCX, NeuN and NCAM. Cultures stained for TuJ1, DCX and NCAM were not infected with eGFP (Bar scale=50μm)
Since low oxygen has been reported to overcome senescence and affect cell proliferation in various systems (Betts et al., 2008; Parrinello et al., 2003; Studer et al., 2000), we explored whether a change in cell proliferation during reprogramming could be accountable for the observed effects. Following the same experimental design as previously described, the day of infection cells derived in high O2 were either maintained in high O2 (High-High), or switched to low O2 (High-Low) conditions (Figure. 2A). We then determined the percentage of Ki67-positive cells at various time points in the two different conditions. Surprisingly, we observed no difference between the High-High and the High-Low conditions before and 1 day after infection and 2 days after Dox (Figure. 2B). Consistent with our previous observation that the vast majority of murine iN cells become postmitotic soon after transgene induction, the percentage of Ki67-positive cells was dramatically decreased 2 days post Dox in both conditions (Figure. 2B) (Vierbuchen et al., 2010). An Ascl1/ Ki67 co-staining revealed a mutually exclusive expression demonstrating that the few remaining cycling cells were not infected (Figure. 2D). We also found that the total number of cells were unchanged between the High-High and High-Low oxygen condition (Figure. 2C). This suggests that also the rate of apoptosis due to the stress of viral infection was similar in both conditions. If low oxygen had a general impact on apoptosis one might have expected an increase in reprogramming efficiency also in the Low-Low oxygen condition. Finally, we also excluded the possibility of an intermediate neural precursor-like cell in the starting population as there was complete absence of Sox2-positive cells (data not shown). Thus, we cannot attribute the increase in Map2-positive cells to differences in cell numbers prior to the induction of transgenes or initial neural precursor populations.
Figure 2. The High-Low oxygen condition does not impact cell proliferation and survival and can be mimicked by inhibitors of GSK3β and SMAD signaling pathways.
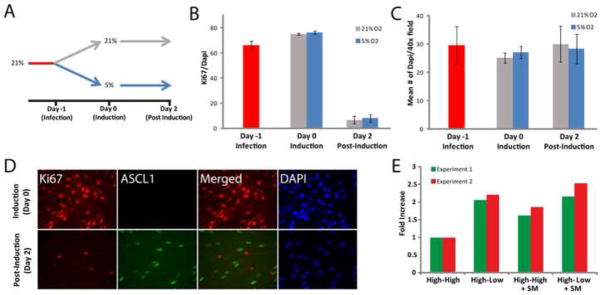
(A) Cartoon representation of the experimental design. (B) Ki67 quantification shows no significant difference between the High-High and the high-low conditions. Bar graph represents the percentage of Ki67 positive cells (n=4). (C) Bar graph showing average number of Dapi staining per 40x field of view (n=4) showing similar numbers of cells in the High-High and the High-Low conditions. (D) Ki67 and Ascl1 immunoflurescent staining showing no overlap after doxycycline. (E) Based on (Ladewig et al., 2012) inhibitors of GSK3β and SMAD signaling pathways were tested for synergistic interaction with low O2 reprogramming condition. Bar graph represents fold increase of Map2 positive cells from two independent experiments. No apparent synergistic effect was apparent.
Recently, the addition of four small molecules to inhibit the GSK3β and Smad signaling pathways were reported to increase the efficiency of iN cell reprogramming (Ladewig et al., 2012). We therefore wanted to address whether the effects of low O2 were synergistic with blockade of these signaling pathways. To address this question, we transduced HPFs with BAMN and grew them in the High-High or High-Low conditions in the presence or absence of the small molecules. Importantly, we could reproduce an increase in reprogramming efficiency in response to the small molecules albeit much less dramatic than reported perhaps due to the more stringent Map2 staining utilized in our study and line-to-line variability. Second, we observed that the increase in reprogramming efficiency was similar between the High-Low condition and the small molecule treatment (Figure. 2E). Furthermore, there was no additional reprogramming enhancement when the molecules were combined with the High-Low condition suggesting the involvement of the GSK3β and Smad signaling pathways in low oxygen reprogramming.
Finally, we performed electrophysiological characterizations of the cells in order to determine whether low oxygen has an effect on the quality and functional maturation of iN cells. Current-clamp recordings from EGFP co-infected cells with neuronal morphology derived from four different HPF lines revealed their ability to generate action-potentials (APs) in response to a brief current pulse (Figure. 3). Around 50% of the iN cells patched from the High-High condition fired mature APs (single or multiple) upon current injection but the other 50% failed to do so (Figure. 3A–B, S1C). Interestingly, almost 100% of the cells patched from High-Low condition successfully generated APs under the same protocol (Figure. 3A–B, S1C). To test whether there were intrinsic differences between the Map2-positive neuronal like cells from the High-High condition and those in the High-Low condition we took a systematic approach and tested various parameters that would address maturity and viability of the cells. First, we measured neurite length of Map2-positive cells in both conditions, as described previously no difference was detected (Figure. 1D, S1B). We next compared the intrinsic properties of iN cells that were able to generate mature APs in High-High and High-Low conditions, but found no significant change in any of the parameters tested (Figure. 3C). Furthermore, in voltage-clamp mode we measured the fast, inactivating, inward Na+-current and outward K+-currents from iN cells with APs and found no significant difference between High-High and High-Low conditions (Figure. S1D–E).
Figure 3. iN cells reprogrammed in low and high oxygen are functionally equivalent.
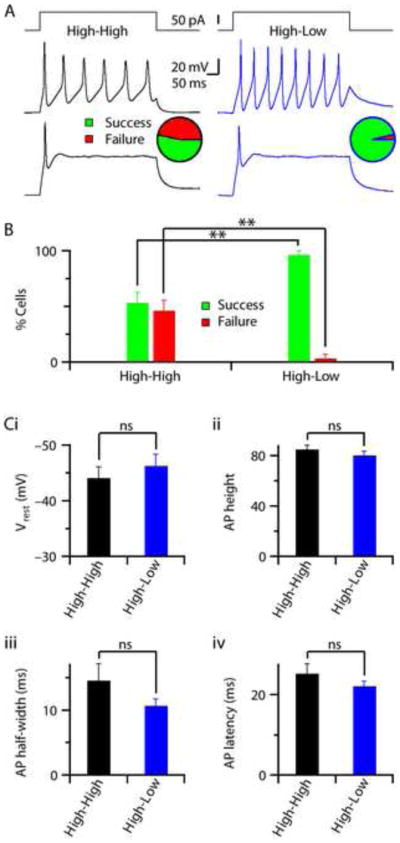
(A) Cells patched under current-clamp mode and ability of firing AP was determined by a 100 pA current pulse (top panels). Example traces showing the presence of multiple (middle panels) or single (bottom panels) AP-firing cells, both in High-High (black, left) or High-Low (blue, right) conditions. Pie-charts indicate the relative fraction of cells that were able to generate AP (Success, green) or the cells that did not (Failure, red), calculated from the total population of cells patched from four independent experimental batches each with a different HPF line (n = 28 cells / condition). (B) Bar-graphs represent average percentages of iN cells capable of firing AP (Success, green) or failed to do so (Failure, red), calculated from independent experimental batches from different HPF lines presented in Fig.3 A. The High-Low condition showed significantly (n = 4, P < 0.005) higher percentages of cells capable of firing AP and a lower failure rate, when compared to High-High condition. (C) Average bar-graphs demonstrate average values of the intrinsic properties measured from iN cells those are capable of firing AP in High-High (n = 15 cells / 4 batches) and High-Low (n = 27 cells / 4 batches) conditions. No significant differences found in resting membrane-potential (Vrest, P > 0.4) (i), AP height (P > 0.3) (ii), AP half-width (P > 0.1) (iii), and AP latency (P > 0.1), calculated as the time from pulse-start to peak amplitude of AP (iv).
CONCLUSION
In summary, this study demonstrates that the reprogramming of fibroblasts in low O2, which were established in high O2, yields more functional iN cells but the maturation stage of the cells is equivalent to those established and reprogrammed in high O2. Although iPSC reprogramming is a fundamentally different process, low O2 also increased the efficiency of iPSCs formation to a remarkably similar (2.5-fold) extent as reported here (Yoshida et al., 2009).
Changes in O2 tension have long been thought to have diverse effects on cell survival ranging from changes in metabolism to genetic stability (Bristow and Hill, 2008). For example, hypoxia inducible factors (HIFs) are extremely sensitive to O2 tension changes and have been shown to regulate pluripotency and proliferation in human embryonic stem cells (Forristal et al., 2010). This family of transcription factors also affect neuronal differentiation (Wang et al., 2013). Furthermore, human fibroblasts grown under 5% O2 have increased cell life span and begin to express low levels of Sox2, Oct4 and Nanog (Page et al., 2009), potentially becoming more easy to reprogram. This would partially explain the increase in iPSC reprogramming observed by Yoshida et al 2008 under low O2 conditions. In mouse embryonic fibroblast mild hypoxic conditions down regulate P53 pathways (Parrinello et al., 2003), which has been shown to inhibit reprogramming of iPSCs (Hong et al., 2009). These pathways should be further studied in the context of iN cell reprogramming and might serve to enhance reprogramming levels.
Our results suggest that transitioning cells into a hypoxic environment can serve as a universal catalyst for the dramatic cellular changes which occurs during any type of cellular reprogramming. Thus, while reprogramming at low O2 is a critical and simple way to increase iN cell reprogramming efficiency, it may very well be important for additional direct lineage conversions. It is worth testing especially in those reprogramming attempts that have failed so far such as the conversion of fibroblasts to clinically relevant cell types like insulin-producing cells.
Supplementary Material
(A) Quantification of Map2 positive cells from three individual hFP lines. Bar graphs represent average number of Map2 positive cells in 20x field with normal neuronal morphology. Counts from each HPF line are based on twenty 20x fields from two replicate wells. The High-Low condition consistently showed higher numbers of Map2+ cells, when compared to the High-High condition. (B) Quantification of neurite length based on Map2 staining from three individual hFP lines. (C) The table shows the number of cells that fired action potentials from each four individual HPF lines. (D) Voltage-clamp recordings were made from HPF iN cells and a 60 mV voltage-pulse protocol (top panel) was used to measure sodium (green dotted box, left panel) and potassium currents (red bar, left panel). Insets show expanded view of sodium currents for High-High (left panels, black) and High-Low (right panels, blue) conditions. (E) No significant differences (P > 0.7) found between the average values of sodium (INa) and potassium (IK) currents recorded from cells capable of firing AP in High-High (n = 15 cells / 4 batches) and High-Low (n = 27 cells / 4 batches) conditions.
Highlights.
Our results show that lowering the O2 tension at initiation of reprogramming is a simple and efficient manner to enhance the production of iN cells.
The acute drop in O2 tension did not affect cell proliferation or cell survival.
Growth in low O2 yields more functional iN cells but the maturation stage of the cells is equivalent to those established and reprogrammed under normoxic conditions.
Blockade of GSK3β and Smad-mediated pathways during iN reprogramming does not act synergistically with reprogramming under hypoxic conditions.
Acknowledgments
We would like to thank D. Solow-Cordero from Stanford’s High Throughput Bioscience Center for important help regarding image analysis. Our work on iN cell reprogramming is supported by the NIH grants AG010770-18A1, R01MH092931, RC4 NS073015-01 and the CIRM grant RT2-02061. M.W. is a New York Stem Cell Foundation-Robertson Investigator.
Footnotes
Conflict of interest: There are no conflicts of interest.
Author contributions:
J.D.: Conception and design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
S.C.: Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing.
C.E.A.: Collection and/or assembly of data, Data analysis and interpretation.
T.C.S.: Financial support.
M.W.: Data analysis and interpretation, Financial support, Manuscript writing, Final approval of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–8. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts DH, Perrault SD, King WA. Low oxygen delays fibroblast senescence despite shorter telomeres. Biogerontology. 2008;9:19–31. doi: 10.1007/s10522-007-9113-7. [DOI] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–76. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Su SC, Wang H, Cheng AW, Cassady JP, Lodato MA, Lengner CJ, Chung CY, Dawlaty MM, Tsai LH, Jaenisch R. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell. 2011;9:413–9. doi: 10.1016/j.stem.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kögler G, Müller FJ, Koch P, Brüstle O. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575–8. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, Alagappan R, Frampton GM, Xu P, Muffat J, Santagata S, Powers D, Barrett CB, Young RA, Lee JT, Jaenisch R, Mitalipova M. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell. 2010;141:872–83. doi: 10.1016/j.cell.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Südhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–82. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Tanishita K. Oxygen transport in brain tissue. J Biomech Eng. 2009;131:074002. doi: 10.1115/1.3184694. [DOI] [PubMed] [Google Scholar]
- Page RL, Ambady S, Holmes WF, Vilner L, Kole D, Kashpur O, Huntress V, Vojtic I, Whitton H, Dominko T. Induction of stem cell gene expression in adult human fibroblasts without transgenes. Cloning Stem Cells. 2009;11:417–26. doi: 10.1089/clo.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–8. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–3. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–7. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–71. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–18. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–83. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang J, Li H, Wang X, Zhu L, Fan M. Hypoxia promotes dopaminergic differentiation of mesenchymal stem cells and shows benefits for transplantation in a rat model of Parkinson’s disease. PLoS One. 2013;8:e54296. doi: 10.1371/journal.pone.0054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–31. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–41. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhu L, Fan M. Oxygen, a Key Factor Regulating Cell Behavior during Neurogenesis and Cerebral Diseases. Front Mol Neurosci. 2011;4:5. doi: 10.3389/fnmol.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Quantification of Map2 positive cells from three individual hFP lines. Bar graphs represent average number of Map2 positive cells in 20x field with normal neuronal morphology. Counts from each HPF line are based on twenty 20x fields from two replicate wells. The High-Low condition consistently showed higher numbers of Map2+ cells, when compared to the High-High condition. (B) Quantification of neurite length based on Map2 staining from three individual hFP lines. (C) The table shows the number of cells that fired action potentials from each four individual HPF lines. (D) Voltage-clamp recordings were made from HPF iN cells and a 60 mV voltage-pulse protocol (top panel) was used to measure sodium (green dotted box, left panel) and potassium currents (red bar, left panel). Insets show expanded view of sodium currents for High-High (left panels, black) and High-Low (right panels, blue) conditions. (E) No significant differences (P > 0.7) found between the average values of sodium (INa) and potassium (IK) currents recorded from cells capable of firing AP in High-High (n = 15 cells / 4 batches) and High-Low (n = 27 cells / 4 batches) conditions.