Abstract
Myelodysplastic syndromes with myelofibrosis (MDS-F) is a poor prognostic hematopoietic disorder. Azacitidine was shown to prolong survival of high-risk MDS patients. However, the effects of azacitidine on MDS-F have yet to be elucidated. Azacitidine was administered to a 74-year-old man with MDS-F at a dose of 75 mg/m2/daily subcutaneously for 7 days every 28 days. Hematologic improvements were observed according to the International Working Group 2006 criteria after 8 cycles of the azacitidine treatment, and complete remission was achieved after 14 cycles. The grade of myelofibrosis was also improved. The therapeutic activity of azacitidine was confirmed in our MDS-F patient.
Keywords: MDS, Myelofibrosis, Azacitidine, IWG 2006 criteria, Hematologic improvement
Highlights
-
•
We here reported the successful response of a MDS-F patient treated with azacitidine.
-
•
Complete remission was achieved after 14 cycles of the azacitidine treatment.
-
•
The grade of myelofibrosis was improved significantly.
-
•
Tri-lineage dysplasia with severe dysmegakaryopoiesis still remains.
-
•
The therapeutic activity of azacitidine was confirmed in our MDS-F patient.
1. Introduction
Myelodysplastic syndromes (MDS) are clonal hematopoietic disorders characterized by ineffective hematopoiesis and potential transformation to acute myeloid leukemia. The mainstay for the treatment of MDS for decades was supportive care. However, supportive care does not alter the natural course of MDS. The only curative treatment for MDS is allogeneic stem cell transplantation (SCT).
Epigenetic changes play an important role in the pathogenesis of myeloid neoplasms. Hence, the role of DNA methyltransferase inhibitors such as azacitidine in achieving promising outcomes for patients with MDS has been investigated. Azacitidine was shown to significantly prolong the survival of high-risk MDS patients [1]. However, the characteristics of MDS are very heterogeneous. Significant degrees of myelofibrosis have been reported in approximately 10% of MDS patients. Myelofibrosis was previously shown to be a poor prognostic factor in MDS. The overall survival (OS) achieved with allogeneic SCT was shown to be shorter in MDS patients with severe myelofibrosis than that in those without myelofibrosis [2]. Azacitidine has been shown to have limited therapeutic activity in patients with primary myelofibrosis and post-essential thrombocythemia (ET) / polycythemia vera (PV) myelofibrosis [3]. Thalidomide, lenalidomide, and pomalidomide have also been shown to exhibit the therapeutic activity in patients with myelofibrosis with myeloid metaplasia, primary myelofibrosis, or post-ET / PV myelofibrosis [4–6]. Previous studies suggested that the etiology of primary myelofibrosis may be associated with the activation of Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling [7] and an increase in inflammatory cytokine levels [8]. Ruxolitinib, a potent JAK1 and 2 inhibitor, has demonstrated reductions in splenomegaly and disease-related symptoms, and an improvement in overall survival in patients with myelofibrosis [9]. Myelofibrosis with MDS may also be associated with the activation of JAK–STAT signaling and an increase in cytokine levels.
However, the effects of azacitidine on MDS with myelofibrosis (MDS-F) remain unknown. We here reported the successful response of a MDS-F patient treated with azacitidine.
2. Case report
A 74-year-old man was transferred to our hospital with cytopenia. An initial peripheral blood examination revealed anemia: hemoglobin (Hb) 9.5 g/dL, platelets (Plt) 71×109/L, and white blood cells (WBC) 1.84×109/L with 0% blasts, 10% band forms, 23% segmented neutrophils, 2% monocytes, and 65% lymphocytes. His ferritin serum level was 197 ng/mL (30–310).
Bone marrow biopsy showed hyperplastic bone marrow with myelofibrosis and an increased number of megakaryocytes (Fig. 1A). Differential counts were 29.0% erythroblasts, 10.3% myeloblasts, 2.3% promyelocytes, 7.0% myelocytes, 1.7% metamyelocytes, 8.6% band forms, 10.5% segmented neutrophils, 1.3% monocytes, 1.6% eosinophils, 0.6% basophils, and 25.9% lymphocytes. Blast cells in his bone marrow stained positive for myeloperoxidase and, based on an immunohistochemical analysis, were also found to express CD34. Dysplasia was observed in tri-lineage cells, and was severe in megakaryocytes and mild in neutrophils and erythroblasts (Fig. 1B and C). A cytogenetic examination of bone marrow cells by G-banding analysis showed the normal karyotype, 46,XY[20/20]. The JAK2-V617F mutation was not detected. He was diagnosed as refractory anemia with an excess of blasts (RAEB)-2 with myelofibrosis according to the 2008 World Health Organization (WHO) classification. The risk group was classified as intermediate-2 in the International Prognostic Scoring System (IPSS), high in the revised IPSS, and high in the WHO classification-based prognostic scoring system (WPSS). This myelofibrosis was assessed as grade 3 following the European consensus guidelines. Allogeneic SCT was judged to be an unsuitable treatment because of his age and complications (type 2 diabetes mellitus, and angina pectoris). Therefore, he was treated with azacitidine. He required a red blood cell transfusion before being treated with azacitidine. The risk group was classified as low in the prognostic score for the azacitidine treatment [10].
Fig. 1.
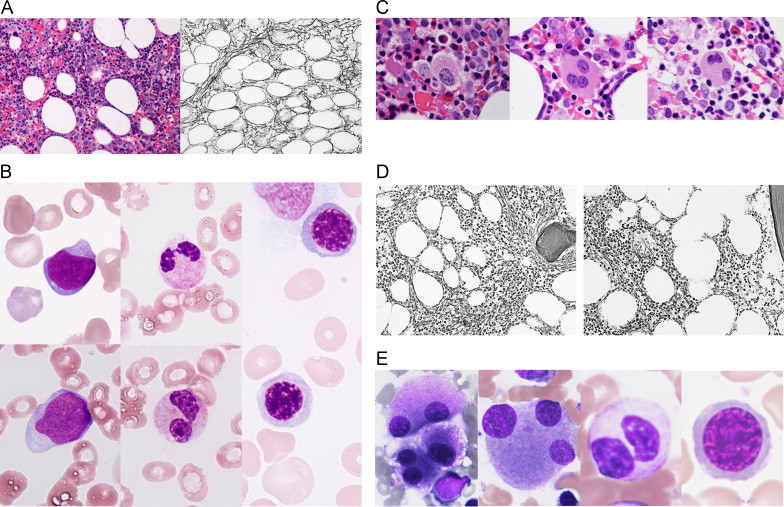
A. Bone marrow biopsy before azacitidine treatment. Left: hyperplastic marrow with myeloid hyperplasia and an increased number of megakaryocytes (Hematoxylin–Eosin stain×200). Right: dense reticulin fibers with bundles of collagen. The grade of myelofibrosis was assessed as grade 3 (Gomori׳s silver impregnation technique×200). B. Bone marrow aspirate smear before the azacitidine treatment (May–Giemsa stain×1000). Left: myeloblasts. Center: hyposegmented mature neutrophils. Right: mild megaloblastic changes in erythroblasts. C. Bone marrow biopsy before the azacitidine treatment (Hematoxylin–Eosin stain×1000). Multinucleated and hypolobated nuclei megakaryocytes. D. Bone marrow biopsy after the azacitidine treatment (Gomori׳s silver impregnation technique×200). Left: after 4 cycles of the azacitidine treatment, reticulin fibers with focal bundles of collagen were assessed as grade 2. Right: after 8 cycles, a loose meshwork of thin reticulin fibers was observed with grade 1 myelofibrosis. E. Bone marrow aspirate smear after 20 cycles of the azacitidine treatment (May–Giemsa stain×1000). Multinucleated and hypolobated nuclei megakaryocytes, hyposegmented mature neutrophils, and mild megaloblastic changes in erythroblasts.
Azacitidine was administered at a dose of 75 mg/m2/daily subcutaneously for 7 consecutive days every 28 days. Transient neutropenia was observed until the seventh cycle of the azacitidine treatment was completed. A gradual increase in his blood cell count was noted and the patient eventually became transfusion independent. Hematological responses after 8 cycles of the azacitidine treatment were considered to be hematologic improvements, with erythroid (HI-E) and platelet (HI-P) responses according to the International Working Group (IWG) 2006 criteria (Table 1). A reduction in myelofibrosis occurred after 4 cycles and repeated bone marrow biopsies revealed a significant improvement in the grade of myelofibrosis (Fig. 1D). The percentage of myeloblasts after 8 cycles was 4.5%. However, the absolute neutrophil count (ANC) was less than 1.0×109/L. The efficacy of the azacitidine treatment was judged to be marrow complete remission (marrow CR) with HI-E and HI-P responses. The ANC increased gradually and exceeded 1.0×109/L. CR was obtained after 14 cycles of the azacitidine treatment (Table 1). A total of 20 cycles of this treatment have been completed to date. Hyperplastic bone marrow with mild myelofibrosis, an increased number of megakaryocytes, and tri-lineage dysplasia with severe dysmegakaryopoiesis still remains (Fig. 1E). Hematologic improvements have been ongoing for 12 months, during which time 12 cycles of the azacitidine treatment have been completed (Table 1).
Table 1.
Hematologic improvements and the International Working Group (IWG) 2006 response criteria by cycles of the azacitidine treatment.
| Cycles of the azacitidine treatment | Hb (g/dL) | Plt (×104/µL) | ANC (/µL) | Transfusion (Red blood cell) | BM blast (%) |
Hematologic improvements |
IWG 2006 response criteria | ||
|---|---|---|---|---|---|---|---|---|---|
| HI-E (increase by ≧1.5 g/dL) | HI-P (increase of ≧3×104/µL) | HI-N (100% increase) | |||||||
| Pre-treatment | 9.5 | 7.1 | 607 | + | 10.3 | − | − | − | – |
| 1 | 8.9 | 7.3 | 396 | + | ND | − | − | − | SD |
| 2 | 8.3 | 7.3 | 332 | + | 10.1 | − | − | − | SD |
| 3 | 8.5 | 9.9 | 442 | + | ND | − | − | − | SD |
| 4 | 11.1 | 11.3 | 580 | + | 9.6 | − | + | − | SD |
| 5 | 10.1 | 8.4 | 442 | + | ND | − | − | − | SD |
| 6 | 9.6 | 9.4 | 435 | − | ND | − | − | − | SD |
| 7 | 9.5 | 10.7 | 365 | − | ND | − | + | − | SD |
| 8 | 11.4 | 17.9 | 976 | − | 4.5 | + | + | − | marrow CR with HI-E and HI-P |
| 9 | 11.0 | 17.0 | 663 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 10 | 11.6 | 20.2 | 638 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 11 | 11.8 | 18.6 | 865 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 12 | 12.2 | 22.3 | 496 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 13 | 14.6 | 23.8 | 577 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 14 | 12.2 | 23.4 | 1012 | − | ND | + | + | − | CR |
| 15 | 12.6 | 18.6 | 931 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 16 | 12.0 | 18.2 | 975 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 17 | 13.2 | 21.0 | 889 | − | ND | + | + | − | marrow CR with HI-E and HI-P |
| 18 | 12.0 | 19.7 | 1573 | − | ND | + | + | + | CR |
| 19 | 12.1 | 15.1 | 1660 | − | ND | + | + | + | CR |
| 20 | 11.7 | 15.1 | 2068 | − | 2.2 | + | + | + | CR |
Peripheral blood counts and responses were evaluated just before the next cycle of the azacitidine treatment.
Abbreviations: Hb: hemoglobin, Plt: platelets, ANC: absolute neutrophil count, BM: bone marrow, HI-E: hematological improvement-erythroid, HI-P: hematological improvement-platelet, HI-N: hematological improvement-neutrophil, ND: no data, SD: stable disease, CR: complete remission.
3. Discussion
The pathogenesis of myelofibrosis in MDS patients is unknown. Clinical trials on azacitidine demonstrated the significant and clinically meaningful prolongation of OS in high-risk MDS patients [1]. However, the successful response of MDS-F patients to the azacitidine treatment has not yet been reported.
Although thalidomide was previously shown to have anti-fibrotic effects in a MDS-F patient [11], suitable therapeutic agents for MDS-F patients have not yet been established. Allogeneic SCT is currently the only curative treatment for MDS patients with/without myelofibrosis. The grade of myelofibrosis is known to affect the engraftment of allogeneic SCT in MDS patients; however, only severe myelofibrosis has been shown to influence survival due to the higher risk of relapse [2]. We showed that azacitidine exhibited therapeutic activity in our MDS-F patient. If the severity of myelofibrosis can be reduced by azacitidine, this treatment may lead to significant prolongation of OS after allogeneic SCT in MDS-F patients.
Epigenetic changes play an important role in the pathogenesis of myeloid neoplasms. Decitabine was previously shown to be effective in an idiopathic myelofibrosis patient [12]. However, the pathogenesis of myelofibrosis in ET / PV patients remains unclear. A previous study that evaluated the activity of azacitidine in patients with myelofibrosis (primary and post-ET / PV) reported that 19 (70%) of 27 patients had the JAK2 (V617F) mutation [3]. Responses included limited clinical improvements in 7 (21%) patients and a partial response in only 1 (3%) patient. These findings indicated that azacitidine exhibited limited therapeutic activity for myelofibrosis in patients with myeloproliferative neoplasms such as primary and post-ET / PV myelofibrosis.
Whereas, ruxolitinib, a potent and selective JAK1 and 2 inhibitor, as compared with the best available therapy, in patients with myelofibrosis (primary and post-ET / PV) has demonstrated rapid reductions in splenomegaly, marked improvement in myelofibrosis-associated symptoms, and the prolongation of overall survival regardless of their status with respect to the JAK2 (V617F) mutation [9]. JAK1 and 2 inhibitor therapy was considered for our patient with myelofibrosis if azacitidine treatment was not successful.
Our patient did not have myeloproliferative neoplasms such as splenomegaly or the JAK2 (V617F) mutation. Therefore, he was diagnosed with MDS-F, and achieved CR with reduced myelofibrosis with the azacitidine treatment. These findings indicate that azacitidine may affect an MDS clone and indirectly improve myelofibrosis.
This is the first case of MDS-F that was successfully treated with azacitidine. Our patient had RAEB-2 with myelofibrosis without the JAK2 (V617F) mutation. Severe dysplasia was observed in his megakaryocytes. The grade of myelofibrosis was improved and CR was obtained after the long-term treatment with azacitidine; however, tri-lineage dysplasia remains. The delay observed in hematologic improvements in our patient may be attributed to severe myelofibrosis. Therefore, these results indicate that a subsequent and long-term treatment with azacitidine may be required to obtain hematologic improvements and reduce myelofibrosis in patients with MDS-F.
A large scale study is needed to clarify the relationship between anti-fibrotic effects and treatment with azacitidine in MDS-F patients.
References
- 1.Fenaux P., Mufti G.J., Hellstrom-Lindberg E., Santini V., Finelli C., Giagounidis A. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroger N., Zabelina T., van Biezen A., Brand R., Niederwieser D., Martino R. Allogeneic stem cell transplantation for myelodysplastic syndromes with bone marrow fibrosis. Haematologica. 2011;96:291–297. doi: 10.3324/haematol.2010.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintas-Cardama A., Tong W., Kantarjian H., Thomas D., Ravandi F., Kornblau S. A phase II study of 5-azacitidine for patients with primary and post-essential thrombocythemia/polycythemia vera myelofibrosis. Leukemia. 2008;22:965–970. doi: 10.1038/leu.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchetti M., Barosi G., Balestri F., Viarengo G., Gentili S., Barulli S. Low-dose thalidomide ameliorates cytopenias and splenomegaly in myelofibrosis with myeloid metaplasia: a phase II trial. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:424–431. doi: 10.1200/JCO.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A., Cortes J., Verstovsek S., Mesa R.A., Thomas D., Lasho T.L. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108:1158–1164. doi: 10.1182/blood-2006-02-004572. [DOI] [PubMed] [Google Scholar]
- 6.Tefferi A., Verstovsek S., Barosi G., Passamonti F., Roboz G.J., Gisslinger H. Pomalidomide is active in the treatment of anemia associated with myelofibrosis. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:4563–4569. doi: 10.1200/JCO.2008.21.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komura E., Chagraoui H., Mansat de Mas V., Blanchet B., de Sepulveda P., Larbret F. Spontaneous STAT5 activation induces growth factor independence in idiopathic myelofibrosis: possible relationship with FKBP51 overexpression. Exp Hematol. 2003;31:622–630. doi: 10.1016/s0301-472x(03)00085-7. [DOI] [PubMed] [Google Scholar]
- 8.Panteli K.E., Hatzimichael E.C., Bouranta P.K., Katsaraki A., Seferiadis K., Stebbing J. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol. 2005;130:709–715. doi: 10.1111/j.1365-2141.2005.05674.x. [DOI] [PubMed] [Google Scholar]
- 9.Cervantes F., Vannucchi A.M., Kiladjian J.J., Al-Ali H.K., Sirulnik A., Stalbovskaya V. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 10.Itzykson R., Thepot S., Quesnel B., Dreyfus F., Beyne-Rauzy O., Turlure P. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–411. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 11.Tsirigotis P., Venetis E., Rontogianni D., Dervenoulas J., Kontopidou F., Apostolidis P. Thalidomide in the treatment of myelodysplastic syndrome with fibrosis. Leuk Res. 2002;26:965–966. doi: 10.1016/s0145-2126(02)00036-x. [DOI] [PubMed] [Google Scholar]
- 12.Danilov A.V., Relias V., Feeney D.M., Miller K.B. Decitabine is an effective treatment of idiopathic myelofibrosis. Br J Haematol. 2009;145:131–132. doi: 10.1111/j.1365-2141.2008.07541.x. [DOI] [PubMed] [Google Scholar]


