Abstract
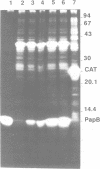
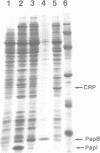
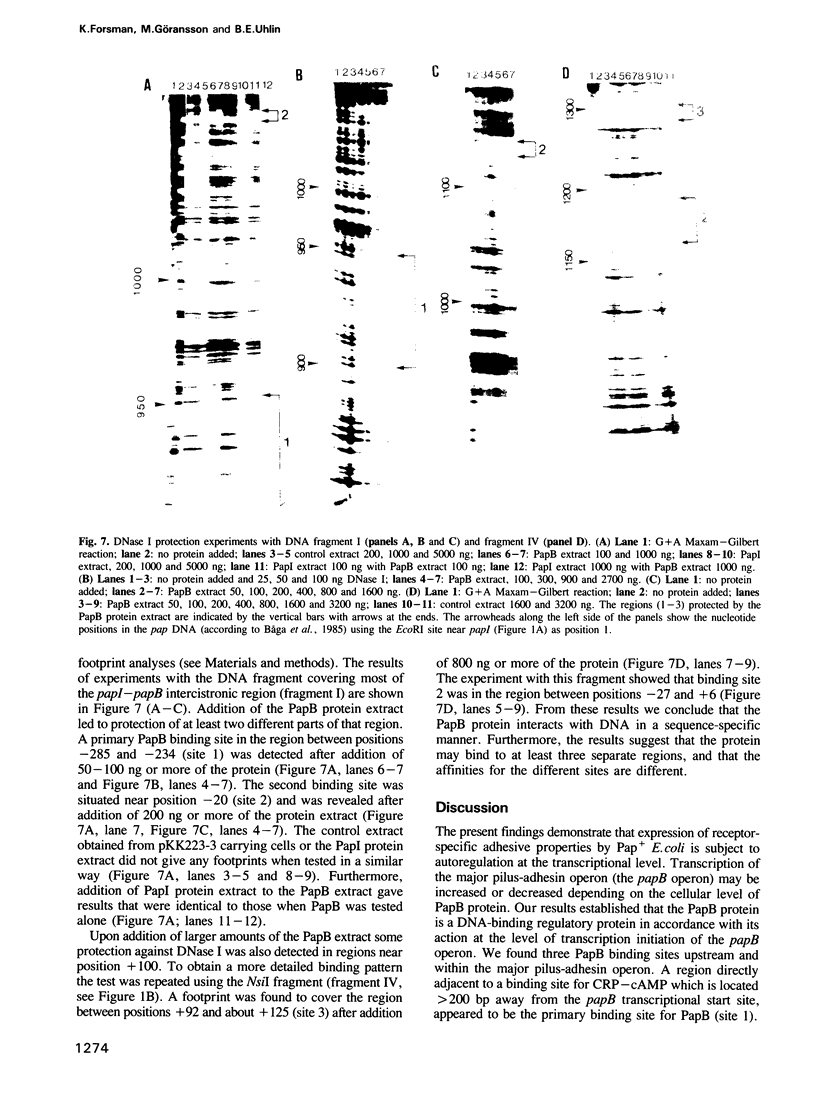
An operon mediating biogenesis of digalactoside-binding pilus-adhesin of serotype F13 in uropathogenic Escherichia coli includes the regulatory gene papB. The papB gene product was found to act as transcriptional activator of an operon which includes the papB gene and several pap cistrons encoding the proteins of the pilus polymer. Studies of how pap gene expression was affected by increasing amounts of PapB protein in the cells showed that high levels did not stimulate transcription but caused repression. Results from in vitro studies demonstrated that the PapB protein was a sequence-specific DNA-binding protein. Binding studies using gel mobility shift assays and DNase I protection (footprinting) showed that PapB protein binds to three separate sites. A sequence greater than 200 bp upstream of the promoter, and directly adjacent to a binding site for the cAMP receptor protein-cAMP complex, appeared as a preferential PapB binding site. A second site was localized to sequences overlapping the -10 region of the promoter and a third binding site was found within the coding sequence of the papB gene itself. The data suggest that the PapB protein has a dual function as activator/repressor of pilus-adhesin transcription and that its autoregulatory mode of action involves differential binding to separate sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Fujimoto S., Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982 Feb 25;10(4):1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell. 1988 Jan 29;52(2):197–206. doi: 10.1016/0092-8674(88)90508-9. [DOI] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Göransson M., Forsman K., Uhlin B. E. Functional and structural homology among regulatory cistrons of pili-adhesin determinants in Escherichia coli. Mol Gen Genet. 1988 Jun;212(3):412–417. doi: 10.1007/BF00330844. [DOI] [PubMed] [Google Scholar]
- Göransson M., Forsman K., Uhlin B. E. Regulatory genes in the thermoregulation of Escherichia coli pili gene transcription. Genes Dev. 1989 Jan;3(1):123–130. doi: 10.1101/gad.3.1.123. [DOI] [PubMed] [Google Scholar]
- Göransson M., Uhlin B. E. Environmental temperature regulates transcription of a virulence pili operon in E. coli. EMBO J. 1984 Dec 1;3(12):2885–2888. doi: 10.1002/j.1460-2075.1984.tb02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L., Martin K. J., Schleif R. Alternative DNA loops regulate the arabinose operon in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5444–5448. doi: 10.1073/pnas.85.15.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Keegan L., Gill G., Ptashne M. Separation of DNA binding from the transcription-activating function of a eukaryotic regulatory protein. Science. 1986 Feb 14;231(4739):699–704. doi: 10.1126/science.3080805. [DOI] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Liu-Johnson H. N., Gartenberg M. R., Crothers D. M. The DNA binding domain and bending angle of E. coli CAP protein. Cell. 1986 Dec 26;47(6):995–1005. doi: 10.1016/0092-8674(86)90814-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Spears P. A., Schauer D., Falkow S. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J Bacteriol. 1985 Oct;164(1):321–330. doi: 10.1128/jb.164.1.321-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal E., Boots M., de Graaf F. K. Two novel genes, fanA and fanB, involved in the biogenesis of K99 fimbriae. Nucleic Acids Res. 1987 Aug 11;15(15):5973–5984. doi: 10.1093/nar/15.15.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Gander I., Müller U., Mertz R., Winnacker E. L. A sensitive and rapid gel retention assay for nuclear factor I and other DNA-binding proteins in crude nuclear extracts. Nucleic Acids Res. 1986 Feb 11;14(3):1303–1317. doi: 10.1093/nar/14.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Båga M., Göransson M., Lindberg F. P., Lund B., Norgren M., Normark S. Genes determining adhesin formation in uropathogenic Escherichia coli. Curr Top Microbiol Immunol. 1985;118:163–178. doi: 10.1007/978-3-642-70586-1_9. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Ree J. M., Schwillens P., van den Bosch J. F. Monoclonal antibodies raised against Pap fimbriae recognize minor component(s) involved in receptor binding. Microb Pathog. 1987 Feb;2(2):113–121. doi: 10.1016/0882-4010(87)90103-3. [DOI] [PubMed] [Google Scholar]