Introduction
Paget's disease of the vulva is a rare vulvar neoplasm most commonly seen in postmenopausal women. Clinically, it presents as a pink eczematoid lesion with white islands of hyperkeratosis accompanied by pruritus. Pathologically it resembles mammary Paget's disease of the nipple and areola, first described by Sir James Paget in 1874 (Paget). The mean age at diagnosis of Paget's disease of the vulva has been reported to be between 50 and 80 years, and it is most common in Caucasian women (Preti et al., 2000). Disease is often limited to the epidermis and mucosa, without invasion. The optimal management of Paget's disease of the vulva remains unclear. Surgical excision is usually the primary therapy; however, 30% to 60% of patients develop recurrent disease. Furthermore, the lesions often extend past clinically apparent borders and surgical excision is limited by the anatomy of the vulva. In addition, the disease is often multifocal. Immunohistochemistry (IHC) can help distinguish Paget's disease from other vulvar conditions. Cytokeratin 7 (CK7), cytokeratin 20 (CK20), carcinoembryonic antigen (CEA), and gross cystic disease fluid protein-15 (GCDFP-15) are common markers for Paget's disease.
Approximately 10% of patients with Paget's disease of the vulva have an underlying second malignancy. These include colorectal cancer, cervical cancer, breast cancer, as well as carcinoma of the transitional epithelium from the renal pelvis to urethra. Routine screening with colonoscopy, Pap test, and mammogram is therefore recommended. In addition, in rare instances, Paget's disease may become invasive, infiltrating the dermis and even metastasizing to lymph nodes and distant sites (Reddy et al., 2012). In this report, we describe the case of a woman with noninvasive Paget's disease of the vulva who developed invasive Paget's disease of the bladder 18 years after her original diagnosis of Paget's disease of the vulva.
Case report
The patient is a 60 year-old woman who was diagnosed with noninvasive Paget's disease of the vulva at age 41. She initially underwent a wide local excision of the vulva in 1994 with positive margins. She underwent re-excision with negative margins and was observed. However, between 1995 and 2013, she developed several recurrences and underwent multiple interventions including repeat surgical excision (n = 8), skinning vulvectomy (n = 3), laser ablation (n = 1), as well as topical treatment with imiquimod. Pathology from all biopsies and excisions showed Paget's disease with no evidence of invasion. During this period, the patient underwent regular screening mammography, colonoscopy, and Pap tests with negative findings.
In 2010, the patient presented with abdominal pain and postmenopausal bleeding. Cervical and endometrial biopsies were obtained and were consistent with Paget's disease. She underwent a laparoscopic hysterectomy and a bilateral salpingo-oophorectomy. Final pathology showed Paget's disease involving the ectocervix with no evidence of invasion, and no residual disease in the endometrium (Fig. 1). During routine follow-up, she was noted to have a left lung nodule. Biopsy showed moderately differentiated adenocarcinoma, with IHC studies confirming a lung primary. She was treated with stereotactic radiotherapy and has been without evidence of lung disease for three years.
Fig. 1.
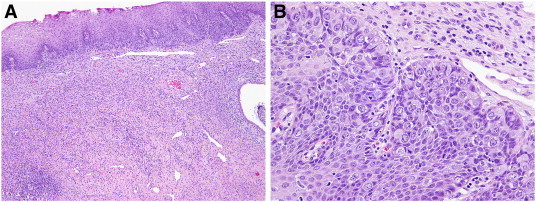
A) Cervix (H&E, 4 ×) demonstrating a concentration of Paget cells in the basal layer; B) higher power image (H&E, 20 ×) shows the large Paget cells with abundant, eosinophilic cytoplasm with a slightly eccentric nucleus.
In 2012 she presented with persistent dysuria and urinary frequency. She underwent cystoscopy and multiple bladder nodules were noted. A transurethral resection was performed and poorly differentiated adenocarcinoma consistent with Paget's disease was diagnosed (Fig. 2). IHC staining was positive for CK7, GCDFP-15, and estrogen receptor, and negative for CK20 and progesterone receptor. The patient was treated with two cycles of docetaxel and carboplatin (Hegarty et al., 2011). She has been followed with regular exams and cystoscopy, with no evidence of disease in the bladder with 10 months of follow-up. However, the patient continues to have persistent Paget's disease of the vulva with her most recent surgical excision performed in March 2013.
Fig. 2.
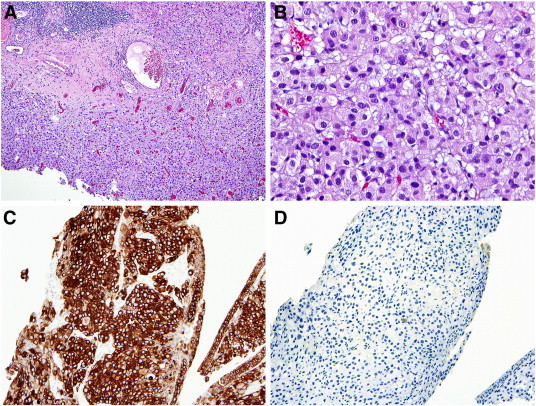
A) Bladder biopsy (H&E, 4 ×) with increased cellularity involving the full tissue thickness; B) higher power image (H&E, 20 ×) shows the cellular proliferation to be composed of eosinophilic cells with eccentric nuclei; C) the cells are diffusely, and strongly cytokeratin 7 positive; and D) negative cytokeratin 20 stain.
Discussion
Paget's disease of the vulva is generally regarded as an indolent, slow growing tumor. Occasionally, Paget cells extend from the epidermis to the dermis and can produce metastases (Reddy et al., 2012; Morikawa et al., 2010; Kay and Southwood, 1964; Kuroda et al., 1987; Koga et al., 1997; Inoue et al., 2012). The most common site of metastases is the regional lymph nodes. Other sites of metastasis include the bone, lung, liver, and adrenal gland. Metastases to the urinary bladder from Paget's disease are extremely rare. Metastatic disease to the urinary bladder from other primary cancers is also uncommon, accounting for approximately 2% of surgically resected bladder tumors. Most metastatic disease to the bladder represents direct invasion by tumors originating from an adjacent site, most commonly the colon. A smaller proportion represent hematologic metastases, most commonly from the stomach.
There are few previously reported cases of Paget's disease of the vulva involving the bladder. In 1964, Kay and Southwood (1964) described the case of a 43 year-old woman with Paget's disease of the vulva who developed a pelvic mass and multiple bladder tumors four years after her original diagnosis of Paget's disease of the vulva. Biopsies of the pelvic mass and bladder nodules showed Paget's disease of the vulva. She underwent exploratory laparotomy and the tumor was deemed inoperable. She died of the disease six months later. Autopsy findings showed the liver, bone, and lymph node metastases consistent with Paget's disease.
In 1987, Kuroda et al. (1987) reported the case of an 83 year-old woman who was diagnosed with Paget's disease of the vulva and treated with radiation therapy. Twelve years after her original diagnosis, she presented with left flank pain and was found to have bilateral hydronephrosis and large nodular tumors filling the bladder. Transurethral biopsies of the bladder revealed Paget's cells.
A subsequent report by Koga et al. (1997) described the case of a 70 year-old female with Paget's disease of the vulva and invasive bladder cancer. She underwent a pelvic exenteration with vulvectomy. Histopathological examination revealed transitional cell carcinoma of the bladder extending thru the urethra to the clitoral skin where it formed a Pagetoid skin lesion on the vulva. Carcinoembryonic antigen (CEA) and cytokeratin (CK) staining demonstrated an identical expression pattern in the bladder cancer cells and the Paget's cells of the vulva, suggesting a similar etiology. In 2012, Inoue et al. (2012) reported the case of a 72 year-old woman diagnosed with Paget's disease of the vulva involving the urethra and bladder. She ultimately underwent vulvar excision and cystourethrectomy showing Paget cell infiltration from the external urethral meatus to the bladder mucosa.
As in the present case, the patients in the above reports were diagnosed with bladder disease several years after the initial diagnosis of Paget's disease of the vulva. In addition, they had noninvasive disease in the vulva but many had invasive disease in the bladder. It remains unclear in all cases if metastatic disease is from the genital tract or vulva to the bladder, or from the bladder to the vulva, or synchronous primary tumors. Unlike the reported cases, our case had involvement of the vagina, cervix and endometrium. In addition, some reported cases showed transitional cell carcinoma of the bladder while our case showed adenocarcinoma.
Conclusion
Paget's disease of the vulva may have synchronous or metastatic disease in the bladder. However, these cases are rare and there is insufficient evidence to recommend routine cystoscopy in this population. However, cystoscopy should be performed in patients with urinary symptoms as bladder involvement can mimic the symptoms of a bladder infection. In our case and others, surgical resection appears to be the primary treatment for Paget's disease involving the bladder. Making the correct diagnosis can be difficult and previous reports suggest that IHC staining may be beneficial in differentiating Paget's disease from other primary and metastatic bladder tumors.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Informed consent
Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent forms is available for review by the Editor-in-Chief of this journal upon request.
References
- Hegarty P.K. Penoscrotal extramammary Paget's disease: the University of Texas M. D. Anderson Cancer Center contemporary experience. J. Urol. 2011;186(1):97–102. doi: 10.1016/j.juro.2011.02.2685. [DOI] [PubMed] [Google Scholar]
- Inoue S., Shiina H., Igawa M. Paget's disease of the vulva with bladder invasion: a case report. Arch. Gynecol. Obstet. 2012;285(5):1493–1496. doi: 10.1007/s00404-011-2145-x. [DOI] [PubMed] [Google Scholar]
- Kay J.M., Southwood W.F. Paget's disease of the vulva associated with an unusual bladder tumour. Br. J. Cancer. 1964;18:233–237. doi: 10.1038/bjc.1964.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga F., Gotoh S., Suzuki S. A case of invasive bladder cancer with Pagetoid skin lesion of the vulva and anogenital Paget's disease. Nihon Hinyokika Gakkai Zasshi. 1997;88(4):503–506. doi: 10.5980/jpnjurol1989.88.503. [DOI] [PubMed] [Google Scholar]
- Kuroda J., Takemura T., Kanokogi M. A case of Paget's disease of the vulva with bladder metastasis. Hinyokika Kiyo. 1987;33(5):774–778. [PubMed] [Google Scholar]
- Morikawa T. Urinary bladder metastasis from extramammary Paget's disease in a patient with a past history of colon and gastric cancers. Pathol. Int. 2010;60(2):145–146. doi: 10.1111/j.1440-1827.2009.02493.x. [DOI] [PubMed] [Google Scholar]
- Paget J.S. disease of the mammary areola preceding cancer of the mammary gland. 1874. pp. 87–89. [Google Scholar]
- Preti M. Vulvar Paget's disease. Clinico-pathologic review of the literature. Minerva Ginecol. 2000;52(5):203–211. [PubMed] [Google Scholar]
- Reddy I.S. Primary extramammary Paget's disease with extensive skeletal metastases. Indian J. Dermatol. Venereol. 2012;78(1):89–92. doi: 10.4103/0378-6323.90954. [DOI] [PubMed] [Google Scholar]


