Abstract
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of liver disease in the Western world and is closely associated with metabolic syndrome, which includes hypertension, central obesity, dyslipidemia and insulin resistance. NAFLD includes a wide spectrum of liver alterations, ranging from simple hepatic steatosis to variable degrees of fibrosis, cirrhosis and even hepatocellular carcinoma. Although the etiology and progression of the disorder remain poorly understood, insulin resistance is considered to play a pivotal role in the pathogenesis. Insulin sensitizers such as biguanides, thiazolidinediones (TZDs), glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase 4 inhibitors have been studied as therapeutic approaches for NAFLD in recent years. Metformin improves insulin sensitivity and serum alanine transaminase and aspartate transaminase (ALT/AST) levels in the majority of subjects; however, it has no significant effect on liver histology. TZDs improve insulin sensitivity, serum ALT/AST levels and histology in some cases, but there are some concerns about the safety of long-term therapy. Selection of appropriate patients for avoiding side effects and the treatment of underlying disease are the main points. These drugs are the best choice for the treatment of NAFLD in patients with type 2 DM who are also candidates for treatment with an insulin sensitizer. The present review provides an overview of insulin sensitizers in the treatment of NAFLD.
Keywords: Insulin sensitizers, Metformin, Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Thiazolidinediones
Core tip: Non-alcoholic fatty liver disease (NAFLD) is increasing significantly due to the obesity epidemic. Insulin resistance, mainly caused by obesity, plays a primary role in NAFLD pathogenesis. Medications that improve insulin sensitivity are theorized to be useful in the treatment of NAFLD. Therefore, recent studies have explored the role of insulin sensitizers to improve biochemical and histological features of NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) was first described by Ludwig et al[1] as a liver disease that mimicked alcoholic hepatitis in histopathological features, but without a history of excessive drinking. A recent definition of NAFLD consists of evidence of hepatic steatosis either by imaging or by histology and no secondary hepatic fat accumulation from causes such as alcohol consumption, hereditary disorders and steatogenic medication[2]. The spectrum of the disease reaches from simple hepatic steatosis to lobular inflammation (non-alcoholic steatohepatitis or NASH), fibrosis, cirrhosis and hepatocellular carcinoma[3,4]. It is the most common cause of chronic liver disease in all industrialized regions of the world[5-7].
BACKGROUND
The estimated prevalence of NAFLD varies in a wide range depending on the population studied and methods used for diagnosis. The disease has been reported in up to 10%-15% of normal weight individuals and 90% of obese persons[8-10]. The prevalence of NAFLD in patients with type 2 diabetes mellitus and hyperlipidemia is approximately 70% and 50%, respectively[11]. Ageing, male gender and ethnicity, such as being Hispanic, are associated factors that increase the prevalence of NAFLD. Elevated liver enzymes, histopathology of liver biopsy and imaging techniques such as ultrasound and magnetic resonance spectroscopy are different methods used for definition.
Previous studies have shown that 40% of patients with NAFLD may go on to develop NASH. The most common cause of cryptogenic cirrhosis is NASH and it progresses to advanced fibrosis in 32% to 37% of patients[12]. The patients with advanced fibrosis and cirrhosis also have higher risk of hepatocellular carcinoma[13-15]. Patients with NAFLD and NASH have increased cardiovascular mortality as well as liver-related mortality[16-18]. This is due to increased pericardial fat, increased carotid intima thickness and abnormal electrocardiogram changes[19-21]. Ramilli et al[22] showed that the prevalence of carotid plaques was close to 60% in patients with NAFLD, while it was 38% in patients without NAFLD.
Type 2 DM, obesity and the associated insulin resistance have been shown as independent factors for fibrosis progression[23]. Although a lot of risk factors have been defined, the major underlying mechanisms of the disease progression have not been clearly understood. The pathogenesis of NAFLD is closely related to obesity that leads to insulin resistance and significant metabolic alterations in liver occur in the setting of insulin resistance[24] (Figure 1). The prevailing hypothesis for explaining the pathway and involved mechanisms is the so-called two hit model[25,26]. At the first hit, hepatic steatosis develops due to insulin resistance and liver fat accumulation induced by excessive free fatty acid production, increased fatty acid oxidation and decreased hepatic triglyceride export. Following this step, the second hit includes increased oxidative stress which is characterized by excessive reactive oxygen species (ROS) in the liver. Progression from NAFLD to NASH is promoted by ROS through lipid peroxidation, cytochrome P450 activation and pro-inflammatory cytokine production[27]. Considering the complexity and unexpected progression of the disease, environmental and genetic factors are approved as contributors of a third hit.
Figure 1.
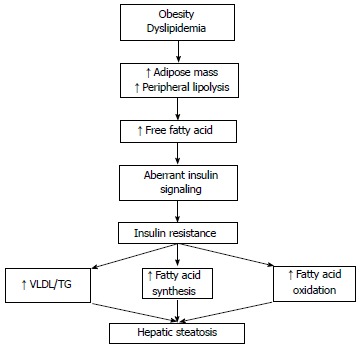
The relationship between obesity, insulin resistance and hepatic steatosis. VLDL: Very low-density lipoprotein; TG:
Insulin resistance is the most specific metabolic risk and pathophysiological feature of NAFLD, with most patients having insulin resistance. In diabetic patients, a correlation between the severity of insulin resistance and grade of hepatic steatosis has also been shown[28]. On the basis of these data, studies of NAFLD treatment are mostly focused on improving insulin resistance and a pharmacological approach targeting improving insulin resistance are the more promising therapeutic candidates among categories that include antioxidants, lipid-lowering agents and anti-obesity drugs.
INSULIN-SENSITIZING MEDICATIONS
Metformin
Metformin was first used in medical practice in the 1950s and has been considered the first-line treatment of type 2 diabetes after receiving approval by the United States Food and Drug Administration (FDA) in 1994. Metformin belongs to a class of insulin-sensitizer drugs and acts through reducing hepatic glucose output, increasing insulin-stimulated glucose uptake in peripheral tissue and stimulating fatty acid oxidation in adipose tissue[29]. Adenosine monophosphate-activated protein kinase is the main player in mediating metformin effects.
Animal studies demonstrated that metformin reverses aminotransferase abnormalities, steatosis and inflammation in mouse models of NAFLD and NASH[30,31]. During last decade, many clinical trials have evaluated the useful effects of metformin on patients with NAFLD and NASH[32-41] (Table 1). Only a few of these studies were randomized and the results are conflicting.
Table 1.
Summary of metformin trials in adult patients with non-alcoholic fatty liver disease/non-alcoholic steatohepatitis
| Ref. | Study type | Subject number | Therapy | Compared with | Duration | NAFLD vs NASH | Liver enzymes | Histology |
| Marchesini et al[32] | Open label, single arm | 20 | Metformin | Baseline | 4 mo | NASH | Improved | Not assessed |
| Nair et al[33] | Open label, Single arm | 15 | Metformin | Baseline | 48 wk | NAFLD | Transiently improved | Mildly improved |
| Uygun et al[34] | Open label, RCT | 36 | Metformin | Diet/Exercise | 6 mo | NASH | Improved | Not improved |
| Bugianesi et al[35] | Open label, RCT | 110 | Metformin | Vitamin E/Diet | 12 mo | NAFLD | Improved | Improved |
| Duseja et al[36] | Open label, RCT | 50 | Metformin | Diet | 6 mo | NAFLD | Improved | Not assessed |
| de Oliveira et al[37] | Open label, Single arm | 20 | Metformin and NAC | Baseline | 12 mo | NASH | Improved | Improved |
| Loomba et al[38] | Open label, Single arm | 28 | Metformin | Baseline | 48 wk | NASH | Improved | Improved |
| Haukeland et al[39] | Open label, RCT | 48 | Metformin | Diet/Exercise | 6 mo | NAFLD | Improved | Not improved |
| Garinis et al[40] | Open label, RCT | 50 | Metformin | Diet | 6 mo | NAFLD | Improved | Not assessed |
| Shargorodsky et al[41] | Open label, RCT | 63 | Metformin | Placebo | 12 mo | NAFLD | Not improved | Not assessed |
NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; RCT: Randomized controlled trials.
The first nonrandomized study was carried out by Marchesini in 2001 and it included 20 biopsy proven NASH patients. For 4 mo the patients were treated with 1.5 g/d metformin and they observed a decrease in aminotransferase levels[32]. The limitation of the study was the lack of histological evaluation and a control group. In 2004, Uygun et al[34] conducted the first randomized control trial comparing dietary modification to dietary modification plus metformin for six months. Aminotransferase levels and insulin sensitivity improved in the metformin treated group but there was no significant differences in necroinflammatory activity or fibrosis between groups. Bugianesi et al[35] presented an open label trial consisting of 110 patients who were randomized to receive either metformin 2 g/d (55 patients), vitamin E 800 IU/d (28 patients) or dietary-induced weight loss (27) patients for 12 mo. Liver transaminase levels were significantly decreased in the metformin group and there was also a histological improvement in hepatic steatosis, inflammation and fibrosis in the subset of 17 patients taking metformin. Haukeland et al[39] demonstrated that treatment with metformin for 6 mo was no better than placebo in terms of improvement in liver histology in patients with NAFLD. Another recent randomized control trial showed that metformin only transiently improved aminotransferase levels in patients with NASH[41]. A meta-analysis including five randomized controlled trials (RCT) concluded that metformin did not improve steatosis, lobular inflammation, hepatocellular ballooning and fibrosis in patients with NASH[42]. These results were independent of drug dose, treatment duration or diabetic state. Therewithal, a recent guideline indicates that metformin has no significant effect on liver histology and therefore it is not recommended as a specific treatment for liver disease in adults with NASH[2]. The largest RCT, “The Treatment of NAFLD in Children” (TONIC), investigated the effects of metformin in a pediatric population. The results of this study demonstrated that metformin was not associated with improvement in histology and reduction in serum alanine transaminase (ALT) levels[43]. On the basis of all these data, the AASLD guideline for the diagnosis and treatment of NAFLD concluded that metformin has no significant effect on liver histology and is not recommended as a specific treatment of NASH. A position statement on NAFLD/NASH based on an EASL special conference has not recommended metformin for specific liver-directed therapy of NASH[44] .
Some studies demonstrated that high insulin and IGF levels have important roles in hepatic fibrosis and hepatocellular carcinoma[45,46]. An inverse association between cancer risk and long term metformin therapy has been found in previous studies. A recent meta-analysis showed that metformin was associated with an estimated 62% reduction in the risk of liver cancer among patients with type 2 diabetes[47]. Chen et al[48] demonstrated that each incremental year increase in metformin use results in 7% reduction in the risk of hepatocellular cancer. While the main anti-tumor effect of metformin is not clear in reducing lipogenesis and lipogenic expression, inhibition of hepatocyte proliferation and induction cell cycle arrest at G0/G1 phase via adenosine monophosphate (AMP)-activated protein kinase, reduction endogenous reactive oxygen species are possible estimated mechanisms.
In summary, metformin improves insulin sensitivity and serum ALT and aspartate transaminase levels in the majority of subjects; however, it has no significant effect on liver histology. The precise dose and duration of treatment is unknown and the beneficial effects on serum ALT only continued during treatment. Metformin has no apparent increase in the risk of lactic acidosis[49] and unlike the thiazolidinediones, it is not encumbered by weight gain or potential hepatotoxicity. According to current data, it cannot be suggested for the specific treatment of NAFLD or NASH but can be given in patients with both NAFLD/NASH and type 2 DM.
Thiazolidinediones
Thiazolidinediones (TZDs) are a class of oral anti-diabetic drugs that induce a nuclear transcription factor, peroxisome proliferator activated receptor-γ (PPAR-γ), by binding selective ligands[50]. PPAR-γ is predominantly expressed in adipose tissue and leads to decreased hepatic fat content and improves glycemic control with insulin sensitivity. TZDs also increase plasma adiponectin levels, activate AMP-activated protein kinase and induce fatty acid stimulation[51].
A lot of human and animal studies have investigated the effect of TZDs on liver enzymes and histology to date. In rat models, pioglitazone and rosiglitazone prevented activation of hepatic stellate cells in vitro and improved hepatic steatosis and fibrosis in vivo[52].
The first human study was conducted by Caldwell in 2001[53]. Troglitazone was studied in 10 patients who had biopsy-proven NASH and the results were associated with improved aminotransferase levels. There was no change in histology and the drug was withdrawn from clinical use due to severe idiosyncratic hepatotoxicity. Neuschwander-Tetri et al[54] showed that rosiglitazone (4 mg twice daily for 48 wk) significantly decreased liver enzymes and improved steatosis, ballooning and inflammation scores in 30 patients who had biopsy-proven NASH. In addition to no change in fibrosis, the liver enzymes levels reverted to pretreatment values 6 mo after withdrawal of the drug. Clinical trials evaluating the effect of thiazolidinediones on patients with NAFLD and NASH are summarized in Table 2[53-61]. Omer et al[60] conducted an open label RCT including biopsy-proven NAFLD individuals to compare rosiglitazone with metformin. After 12 mo treatment, study results reported that rosiglitazone was more effective in metabolic control and histological improvement but fibrosis did not change significantly.
Table 2.
Summary of thiazolidinedione trials in adult patients with non-alcoholic fatty liver disease/non-alcoholic
| Ref. | Study type | Subject number | Therapy | Compared with | Duration | NAFLD vs NASH | Liver enzymes | Histology |
| Caldwell et al[53] | Open label, single arm | 10 | Troglitazone | Baseline | < 6 mo | NASH | Improved | Mildly improved |
| Neuschwander-Tetri et al[54] | Open label, single arm | 30 | Rosiglitazone | Baseline | 48 wk | NASH | Improved | Improved |
| Promrat et al[55] | Open label, single arm | 18 | Pioglitazone | Baseline | 48 wk | NASH | Improved | Improved |
| Sanyal et al[56] | Open label, RCT | 20 | Pioglitazone + Vitamin E | Vitamin E | 6 mo | NASH | Improved | Improved |
| Belfort et al[57] | Blinded, RCT | 55 | Pioglitazone | Placebo | 6 mo | NASH | Improved | Improved |
| Idilman et al[58] | Open label, RCT | 74 | Rosiglitazone | Metformin/life style modification | 48 wk | NASH | Improved | Improved |
| Ratziu et al[59] | Blinded, RCT | 63 | Rosiglitazone | Placebo | 12 mo | NASH | Improved | Improved |
| Omer et al[60] | Open label, RCT | 64 | Rosiglitazone | Metformin | 12 mo | NAFLD | Improved | Improved |
| Sanyal et al[61] | Blinded, RCT | 274 | Pioglitazone | Placebo and vitamin E | 24 mo | NASH | Improved | Improved |
NAFLD: Non-alcoholic fatty liver disease; NASH: Non-alcoholic steatohepatitis; RCT: Randomized controlled trials.
An early study with pioglitazone in 18 patients who had biopsy-proven NASH resulted in a decrease in aminotransferase levels with histological improvement[55]. The first double-blind, placebo-controlled trial using pioglitazone compared with placebo included 55 patients with NASH for 6 mo[57]. Insulin sensitivity, serum ALT levels, steatosis and necroinflammation, except fibrosis, were significantly ameliorated in the pioglitazone group.
In the largest trial completed to date for evaluation of the role of pioglitazone, 247 subjects with biopsy proven NASH were randomized to vitamin E, pioglitazone or placebo for 96 wk[61]. Compared with placebo, both agents, pioglitazone and vitamin E, were associated with reductions in liver steatosis, lobular inflammation, hepatocellular ballooning and improvement in insulin resistance and serum aminotransferase levels. However, there was no improvement in fibrosis scores in the pioglitazone treated group. The “Fatty Liver Improvement with Rosiglitazone Therapy” (FLIRT) trial compared rosiglitazone with placebo in 63 patients[59]. Rosiglitazone improved serum aminotransferase levels, insulin sensitivity and hepatic steatosis. The two year extended trial (FLIRT2) demonstrated that improvement in liver enzyme levels continued but there was no further improvement in liver histology[62].
A meta-analysis including six trials demonstrated reduction in steatosis and hepatocyte ballooning but no improvement in inflammation or fibrosis compared with control[63]. In contrast to this study, Mahady et al[64] found improvement in inflammation and fibrosis in addition to reduction in steatosis and hepatocyte ballooning in a meta-analysis including seven randomized trials.
The largest meta-analysis that included 11 RCTs (862 participants, 38% diabetic) showed that TZDs improve steatosis, hepatocellular ballooning and necroinflammation, delay fibrosis progression and ameliorate hepatic, muscle and adipose tissue insulin resistance with more consistent cardiovascular benefits with pioglitazone[42].
Although the results of studies suggest some benefits from TZDs, a major problem also emerges: safety of long-term therapy and adverse effects. The use of rosiglitazone has been highly restricted in the United States and prohibited in Europe due to the increased risk of coronary events. On the other hand, pioglitazone is associated with adverse events such as bladder cancer, bone loss, weight gain, painful swollen legs and congestive heart failure. After evaluation of the overall results, it would be a good choice to use TZDs for the treatment of NAFLD only in patients with type 2 DM who are also candidates for treatment with a TZD. The AASLD guideline recommended that pioglitazone can be used to treat only patients with biopsy-proven NASH; however, it also raised the concern about its long term safety and efficacy in patients with NASH[2]. The guideline also stressed that most of the clinical studies had been done in non-diabetic patients and thus the effect of TZDs on NASH of diabetic patients was not established. The position statement of a special EASL conference has recommended that pharmacological therapy of NASH could be a 1-2 year course of therapy with glitazone[44].
Dipeptidyl peptidase 4 inhibitors
Dipeptidyl peptidase 4 (DPP4) inhibitors are a new class of drugs and include sitagliptin, vildagliptin and saxagliptin. DPP4 is a membrane associated peptidase with a widespread organ distribution and deactivates a variety of bioactive peptides such as glucagon like peptide-1 (GLP-1). Inactivation of GLP-1 causes glucose intolerance, diabetes mellitus and hepatic steatosis. In a study including 31 NASH patients, Balaban et al[65] reported that serum DPP-4 levels were higher in patients with NASH compared to controls. Furthermore, the serum DPP-4 activity and staining intensity in liver were correlated with histopathological grade of NASH and hepatosteatosis.
In rat models, DPP-4 inhibitors improve hepatic steatosis by increasing insulin sensitivity and decreasing hepatic triglyceride levels[66,67]. To date, there is no published controlled trial with these agents in humans.
GLP-1, a hormone excreted by intestinal L cells, regulates blood glucose by stimulation of glucose-dependent insulin release. GLP-1 has a direct effect on hepatocytes by inducing genes responsible for fatty acid oxidation and insulin sensitivity[68]. GLP-1 analogs (exenatide, liraglutide) have been approved by the FDA for treatment of patients with type 2 diabetes mellitus. Ding et al[69] demonstrated that exenatide improves insulin sensitivity and reduces hepatosteatosis in rats with fatty liver. In another animal study liraglutide treatment reduced hepatic steatosis[70]. A case series including 8 patients with type 2 diabetes and biopsy-proven NAFLD showed that exenatide improves serum liver enzyme levels but has no effect on histopathology[71]. A recent meta-analysis including 4442 patients indicated that liraglutide decreased aminotransferase levels and that this effect was dose-dependent[72]. However, controlled studies are needed to show the efficacy of GLP-1 analogs in NAFLD and NASH treatment.
CONCLUSION
NAFLD is a complex, multifactorial and major public health problem with an increasing prevalence worldwide. Insulin resistance is very common in this disease and the goal of the therapy should include improving insulin sensitivity. Insulin sensitizing agents could be convenient drugs to reach this target. Metformin has been accepted to have no significant effect on liver histology and is not recommended as a specific treatment for liver disease in adults with NAFLD. TZDs have been most extensively evaluated in published trials to date and they have modest effects on liver histology. The long term safety and efficacy of TZDs in patients with NAFLD is lacking. Selection of appropriate patients to avoid side effects and treatment of the underlying disease causing insulin resistance, such as obesity, are crucial main points. NAFLD patients with metabolic syndrome and obesity are likely to be the best candidates to be treated with TZDs. According to current data, unfortunately insulin sensitizers do not satisfy expectations for the treatment of NAFLD. Future RCTs with adequate size and duration are still needed to assess the clinical outcomes in patients with NAFLD.
Footnotes
P- Reviewers: Carvalho-Filho RJ, Ido Y, Masaki T, Zhao D S- Editor: Song XX L- Editor: Roemmele A E- Editor: Wu HL
References
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162–168. doi: 10.1159/000282081. [DOI] [PubMed] [Google Scholar]
- 4.Day CP. Genetic and environmental susceptibility to non-alcoholic fatty liver disease. Dig Dis. 2010;28:255–260. doi: 10.1159/000282098. [DOI] [PubMed] [Google Scholar]
- 5.Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–793. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 6.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 7.Larter CZ, Chitturi S, Heydet D, Farrell GC. A fresh look at NASH pathogenesis. Part 1: the metabolic movers. J Gastroenterol Hepatol. 2010;25:672–690. doi: 10.1111/j.1440-1746.2010.06253.x. [DOI] [PubMed] [Google Scholar]
- 8.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 9.Boza C, Riquelme A, Ibañez L, Duarte I, Norero E, Viviani P, Soza A, Fernandez JI, Raddatz A, Guzman S, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obes Surg. 2005;15:1148–1153. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- 10.Haentjens P, Massaad D, Reynaert H, Peeters E, Van Meerhaeghe A, Vinken S, Poppe K, Velkeniers B. Identifying non-alcoholic fatty liver disease among asymptomatic overweight and obese individuals by clinical and biochemical characteristics. Acta Clin Belg. 2009;64:483–493. doi: 10.1179/acb.2009.084. [DOI] [PubMed] [Google Scholar]
- 11.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 12.Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Managing nonalcoholic fatty liver disease: recommendations for family physicians. Can Fam Physician. 2007;53:857–863. [PMC free article] [PubMed] [Google Scholar]
- 13.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 15.Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9:291–293. [PubMed] [Google Scholar]
- 16.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 17.Hamaguchi M, Kojima T, Takeda N, Nagata C, Takeda J, Sarui H, Kawahito Y, Yoshida N, Suetsugu A, Kato T, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol. 2007;13:1579–1584. doi: 10.3748/wjg.v13.i10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24:1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 19.Bellentani S, Bedogni G, Tiribelli C. Liver and heart: a new link? J Hepatol. 2008;49:300–302. doi: 10.1016/j.jhep.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Yu JH, Lee KS, Lee SY, Hong AR, Park YS. The association of cardiovascular risk factors with nonalcoholic fatty liver disease in health checkup examinees. J Prev Med Public Health. 2008;41:407–412. doi: 10.3961/jpmph.2008.41.6.407. [DOI] [PubMed] [Google Scholar]
- 21.Fracanzani AL, Burdick L, Raselli S, Pedotti P, Grigore L, Santorelli G, Valenti L, Maraschi A, Catapano A, Fargion S. Carotid artery intima-media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121:72–78. doi: 10.1016/j.amjmed.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 22.Ramilli S, Pretolani S, Muscari A, Pacelli B, Arienti V. Carotid lesions in outpatients with nonalcoholic fatty liver disease. World J Gastroenterol. 2009;15:4770–4774. doi: 10.3748/wjg.15.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 24.Choudhury J, Sanyal AJ. Insulin resistance in NASH. Front Biosci. 2005;10:1520–1533. doi: 10.2741/1636. [DOI] [PubMed] [Google Scholar]
- 25.Day CP. Non-alcoholic steatohepatitis (NASH): where are we now and where are we going? Gut. 2002;50:585–588. doi: 10.1136/gut.50.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 27.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 28.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 29.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 30.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 31.Kita Y, Takamura T, Misu H, Ota T, Kurita S, Takeshita Y, Uno M, Matsuzawa-Nagata N, Kato K, Ando H, et al. Metformin prevents and reverses inflammation in a non-diabetic mouse model of nonalcoholic steatohepatitis. PLoS One. 2012;7:e43056. doi: 10.1371/journal.pone.0043056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 33.Nair S, Diehl AM, Wiseman M, Farr GH, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 34.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, Yesilova Z, Gulsen M, Dagalp K. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 35.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 36.Duseja A, Das A, Dhiman RK, Chawla YK, Thumburu KT, Bhadada S, Bhansali A. Metformin is effective in achieving biochemical response in patients with nonalcoholic fatty liver disease (NAFLD) not responding to lifestyle interventions. Ann Hepatol. 2007;6:222–226. [PubMed] [Google Scholar]
- 37.de Oliveira CP, Stefano JT, de Siqueira ER, Silva LS, de Campos Mazo DF, Lima VM, Furuya CK, Mello ES, Souza FG, Rabello F, et al. Combination of N-acetylcysteine and metformin improves histological steatosis and fibrosis in patients with non-alcoholic steatohepatitis. Hepatol Res. 2008;38:159–165. doi: 10.1111/j.1872-034X.2007.00215.x. [DOI] [PubMed] [Google Scholar]
- 38.Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172–182. doi: 10.1111/j.1365-2036.2008.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, Haaland T, Løberg EM, Birkeland K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 40.Garinis GA, Fruci B, Mazza A, De Siena M, Abenavoli S, Gulletta E, Ventura V, Greco M, Abenavoli L, Belfiore A. Metformin versus dietary treatment in nonalcoholic hepatic steatosis: a randomized study. Int J Obes (Lond) 2010;34:1255–1264. doi: 10.1038/ijo.2010.40. [DOI] [PubMed] [Google Scholar]
- 41.Shargorodsky M, Omelchenko E, Matas Z, Boaz M, Gavish D. Relation between augmentation index and adiponectin during one-year metformin treatment for nonalcoholic steatohepatosis: effects beyond glucose lowering? Cardiovasc Diabetol. 2012;11:61. doi: 10.1186/1475-2840-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 43.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Hung CH, Wang JH, Hu TH, Chen CH, Chang KC, Yen YH, Kuo YH, Tsai MC, Lu SN, Lee CM. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265–2271. doi: 10.3748/wjg.v16.i18.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fartoux L, Poujol-Robert A, Guéchot J, Wendum D, Poupon R, Serfaty L. Insulin resistance is a cause of steatosis and fibrosis progression in chronic hepatitis C. Gut. 2005;54:1003–1008. doi: 10.1136/gut.2004.050302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97:2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 48.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 49.Kadayifçi A. Nonalcoholic steatohepatitis: role of leptin in pathogenesis and benefits of metformin in treatment. Am J Gastroenterol. 2003;98:2330; author reply 2330–2331. doi: 10.1111/j.1572-0241.2003.07736.x. [DOI] [PubMed] [Google Scholar]
- 50.Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11:188–195. doi: 10.1111/j.1463-1326.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 52.Duvnjak M, Tomasic V, Gomercic M, Smircic Duvnjak L, Barsic N, Lerotic I. Therapy of nonalcoholic fatty liver disease: current status. J Physiol Pharmacol. 2009;60 Suppl 7:57–66. [PubMed] [Google Scholar]
- 53.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 54.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 55.Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 56.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 57.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 58.Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, Coban S, Erden E, Soykan I, Emral R, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;28:200–208. doi: 10.1111/j.1365-2036.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- 59.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 60.Omer Z, Cetinkalp S, Akyildiz M, Yilmaz F, Batur Y, Yilmaz C, Akarca U. Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:18–23. doi: 10.1097/MEG.0b013e32832e2baf. [DOI] [PubMed] [Google Scholar]
- 61.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 63.Rakoski MO, Singal AG, Rogers MA, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2010;32:1211–1221. doi: 10.1111/j.1365-2036.2010.04467.x. [DOI] [PubMed] [Google Scholar]
- 64.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J Hepatol. 2011;55:1383–1390. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 65.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- 66.Akaslan SB, Degertekin CK, Yilmaz G, Cakir N, Arslan M, Toruner FB. Effects of sitagliptin on nonalcoholic fatty liver disease in diet-induced obese rats. Metab Syndr Relat Disord. 2013;11:243–250. doi: 10.1089/met.2012.0128. [DOI] [PubMed] [Google Scholar]
- 67.Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, et al. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60:1246–1257. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 69.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One. 2011;6:e25269. doi: 10.1371/journal.pone.0025269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kenny PR, Brady DE, Torres DM, Ragozzino L, Chalasani N, Harrison SA. Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: a case series. Am J Gastroenterol. 2010;105:2707–2709. doi: 10.1038/ajg.2010.363. [DOI] [PubMed] [Google Scholar]
- 72.Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234–242. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]


