Abstract
In the present study, virus-like particles (VLPs) were evaluated as a candidate veterinary vaccine against canine influenza virus (CIV) subtype H3N2. Specific pathogen-free (SPF) beagle dogs received a single injection of a VLP vaccine containing hemagglutinin (HA) and M1 protein of CIV H3N2 (H3 HA VLP). The vaccine was tested at 3 different doses with an adjuvant and 1 dose without an adjuvant. To evaluate the immunogenicity and protective efficacy of the H3 HA VLP vaccine, we performed hemagglutination inhibition tests to determine serological immune responses and conducted challenge studies using SPF beagle dogs. The addition of Montanide ISA 25 adjuvant significantly increased the immunogenicity of the H3 HA VLP vaccine. The experimental infection study showed that a single dose of H3 HA VLP vaccine induced protection against wild-type virus challenge in dogs. These results provide support for continued development of the VLP as an animal vaccine against influenza virus.
Keywords: Virus-like particle, Canine influenza, Vaccine, H3N2
1. Introduction
Canine influenza virus (CIV) belongs to the genus Influenza-virus A of the family Orthomyxoviridae. An equine-origin H3N8 influenza virus was first isolated from racing dogs affected with acute respiratory disease in the United States in 2004 [1]. Subsequent outbreaks were reported, and the infection spread rapidly across the United States [2]. Between May 2006 and March 2010, sporadic cases of a different subtype of CIV, namely, H3N2, were identified from sick dogs at animal clinics in China [3,4]. In 2007, CIV H3N2 also caused an outbreak of contagious canine respiratory disease in South Korea [5]. This virus appears to be entirely of avian origin and is, to the best of our knowledge, the first low pathogenic avian influenza (LPAI) virus reported to cause respiratory disease in dogs. CIV H3N2 infection results in clinical outcomes ranging from mild respiratory illness to death. Previous studies found that experimentally infected and contact-exposed beagle dogs shed virus through nasal excretion, seroconverted, and became ill with severe necrotizing tracheobronchitis and bronchioalveolitis with accompanying clinical signs [5,6].
Recently, Lee et al. reported that CIV H3N2 can replicate efficiently in the respiratory system of experimentally infected ferrets and cause acute necrotizing bronchioalveolitis and non-suppurative encephalitis [7]. Furthermore, a novel reassortant CIV H3N1 between pandemic H1N1 and Korean CIV H3N2 has been reported [8]. Therefore, epidemics of CIV H3N2 among dogs in Asian countries have raised concern over the potential for zoonotic transmission of this virus.
Virus-like particles (VLPs), which resemble infectious virus particles in their structure and morphology and display multiple antigenic epitopes, have been proposed as a new generation of non-egg-based, cell culture-derived vaccine candidates against influenza virus [9]. VLP vaccines containing influenza surface proteins are produced easily in insect or mammalian cells via simultaneous expression of hemagglutinin (HA) and neuraminidase along with a viral core protein such as the influenza matrix protein (M1) [10]. The protective mechanism of influenza VLP vaccines is known to be similar to that of the commercial inactivated full virus influenza vaccine; they induce neutralizing antibodies and hemagglutination inhibition (HI) activities and have a solid safety profile [9]. Previous studies have demonstrated strong immunogenicity and high protective efficacy of VLP vaccines in animal influenza challenge models [11–16]. However, there have been no studies of VLP vaccines against influenza virus in dogs.
In the present study, we developed a canine influenza H3 HA VLP vaccine by using a baculovirus expression system and evaluated its immunogenicity and protective efficacy in beagle dogs for the first time.
2. Materials and methods
2.1. Preparation of canine influenza H3 HA VLPs
VLPs containing H3 HA and M1 proteins were produced as described previously [17]. Briefly, to generate the recombinant baculoviruses (rBVs) expressing the influenza proteins, full-length HA cDNA derived from influenza A/canine/Korea/LBM412/2008 (H3N2) virus and M1 cDNA were cloned into the pFastBac vector (Invitrogen, Carlsbad, CA), and then transferred into a bacmid (baculovirus shuttle vector) by transforming the recombinant plasmid DNA into competent DH10Bac Escherichia coli cells (Invitrogen, Carlsbad, CA). Sf9 insect cells were transfected with the recombinant bacmid DNA, and rBVs expressing HA and M1 proteins were plaque purified from culture supernatants of the transfected Sf9 cells. To generate H3 HA VLPs, Sf9 cells (ATCC, CRL-1711) were co-infected with rBVs expressing H3 HA and M1 at multiplicities of infection of 3 and 1, respectively. Culture medium was collected and clarified by low-speed centrifugation (2000 × g, 30 min, 4°C) at 2 days post infection. Culture supernatants were concentrated and filtrated using a QuixStand benchtop system (GE Healthcare, Waukesha, WI) with a hollow fiber cartridge (300,000 Da as the nominal molecular weight cutoff). Further purification was performed by 30% and 60% sucrose gradient ultracentrifugation (28,000 × g, for 60 min) followed by dialysis with HEPES-buffered saline solution, and then, the H3 HA VLP solution was concentrated using a Viva spin (Sartorius, Bohemia, NY) protein concentrator. The final protein concentration of H3 HA VLPs was determined using a protein assay kit (Bio-Rad, Irvine, CA) and the biological activity was determined by a hemagglutination assay as described previously [18]. Units of hemagglutination activity are presented as a factor of dilution that prevents the precipitation of red blood cells. The electron microscopic examination of purified VLPs was carried out. Briefly, VLPs were absorbed onto carbon coated grids and negatively stained with 2% uranyl acetate. The grids were observed by transmission electron microscopy (TEM) at 100,000× magnification.
3. Vaccine and viruses
Vaccines were prepared at 3 different protein concentrations (3.75 μg, 7.5 μg, and 15 μg). Preparations with adjuvant were made by emulsifying VLP solution with Montanide ISA 25 VG (SEPPIC, France) oil adjuvant at a ratio of 75:25 (w/w) or aluminum hydroxide gel adjuvant at a ratio of 80:20 (v/v). To evaluate vaccine efficacy, dogs were challenged intranasally with 107 50% egg infectious dose (EID50) of homologous H3N2 influenza A virus (A/canine/Korea/LBM412/2008).
3.1. Animals and experimental design
To select an effective adjuvant and to determine the immunogenicity of the VLP vaccine, 21 influenza-seronegative adult beagle dogs were divided into 7 groups. Dogs were immunized by intramuscular injection with a single dose of VLP vaccine (3.75 μg, 7.5 μg, or 15 μg) with oil or gel adjuvant or 15 μg of VLP vaccine without an adjuvant. As a non-vaccinated control group, another 3 beagle dogs were injected with PBS. Serum samples were collected prior to vaccination and 2, 3, 4, and 12 weeks after vaccination, and treated with a receptor-destroying enzyme for HI tests. HI tests were performed according to the OIE standard method, by using formalin-inactivated homologous antigen and turkey erythrocytes. The cut-off HI antibody titer for seropositivity was 16.
To determine the protective efficacy of the VLP vaccine, 12 specific pathogen-free beagle dogs were divided into 4 groups. Dogs were immunized with a single dose of VLP vaccine (3.75μg, 7.5 μg, or 15 μg) with ISA 25 VG oil adjuvant. In the non-vaccinated control group, dogs were injected with an emulsified solution of distilled water and ISA 70 prepared at the same ratio as the VLP vaccine. At 4 weeks after vaccination, under Animal Biosafety Level 2 enhanced conditions, dogs were challenged intranasally with the homologous H3N2 virus. We observed the mortality and clinical signs daily for 4 days post inoculation (dpi) and determined viral shedding pattern by using real-time reverse transcriptase polymerase chain reaction (rRT-PCR). At 4 dpi, necropsies were performed on the dogs for pathologic examination. All animal procedures performed in this study were reviewed, approved, and supervised by the Institutional Animal Care and Use Committee of Konkuk University.
4. Virus quantification
To determine viral shedding in the respiratory tract, nasal swab samples were collected at 1, 2, 3, and 4 dpi, and suspended in 1 mL of phosphate-buffered saline (PBS). At 4 dpi, lung tissues were collected from each dog and homogenized in PBS (10% w/v). The suspensions were centrifuged and a clear liquid supernatant was collected. A 0.2-mL aliquot of each sample was used for RNA extraction with an RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. CIV RNA was quantified by the cycle threshold (Ct) method using matrix gene-based rRT-PCR as described previously [19]. For extrapolation of the Ct values to infectious units, known titers of CIV from egg allantoic fluid, measured as EID50, were serially diluted 10 fold. Viral RNA was extracted from these dilutions and quantified by rRT-PCR as described above. To generate a standard curve, Ct values of the viral dilutions were plotted against viral titers. The resulting standard curve was highly correlated (r2 > 0.99) and was used to convert Ct values to EID50.
5. Lung histopathology
The cranial, middle, and caudal lobe tissues were fixed in 10% neutral buffered formalin, processed, embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined by light microscopy.
6. Statistical analysis
Statistical analysis of differences between the vaccinated groups and the unvaccinated control group was performed. One-way ANOVA with a Dunnett’s post hoc test was used for statistical analysis of body temperature and viral shedding. Statistical significance was designated for differences with p values less than or equal to 0.05.
7. Results
7.1. Production of CIV H3 HA VLPs
VLPs containing the HA protein from influenza A/canine/Korea/LBM412/2008 (H3N2) virus were produced in insect cells by using a baculovirus expression system and purified by ultrafiltration and sucrose gradient ultracentrifugation. Purified CIV H3 HA VLPs were found to have approximately 8000 units of hemagglutination activity at a protein concentration of 1.2 mg/mL. This result indicates that the HA protein maintains a biologically active conformation at the receptor-binding site even when it is incorporated into VLPs. The native conformation of the HA receptor-binding site in influenza VLPs was recently shown to be important for maintaining the immunogenicity of the VLPs and for inducing protective immune responses [20]. In addition, negatively stained preparations of CIV H3 HA VLPs examined by TEM revealed particles of 80–100 nm in diameter (Fig. 1).
Fig. 1.
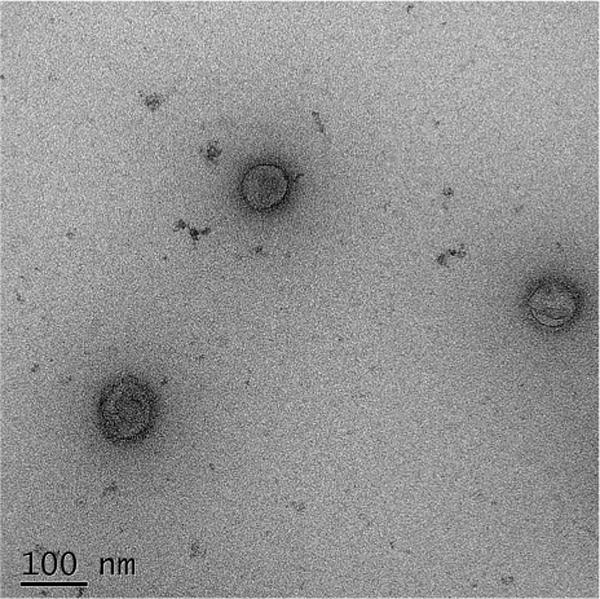
Negative staining transmission electron microscopy examination of purified CIV H3 HA VLP. The bar represents 100 nm.
7.2. Immune responses to vaccination with CIV H3 HA VLPs
To select an effective adjuvant and to determine the immunogenicity of VLP vaccine, we administered single doses of vaccine with or without oil or gel adjuvant to groups of dogs via intramuscular injection and determined serological immune responses 2, 3, 4, and 12 weeks after vaccination. There was no detectable antibody prior to vaccination in all groups. As shown in Fig. 2, as little as 3.75 μg of VLP vaccine (with adjuvant) induced significant levels of virus-specific antibodies. These results suggest that influenza H3 HA VLP vaccines can induce virus-specific functional antibody responses. In the present study, Montanide ISA 25 VG oil adjuvant vaccine produced a higher antibody response than aluminum hydroxide gel adjuvant vaccine. Without adjuvants, low antibody responses were induced even with 15 μg of VLPs. Therefore, the highest immune enhancing effect was achieved by the inclusion of Montanide ISA 25 VG in the H3 HA VLP vaccine.
Fig. 2.
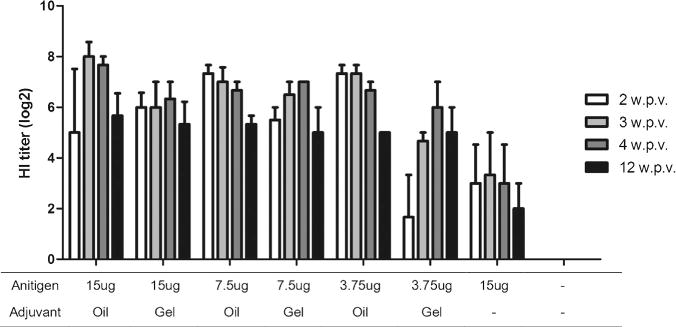
Mean serum hemagglutination inhibition (HI) titers induced in beagle dogs after a single dose of CIV H3 HA VLP vaccine with Montanide ISA 25 VG (oil) or aluminum hydroxide (gel) adjuvant. A total of 21 beagles (3 per group) were intramuscularly immunized with VLP vaccine containing the hemagglutinin of CIV H3N2. HI titers against the homologous antigen were determined 2, 3, 4, and 12 weeks after vaccination.
7.3. Protective efficacy of CIV H3 HA VLPs
In the vaccinated groups, no clinical signs of infection were observed during the observation period following intranasal challenge with CIV H3N2. However, in the non-vaccinated group, all infected dogs exhibited clinical signs of respiratory disease, including nasal discharge, ocular discharge, coughing, anorexia, and depression. Clinical signs were evident as early as 2 dpi (Table 1). Nasal discharge and coughing were the predominant clinical signs. Mucopurulent ocular discharge was observed in all the infected dogs at 2 dpi. Anorexia and depression began at 2 dpi and persisted until 4 dpi. At 1 and 3 dpi, infected dogs developed a transient clinical fever (≥ 39.5°C) (Fig. 3). Mean body temperature of the vaccinated dogs was within the normal range.
Table 1.
Clinical observations of dogs vaccinated with virus-like particle (VLP) vaccine and infected with canine influenza virus (CIV).
| Group | No. of animals | Clinical signs
|
|||
|---|---|---|---|---|---|
| 1 day post challenge | 2 day post challenge | 3 day post challenge | 4 day post challenge | ||
| Non-vaccinated | 1 | – | OD, AN, DE | AN, DE, CO | AN, DE, CO |
| 2 | – | OD, AN, DE | AN, DE, ND | AN, DE, ND | |
| 3 | – | OD, AN, DE | AN, DE | AN, DE, ND | |
| 3.75 μg vaccinated | 4 | – | – | – | – |
| 5 | – | – | – | – | |
| 6 | – | – | – | – | |
| 7.5 μg vaccinated | 7 | – | – | – | – |
| 8 | – | – | – | – | |
| 9 | – | – | – | – | |
| 15 μg vaccinated | 10 | – | – | – | – |
| 11 | – | – | – | – | |
| 12 | – | – | – | – | |
OD, ocular discharge; AN, anorexia; DE, depression; CO, cough; ND, nasal discharge.
Fig. 3.
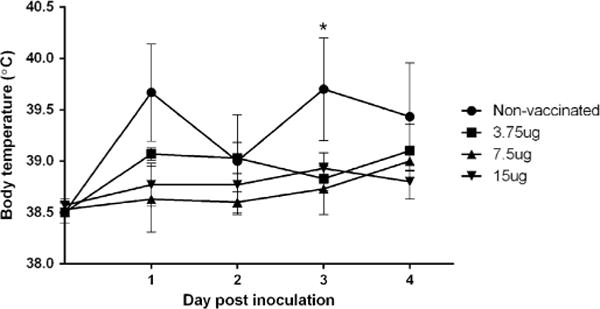
Body temperature of vaccinated and non-vaccinated dogs recorded for 4 days following intranasal challenge with CIV H3N2. Data are expressed as the mean ± SE. *p<0.05 by ANOVA with Dunnett’s post hoc test compared with vaccinated groups.
The non-vaccinated group showed the highest virus titers in the nasal swabs and lung tissues (Fig. 4 and Table 2). Notably, at 4 dpi, all vaccinated groups showed significantly reduced viral excretion compared with the non-vaccinated group.
Fig. 4.
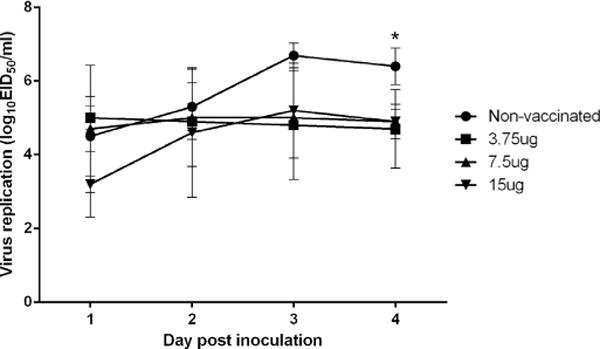
Quantification of virus in the nasal swab samples. To determine viral shedding in the respiratory tract, nasal swab samples were collected at 1, 2, 3, and 4 days post inoculation and suspended in 1 mL of phosphate-buffered saline. CIV RNA was quantified by matrix gene-based real-time reverse transcriptase polymerase chain reaction. The log10EID50 equivalents were determined with the use of rRT-PCR. *p < 0.05 by ANOVA with Dunnett’s post hoc test compared with vaccinated groups.
Table 2.
Histopathological lesions and virus titers in the lungs of dogs vaccinated with VLP vaccine and infected with CIV.
| Group | Animal identification no. | Cranial lobe
|
Middle lobe
|
Caudal lobe
|
|||
|---|---|---|---|---|---|---|---|
| Histopathology scorea | Virus titerb | Histopathology score | Virus titer | Histopathology score | Virus titer | ||
| Non-vaccinated | 1 | 3 | 5.3 | 3 | 5.4 | 3 | 6.6 |
| 2 | 3 | 5.7 | 3 | 4.5 | 3 | 5.0 | |
| 3 | 3 | 3.5 | 3 | 3.1 | 3 | 5.6 | |
| 3.75 μg vaccinated | 4 | 0 | 3.4 | 0 | 4.9 | 2 | 3.7 |
| 5 | 0 | 0.0 | 1 | 0.0 | 1 | 0.0 | |
| 6 | 0 | 2.8 | 2 | 2.2 | 0 | 3.5 | |
| 7.5 μg vaccinated | 7 | 0 | 2.6 | 0 | 1.9 | 0 | 1.9 |
| 8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 9 | 0 | 2.1 | 0 | 3.1 | 0 | 2.4 | |
| 15 μg vaccinated | 10 | 0 | 1.6 | 0 | 0.0 | 0 | 0.0 |
| 11 | 0 | 2.4 | 0 | 0.0 | 0 | 5.8 | |
| 12 | 0 | 3.3 | 0 | 2.9 | 0 | 2.5 | |
Dogs were intranasally with 1 mL of virus suspension with a titer of 107.0 EID50/mL
Histopathological lesion scores were determined as follows: 0, no lesion; 1, mild lesion; 2, moderate lesion; 3, severe lesion.
Virus titers expressed as log10EID50/mL.
Gross necropsy and histopathologic examination revealed diffuse dark red consolidated areas in the lung lobes of non-vaccinated dogs (Fig. 5 and Table 2). They had severe bronchopneumonia and bronchoalveolitis characterized by severe epithelial sloughing and necrotic cellular debris in the bronchi, moderate epithelial necrosis and degeneration with cellular debris in the bronchioles, and infiltration of mononuclear cells and neutrophils in the alveoli. In the group vaccinated with 3.75 μg of VLPs, multifocal-to-diffuse hemorrhagic lesions were observed in the lungs. These dogs had mild-to-moderate bronchopneumonia and alveolitis. Although petechial hemorrhagic lesions were observed in the lungs of dogs vaccinated with 7.5 μg of VLPs, dogs vaccinated with 7.5 or 15 μg of VLPs did not exhibit significant morphologic changes in the lungs.
Fig. 5.
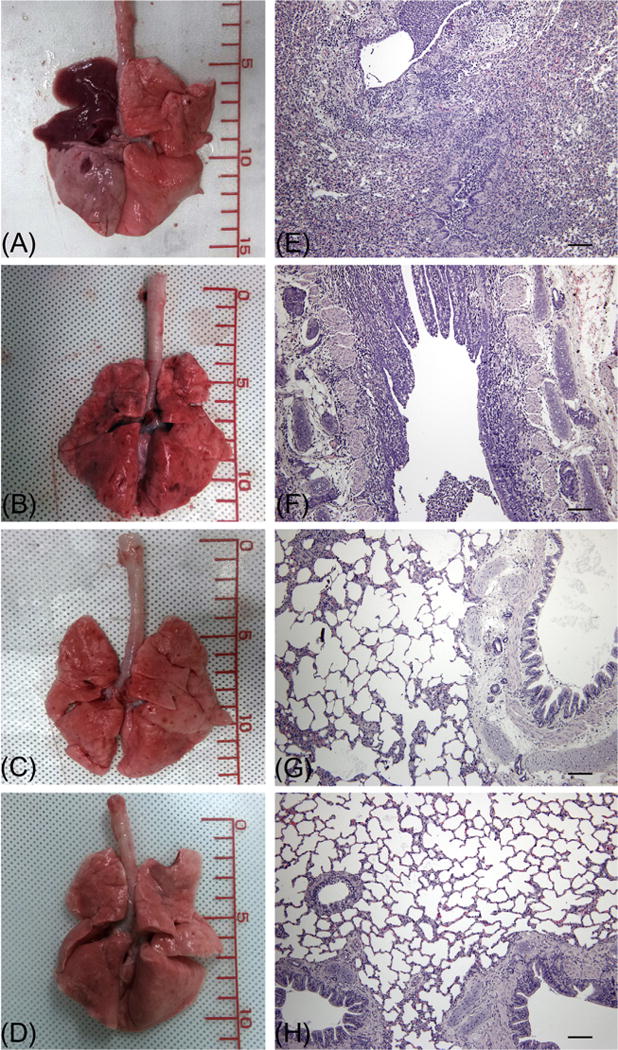
Gross and histopathologic changes in the lungs infected with CIV. Gross lesion and hematoxylin-and-eosin-stained lung sections (scale bar= 100 μm) from dogs at 4 days post infection are shown. (A) and (E), unvaccinated dog. (B) and (F), dog vaccinated with 3.75 μg of CIV H3 HA VLP. (C) and (G), dog vaccinated with 7.5 μg of CIV H3 HA VLP. (D) and (H), dog vaccinated with 15 μg of CIV H3 HA VLP.
8. Discussion
There are advantages to a VLP vaccine approach. VLPs mimic virus particles, presenting multiple antigenic epitopes that stimulate a diverse set of immune responses. In addition, the use of VLP vaccines could help address the safety concerns associated with live-attenuated and inactivated whole-virus vaccines [9,21]. Antibodies directed against the influenza HA protein are known to largely mediate virus neutralization and confer protection against infection. In the present study, we describe the development of an H3 influenza VLP vaccine comprised of only two influenza virus structural proteins, HA and M1, which were derived from CIV H3N2. CIV H3 HA VLP vaccine elicited high levels of antibody to the virus, as shown by HI activity, and provided protection against wild-type virus infection. To the best of our knowledge, this study is the first to generate and evaluate an influenza VLP vaccine for dogs. Our results should provide further support to the possibility of developing VLP vaccines that can reliably induce immunity in animal species.
In the present study, we have purified VLP antigen by filtration with a hollow fiber cartridge and sucrose gradient ultracentrifugation. Purified H3 HA VLPs were found to have approximately 8000 units of hemagglutination activity at a protein concentration of 1.2 mg/mL. In previous studies, the content of HA was approximately 10% of total proteins of influenza VLPs [20,22]. In this study, the purified VLP preparations were quantified in terms of the amount of total protein, not HA antigen. Therefore, the antigen used in this study may contain HA antigen as well as high titers of insect cell and baculovirus vector derived proteins. Since baculovirus is hard to separate from influenza VLPs due to the similar density and enveloped nature they share, it is always present in purified VLP preparations [23,24]. Previous study showed that the enhanced immunogenicity of the baculovirus-derived VLP is caused by contamination with residual baculovirus which activates the innate immune response [25]. In this study, there is possibility that contaminating baculovirus in H3 HA VLP preparations may exhibit significant adjuvant activity in this study.
Montanide ISA 25 VG and aluminum hydroxide have already been demonstrated as safe and effective adjuvants in a canine model [26]. In this study, we found that a lower dose of VLP vaccine (3.75 μg) with an ISA 25 or aluminum hydroxide adjuvant resulted in significantly higher antibody titers than a high dose of VLPs (15 μg) without an adjuvant. These results, combined with the HI test results, suggest that the addition of adjuvant significantly enhances the protective immune response induced by the VLP vaccine, even at low vaccine doses. In addition, the Montanide ISA 25 VG adjuvant vaccine produced a slightly higher antibody response than the aluminum hydroxide adjuvant vaccine. Therefore, we selected Montanide ISA 25 VG as the adjuvant for CIV H3 HA VLP vaccine in the challenge study.
In the present study, a single administration of CIV H3 HA VLP vaccine elicited high level of functional antibodies and protected dogs against a wild-type virus challenge. This vaccine provided protection as demonstrated by preventing clinical signs and reducing pulmonary pathology and viral shedding. Although viral shedding was not eliminated completely in the vaccinated group, virus shedding was significantly reduced compared to the control group. In a previous study conducted by Lee et al. using CIV H3N2 strain by priming and boosting demonstrated reduced clinical signs and pulmonary pathology but not able to completely eliminate viral shedding like our study [27]. Consistent with our findings, virus shedding in nasal discharge began at 1 dpi and continued to 4 dpi in vaccinated beagle dogs. Therefore, improved vaccination strategies against CIV H3N2 are required to provide sterile immunity and eliminate viral shedding completely.
In conclusion, the CIV H3 HA VLP vaccine developed in this study was safe, immunogenic, and protected beagle dogs from wild-type CIV infection, strongly reducing clinical symptoms and pathologic lesions even with a single administration. The results of this study demonstrate that VLP vaccination is a promising strategy for the control of influenza A virus in animals.
Acknowledgments
This work was supported by Konkuk University.
References
- 1.Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EP, Chen L, et al. Transmission of equine influenza virus to dogs. Science. 2005;310:482–5. doi: 10.1126/science.1117950. [DOI] [PubMed] [Google Scholar]
- 2.Payungporn S, Crawford PC, Kouo TS, Chen LM, Pompey J, Castleman WL, et al. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerg Infect Dis. 2008;14:902–8. doi: 10.3201/eid1406.071270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Shi Z, Jiao P, Zhang G, Zhong Z, Tian W, et al. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect Genet Evol: J Mol Epidemiol Evol Genet Infect Dis. 2010;10:1286–8. doi: 10.1016/j.meegid.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng Q, Zhang X, Xu D, Zhou J, Dai X, Chen Z, et al. Characterization of an H3N2 canine influenza virus isolated from Tibetan mastiffs in China. Vet Microbiol. 2012 doi: 10.1016/j.vetmic.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Song D, Lee C, Kang B, Jung K, Oh T, Kim H, et al. Experimental infection of dogs with avian-origin canine influenza A virus (H3N2) Emerg Infect Dis. 2009;15:56–8. doi: 10.3201/eid1501.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song D, Kang B, Lee C, Jung K, Ha G, Kang D, et al. Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. 2008;14:741–6. doi: 10.3201/eid1405.071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YN, Lee DH, Park JK, Yuk SS, Nahm SS, Lee JB, et al. Experimental infection and natural contact exposure of ferrets with canine influenza virus (H3N2) J Gen Virol. 2012 doi: 10.1099/vir.0.042473-0. [DOI] [PubMed] [Google Scholar]
- 8.Song D, Moon HJ, An DJ, Jeoung HY, Kim H, Yeom MJ, et al. A novel reassortant canine H3N1 influenza virus between pandemic H1N1 and canine H3N2 influenza viruses in Korea. J Gen Virol. 2012;93:551–4. doi: 10.1099/vir.0.037739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus-like particles as pandemic vaccines. CurrTop Microbiol Immunol. 2009;333:269–89. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- 10.Haynes JR. Influenza virus-like particle vaccines. Exp Rev Vaccines. 2009;8:435–45. doi: 10.1586/erv.09.8. [DOI] [PubMed] [Google Scholar]
- 11.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83:5726–34. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pushko P, Kort T, Nathan M, Pearce MB, Smith G, Tumpey TM. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine. 2010;28:4771–6. doi: 10.1016/j.vaccine.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 13.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–75. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao P, Luo M, Zhu D, Qu S, Yang Z, Gao M, et al. Virus-like particle vaccine comprised of the HA, NA, and M1 proteins of an avian isolated H5N1 influenza virus induces protective immunity against homologous and heterologous strains in mice. Viral Immunol. 2009;22:273–81. doi: 10.1089/vim.2009.0017. [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Park JK, Lee YN, Song JM, Kang SM, Lee JB, et al. H9N2 avian influenza virus-like particle vaccine provides protective immunity and a strategy for the differentiation of infected from vaccinated animals. Vaccine. 2011;29:4003–7. doi: 10.1016/j.vaccine.2011.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JK, Lee DH, Youn HN, Kim MS, Lee YN, Yuk SS, et al. Protective efficacy of crude virus-like particle vaccine against HPAI H5N1 in chickens and its application on DIVA strategy. Influenza Other Respir Viruses. 2012 doi: 10.1111/j.1750-2659.2012.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, et al. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS One. 2009;4:e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song JM, Kim YC, Lipatov AS, Pearton M, Davis CT, Yoo DG, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010;17:1381–9. doi: 10.1128/CVI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spackman E, Senne DA, Bulaga LL, Myers TJ, Perdue ML, Garber LP, et al. Development of real-time RT-PCR for the detection of avian influenza virus. Avian Dis. 2003;47:1079–82. doi: 10.1637/0005-2086-47.s3.1079. [DOI] [PubMed] [Google Scholar]
- 20.Quan FS, Kim YC, Vunnava A, Yoo DG, Song JM, Prausnitz MR, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84:7760–9. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Exp Rev Vaccines. 2010;9:1149–76. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 22.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83:4489–97. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song JM, Choi CW, Kwon SO, Compans RW, Kang SM, Kim SI. Proteomic characterization of influenza H5N1 virus-like particles and their protective immunogenicity. J Proteome Res. 2011;10:3450–9. doi: 10.1021/pr200086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krammer F, Grabherr R. Alternative influenza vaccines made by insect cells. Trends Mol Med. 2010;16:313–20. doi: 10.1016/j.molmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Margine I, Martinez-Gil L, Chou YY, Krammer F. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS One. 2012;7:e51559. doi: 10.1371/journal.pone.0051559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang YF, Appel MJ, Jacobson RH, Shin SJ, Harpending P, Straubinger R, et al. Recombinant OspA protects dogs against infection and disease caused by Borrelia burgdorferi. Infect Immun. 1995;63:3543–9. doi: 10.1128/iai.63.9.3543-3549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C, Jung K, Oh J, Oh T, Han S, Hwang J, et al. Protective efficacy and immunogenicity of an inactivated avian-origin H3N2 canine influenza vaccine in dogs challenged with the virulent virus. Vet Microbiol. 2010;143:184–8. doi: 10.1016/j.vetmic.2009.11.037. [DOI] [PubMed] [Google Scholar]


