Abstract
Introduction
Most vaccines are administered by intramuscular injection using a hypodermic needle and syringe. Some limitations of this procedure include reluctance to be immunized because of fear of needlesticks, and concerns associated with the safe disposal of needles after their use. Skin delivery is an alternate route of vaccination that has potential to be painless and could even lead to dose reduction of vaccines. Recently, microneedles have emerged as a novel painless approach for delivery of influenza vaccines via the skin.
Areas covered
In this review, we briefly summarize the approaches and devices used for skin vaccination, and then focus on studies of skin immunization with influenza vaccines using microneedles. We discuss both the functional immune response and the nature of this immune response following vaccination with microneedles.
Expert opinion
The cutaneous administration of influenza vaccines using microneedles offers several advantages: it is painless, elicits stronger immune responses in preclinical studies and could improve responses in high-risk populations. These dry formulations of vaccines provide enhanced stability, a property of high importance in enabling their rapid global distribution in response to possible outbreaks of pandemic influenza and newly emerging infectious diseases.
Keywords: cutaneous, immunization, influenza, review
1. Introduction
The modern history of vaccination began in 1796 when Edward Jenner used the cutaneous administration of a virus preparation from a milkmaid for prevention of smallpox [1]. A modification of this process using a bifurcated needle was subsequently employed for the successful global campaign, resulting in eradication of this disease [2]. More recently, a number of studies have been performed using vaccines administered by the intradermal route and have been recently reviewed [3–5]. Two examples of live recombinant viral vectors, simian adenovirus type 63 and modified vaccinia Ankara (MVA) have also been used for skin immunization of mice with a candidate malaria vaccine, and were found to induce antigen-specific CD8+ T-cell responses [6]. Administration of the BCG tuberculosis vaccine via the skin is another example of skin-based vaccination currently being practised.
While hypodermic injections have formed the mainstay for intradermal vaccine delivery for many decades, recently, multiple new technologies have been developed that allow for elegant ways to deliver vaccines into the skin. Influenza virus continues to devastate human lives each year. It produces severe respiratory illness and has the potential to produce pandemics, as evident from the past pandemics of 1918, 1957 and 1968, and the recent 2009 pandemic. A holistic approach to a successful mass influenza vaccination requires synergistic optimization between vaccine dose, logistics of vaccine transportation, and administration to vaccinees. Recent studies have shown that influenza vaccine delivery to the skin not only leads to dose reduction, but the use of innovative technologies can also allow for painless and self-administratered vaccination. In the following sections, we summarize different skin-delivery technologies and focus discussion on the use of microneedles for influenza vaccination.
2. Immunological competence of skin as an attractive compartment for vaccine delivery
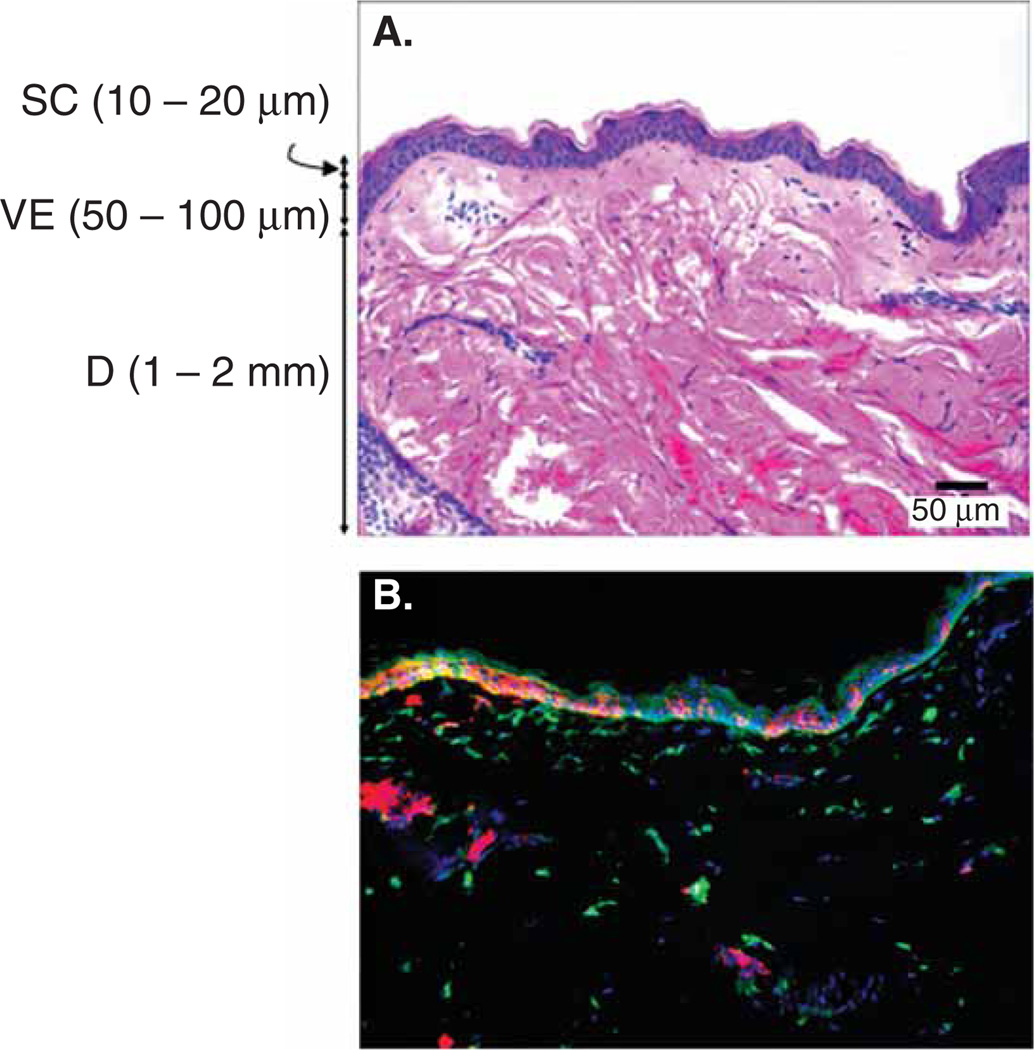
The skin provides a protective barrier around the human body and isolates it from the surrounding environment. The physical barrier is offered predominantly by the upper 10 – 20 µm of the skin layer called the stratum corneum (SC), a layer made of dead cells (Figure 1) [7]. In addition to the physical barrier that prevents pathogen entry, the skin is also endowed with a rich population of immune cells to mount an immune response if its barrier function is compromised due to nicks and cuts [8]. These immune cells include Langerhans cells (LCs) that reside in the viable epidermis and dermal dendritic cells located in the collagen-rich dermal layer of the skin (Figure 1) [8,9]. Both LCs and dermal dendritic cells are potent antigen-presenting cells (APCs), which help to process and present pathogen-derived antigens to mount an adaptive immune response [10,11]. Considering the richness of the skin in APCs, delivery of vaccines to this compartment has the potential to induce potent immune responses.
Figure 1. Skin structure and constituent immune cells.
A. Histological micrograph of hematoxylin and eosin stained macaque skin showing the different layers of the skin. B. Immunohistochemical micrograph of macaque skin stained for Langerhans cells (CD1a, red), dermal dendritic cells (X111a, green), and cell nuclei (blue).
D: Dermis; SC: Stratum corneum; VE: Viable epidermis.
3. Strategies for vaccine delivery to the skin
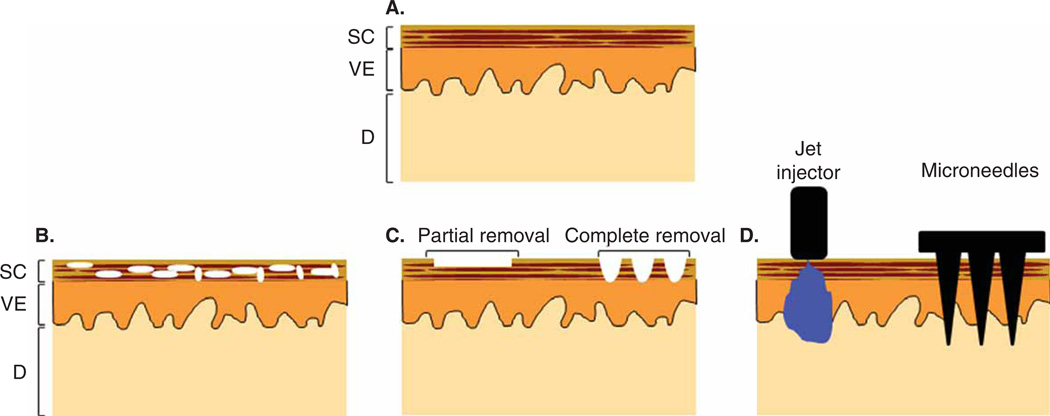
The natural barrier property of the skin’s SC layer, which prevents pathogen entry, also poses a tremendous barrier to delivery of vaccines into the skin. To overcome the barrier posed by SC to vaccine delivery, three basic strategies are employed (Figure 2): i) SC disruption; ii) SC removal; and iii) SC penetration.
Figure 2. Schematic overview of different approaches to increase skin permeability.
A. Intact skin. B. Stratum corneum (SC) disruption (indicated by white space in figure) leads to better transport of vaccine molecules. C. Partial or complete removal of SC leads to reduction or elimination, respectively, of transport barrier. D. Direct penetration across SC bypasses the barrier layer as exemplified with jet injector and microneedles as representative examples.
D: Dermis; SC: Stratum corneum; VE: Viable epidermis.
3.1 SC disruption
The underlying principle of this approach is to transiently modify or disrupt the structure of SC to increase its permeability, allowing successful diffusion of large vaccine molecules into the skin. Multiple techniques have been used to modify or disrupt the SC structure.
3.1.1 Chemical enhancers
Various chemical compounds have been found to disrupt the ordered structure of SC to increase its permeability. Using these compounds, vaccination studies have been performed to demonstrate that antigen-specific antibodies can be generated upon topical application of ovalbumin as a model antigen [12]. However, the low uptake of vaccine antigen necessitates high antigen doses, and delivering viral particles as vaccines using this approach continues to be challenging.
3.1.2 Ultrasound
Low-frequency ultrasound has been successfully used to increase skin permeability. Bubble cavitation in the liquid applied between the skin and the ultrasound probe to obtain acoustic transmission into the skin is now considered to be primarily responsible for this permeabilization effect. Using this approach, tetanus toxoid has been delivered to the skin, and it was found that a 10-fold higher antibody titer was obtained in comparison to a subcutaneous injection of tetanus toxoid [13].
3.1.3 Electroporation
Application of high voltage for short durations has been shown to enhance skin permeability. In one study, DNA expressing poxvirus antigen was delivered to the skin using electroporation, and the resulting immune response was protective against a lethal challenge of vaccinia virus [14]. In another study, electroporation was used to deliver DNA encoding hepatitis B surface antigen to pigs, resulting in antigen-specific serum antibodies [15]. DNA encoding influenza antigen has also been delivered [16]. In this study, intradermal injection was used to inject DNA, while electroporation was used to enhance DNA transfection into cells of the skin.
3.2 SC removal
As SC is the predominant barrier to vaccine delivery to the skin, various techniques have been used to assess whether partial or complete removal of SC can enable delivery of vaccine antigens in to the skin.
3.2.1 Tape stripping
One of the earliest approaches used to remove SC was the sequential application of a tape to skin surface to peel away SC layers. The approach although effective is difficult to standardize. Nonetheless, various studies using this approach of vaccination have shown enhanced delivery of vaccine antigens and increased antibody responses. In one such study, inactivated influenza virus was topically applied to tape-stripped skin to generate protective immunity against lethal virus challenge [17].
3.2.2 Abrasion
In this approach, a rough surface is rubbed across the skin to produce a sandpaper effect and remove SC. A wet patch containing vaccine is then typically applied on the treated skin [9]. In one study, the ability to use a dry patch containing influenza vaccine was tested and was found to be as effective as a wet patch in inducing serum anti-influenza antibodies [18]. The approach, while effective, required co-delivery of adjuvants including heat labile enterotoxin and cholera toxin, both of which can pose safety concerns for humans due to their inherent toxicities.
3.2.3 Thermal ablation
With technological development, novel microdevices have been developed that utilize thermal energy to ablate SC. For example, in one design, electrical resistors are used to heat SC and ablate it. This enables creation of microholes in the SC barrier across which topically applied vaccines can be delivered into the skin. In one such study, influenza vaccine was delivered into the skin with CpG as adjuvant, which provided protection against a lethal dose of pandemic influenza virus [19].
3.2.4 Microdermabrasion
Microdermabrasion is a cosmetic procedure to resurface skin to improve its look by removing scars or acne. Sharp multi-faceted inorganic microcrystals are projected at high velocity cutting away skin layers, and are collected in a waste container. This approach has recently been fine-tuned to allow controlled removal of SC layer [20,21]. The approach has also been used to demonstrate enhanced uptake of MVA virus and generation of antibodies toward it [20].
3.3 SC penetration
In this approach, SC, the barrier layer is penetrated to bypass its barrier properties and to deliver the vaccine molecules directly beneath the SC layer.
3.3.1 Jet injectors
In this approach, a stream of liquid containing the vaccine is discharged into the skin at a high velocity. The momentum of the stream enables the fluid to penetrate across the SC resulting in vaccine delivery into the skin [22]. This approach has been used to deliver millions of vaccine doses especially during mass vaccinations. However, due to risk of cross contamination, its usage for vaccination remains questionable. This is despite multiple innovations in design, which have been made to enhance safety [22]. In addition to various diseases, such as polio, small pox, and tetanus, jet injectors have been successfully used to vaccinate against influenza [22].
3.3.2 Gene gun
In this approach, gold particles a few micrometers in diameter are coated with a DNA expressing the vaccine antigen and bombarded into the skin. The DNA is transiently expressed by keratinocytes and other skin cells, enabling presentation by APCs located in the skin. This strategy has been exploited to deliver vaccines against a host of infectious diseases including influenza [23,24].
3.3.3 Microneedles
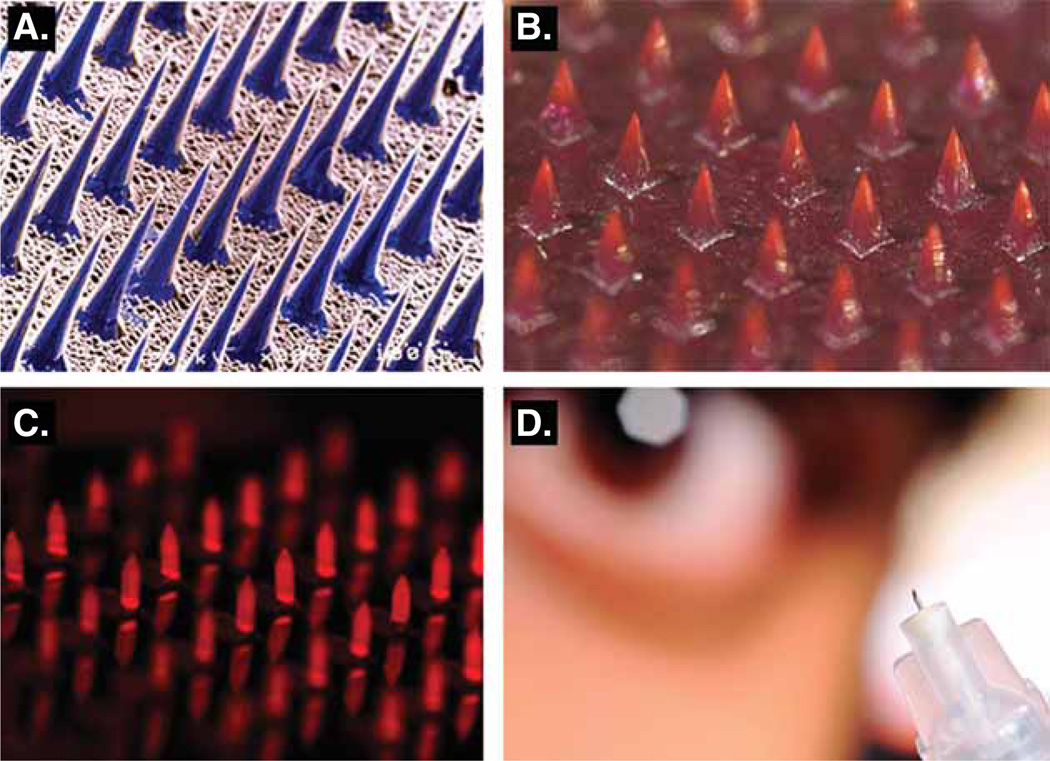
Microneedles are sharp microstructures measuring a few hundred micrometers in length that can painlessly [25] penetrate the SC to deliver vaccines. Both solid and hollow configurations of microneedles have been used for vaccine delivery (Figure 3). In one approach solid microneedles are inserted into the skin to create microholes in the SC, on which a vaccine can be applied [26]. In a second approach vaccine is encapsulated in a polymeric microneedle, which upon insertion in the skin can either dissolve or be separated from the patch leaving the microneedles deposited in the skin. The separated microneedles can then slowly degrade releasing the vaccine [27]. In a third approach the vaccine formulation is coated on solid microneedles, which upon skin insertion can enable dissolution of the coating depositing the vaccine in the skin [28,29]. In another approach, hollow microneedles have been developed that enable injection of vaccine into the skin [27].
Figure 3. Different microneedle configurations.
A. Solid silicon microneedles, each microneedle is 150 µm tall. B. Solid dissolving polymer microneedles encapsulating a model vaccine, each microneedle is 600 µm tall. C. Solid stainless steel microneedles coated with a model vaccine, each microneedle is 700 µm tall. D. Hollow microneedle for injection. Images A, B, D: courtesy of Georgia Institute of Technology. The authors would like to thank the Laboratory of Dr. Mark Prausnitz at the Georgia Institute of Technology for additions to Figure 3.
4. Microneedles for skin immunization against influenza
The delivery of whole inactivated influenza virus (WIV), which is large enveloped particles to the skin by the simple topical application, such as tape abrasion, was ineffective in inducing immune responses because 50 – 100 µg of vaccines were needed to induce detectable levels of antibodies [17]. As an alternative skin delivery method to topical application, intradermal injection of influenza vaccines using needles and syringes or microinjection systems has been widely studied in humans [30,31]. To reduce discomfort associated with needlesticks, microneedles are designed to deliver compounds into the skin. The use of microneedles makes it feasible to deliver high-molecular-weight vaccines through the skin barrier. Such compounds delivered to the skin using microneedles include bovine serum albumin, oligonucleotides or plasmid DNA vaccines, latex particles of viral dimensions, and recombinant bacterial proteins [32–35]. In addition to solid microneedles (Figure 3A–C), hollow microneedle devices (Figure 3D) have also been developed.
Using solid vaccine-coated microneedles, dry coating of WIV onto solid microneedles was evaluated in mice [36]. In this study, 10 µg of inactivated whole influenza H1N1 virus was successfully delivered to the mouse skin, with vaccine delivery efficiency of approximately 90%. Vaccine-specific antibody responses and protection were induced in mice immunized in the skin at a comparable level as the standard intramuscular (IM) vaccination [36]. Also, in a similar study, dry coating of microneedles with inactivated H3N2 influenza virus was used to deliver 3 – 10 µg of WIV vaccine to the skin, which showed comparable immunogenicity to results using standard IM injection [37]. However, requirements for high doses of influenza vaccines were postulated to be due to stability problems associated with dry coating of lipid-enveloped WIV vaccines onto solid microneedles. Therefore, approaches for stabilization of vaccines during coating were investigated as described subsequently.
4.1 Stabilized formulations of microneedle-coated viral vaccines
Influenza vaccines provide a useful system for investigating the stability and optimization of microneedle vaccines by utilizing the easy and reliable assay of hemagglutination activity, which measures the functional and structural integrity of influenza virus hemagglutinin (HA) for receptor binding. Microneedle coating and drying were found to result in a significant loss in the stability of the influenza vaccines, as evidenced by a decrease in influenza vaccine hemagglutination titers, possibly due to the phase change from a liquid to solid formulation [38,39]. New formulations were, therefore, developed to stabilize hemagglutination activity of microneedle influenza vaccines [39,40]. Many carbohydrate compounds were screened, and addition of trehalose (a disaccharide) was found to maintain the hemagglutination activity of influenza vaccines during the microneedle dry coating process. Studies of stabilized and un-stabilized solid microneedle influenza vaccines provided evidence that functional integrity of HA in the influenza vaccine was critically important in determining the quality of host protective immune responses to microneedle vaccination in the skin as well as the protective efficacy [39]. As a result of stabilization, as low as 0.4 µg of inactivated influenza (A/PR8, H1N1) virus vaccine coated onto microneedles was able to confer complete protection, which was superior to IM immunization with the same vaccine [39,40]. Un-stabilized solid microneedle vaccines induced much weaker protection. The trehalose sugar itself does not have an adjuvant effect, as vaccines (not exposed to microneedle coating) induced similar antibody responses after IM immunization in the presence or absence of trehalose [39]. These studies indicate that trehalose plays a critical role as a stabilizing agent in retaining hemagglutination activity of influenza vaccines during coating and drying during preparation of coated microneedles.
Mechanistic studies on stability loss during microneedle influenza vaccine coating demonstrated that crystallization and phase separation of the microneedle coating matrix damages influenza vaccine coated onto microneedles [41]. Transmission electron microscopic examination similarly showed morphological changes of the inactivated virus, suggesting damage to vaccine integrity in unstabilized microneedle vaccines after dry coating. A further study found that trehalose and a viscosity enhancer were beneficial coating excipients, but the inclusion of a surfactant was detrimental to vaccine stability [42]. Retaining the receptor-binding functional activity of the HA protein antigen in influenza vaccines was critical for the effective induction of protective immune responses. Vaccination in the skin of mice using stabilized microneedle patch, therefore, elicits improved protective immunity with reduced vaccine doses.
Co-formulation with the disaccharides trehalose and sucrose was also demonstrated to stabilize viral vector vaccines during the dry-coating process. The inclusion of trehalose and sucrose in the coating formulation for Nanopatch microprojections stabilized, simian adenovirus serotype 63 and the MVA poxvirus expressing malaria antigens, which was successfully applied to humans [6]. In addition, this recombinant adenovirus vector vaccine coated onto the Nanopatch microprojections was stored for 10 weeks at 37°C without much loss in viability [6]. In addition, this solid Nanopatch microprojection was used for skin delivery of a licensed influenza vaccine (Fluvax 2008®) with saponin Quil-A adjuvant [43]. The skin delivery of influenza vaccines and adjuvants showed equivalent immunogenicity to IM immunization [43].
4.2 Microneedle-based delivery of split and subunit influenza vaccines
Despite the fact that whole influenza virus was found to be more immunogenic compared to other forms, its use was discontinued in most countries and replaced with less reactogenic split or subunit influenza vaccines [44–47]. While these split or subunit vaccines have been proven to be safe, several studies have demonstrated decreased immune responses after IM administration, especially in children, the elderly, or immunocompromised individuals [48–50]. The usual target for influenza vaccination is the deltoid muscle. However, this tissue contains limited numbers of APCs, such as dendritic cells and macrophages, and lacks MHC class II expressing cells, resulting in a reduction in T-cell activation as well as in humoral and cellular immune responses [51]. In contrast, the skin represents an ideal target for vaccine delivery because it contains large numbers of immunologically active cells and APCs that will take up the antigen and migrate to the proximal lymph nodes, where naïve T and B cells are activated to initiate adaptive immune responses [37]. Indeed, studies using WIV for skin immunization of experimental animals using antigen-coated metal microneedle patches resulted in strong humoral and cellular immune responses, and improved serological memory and long term protection compared to the conventional routes of vaccine delivery [37,51,52].
In a study using metal microneedles coated with licensed influenza subunit vaccines the magnitude and longevity of immune responses has been investigated [53]. Vaccination of mice by the IM route elicited peak antibody titers at week 8, whereas a parallel MN-immunized group elicited the highest responses at week 12. At a later time, 36 weeks post-immunization, 37.5% of the IM vaccinated animals exhibited a decline in titers below protective levels (HAI < 40). Delivery of subunit influenza vaccine through the skin using MN or through IM injection also induced high levels of neutralizing antibody titers in both vaccinated groups. The antibody responses in the MN group peaked at 12 weeks post-immunization at a threefold higher level when compared to the IM group. These results demonstrate that stronger immune responses are induced after MN delivery of influenza subunit vaccine, compared with IM delivery. To evaluate the longevity and efficacy of subunit influenza vaccine in conferring protection after a single immunization through the skin versus the muscle, cohorts of vaccinated mice were challenged with mouse-adapted virus at intervals post vaccination. At 4 or 12 weeks post immunization, both vaccinated groups were fully protected against lethal challenge and showed little reduction in body weight. However, at week 36 post immunization, the MN group remained fully protected against virus challenge, exhibiting an average body weight loss of 5% and 100% survival. In contrast, the IM group was only partially protected, with a mortality rate of 40% and an average body weight loss of 15% in mice that survived the challenge. These findings demonstrate that skin immunization using metal microneedles coated with an influenza subunit vaccine induces longer-lived immunity capable of conferring full protection and survival against lethal challenge with homologous virus, at least up to 36 weeks after a single immunization.
Similar effectiveness of subunit vaccines was observed in hairless guinea pigs [54]. In this study, microneedles coated with a commercial trivalent vaccine led to hemagglutination inhibition titers similar to those obtained from IM injection of the unprocessed vaccine.
BD Soluvia™ is a prefillable delivery system that is integrated with a 1.5 mm long hollow needle, which enables intradermal delivery of a drug or vaccine. Clinical trials of this device demonstrated that the system was safe and easy to use [55]. Recently, it was demonstrated in several clinical studies that the trivalent split inactivated influenza vaccines can be delivered intradermally using this device, and that a dose-sparing effect was achieved [56,57]. This intradermally delivered vaccine has now been approved for use in Europe and in the US [55], and provides an approach for skin immunization.
4.3 Microneedle-based delivery of influenza virus-like particles
Virus-like particles (VLPs) are multi-subunit protein complexes capable of self-assembly, forming structures that mimic the morphology and structural conformation of native viruses [58]. VLPs do not contain viral genetic material and are non-infectious particles. Compared with vaccines based on attenuated or inactivated viruses, VLP vaccines are considered safer as they help avoid the possible reversion of an attenuated vaccine strain or potential incomplete inactivation of a live virus [58]. The first human vaccine manufactured using recombinant DNA technology was the enveloped hepatitis B vaccine produced in yeast [59]. Hepatitis small envelope protein antigen has the property of self-assembly to form VLPs of approximately 22 nm [60]. Human papillomavirus (HPV) VLPs produced in yeast or insect cells were highly immunogenic and protective, resulting in successful prevention of HPV infection and approval for human use [61]. The repetitive arrays of antigenic target molecules on the surface of VLPs are recognized by the immune system, inducing strong humoral and cellular responses. Thus, VLPs are able to induce B cell-mediated immune responses that produce high titers of neutralizing antibodies and a cytotoxic T lymphocyte response in the absence of adjuvant [62].
Influenza HA is responsible for attachment of the virus to sialic acid-containing receptors on target cells and is the major antigenic target for inducing protective immune responses. Influenza VLPs containing HA were shown to be highly immunogenic, inducing protective immunity [58]. Thus, they were suggested to be promising vaccines alternative to traditional platforms of inactivated influenza virus or live attenuated influenza vaccines. Influenza VLP vaccines derived from the 2009 H1N1 pandemic virus or H5N1 avian-origin pandemic potential virus have been successfully tested in clinical trials [63–65].
Similar to results with whole inactivated virus, use of trehalose sugar in the microneedle coating with influenza HA VLPs (H1N1, A/PR/8/34) preserved hemagglutination activity, providing further evidence that trehalose stabilized the HA protein [66]. Microneedle immunization of mice in the skin with a single dose of stabilized influenza VLPs induced 100% protection against lethal infection, whereas unstabilized microneedle VLP vaccine induced only partial protection [66]. HA stabilization, combined with vaccination in the skin using a solid-formulated microneedle patch, conferred superior protection to IM injection, potential dose sparing effects and rapid recall immune responses against influenza virus [66]. This correlation between the functional integrity of HA and protective immunity against influenza was further supported by studies with other influenza vaccines, such as H1N1 and H5N1 VLP vaccines [38,67,68]. Among the immunologic data, the recall immune responses with microneedle vaccination in the skin were significantly stronger than those found by IM immunization, and were consistently observed with both whole inactivated 2009 H1N1 pandemic influenza virus and VLP vaccines [39,51,69]. Protective immunity by microneedle immunization in the skin with stabilized influenza VLP vaccine was maintained for over 14 months after a single dose [70]. Therefore, the microneedle delivery of vaccines to the skin can be applied to a broad range of influenza vaccines and is effective at inducing long-term protective immunity.
4.4 Microneedle-based delivery of influenza DNA vaccines
Manufacturing of DNA vaccines can be rapid and inexpensive because of simple molecular cloning and bacterial culture techniques, compared with egg-based methods currently used for conventional influenza vaccines. DNA vaccines against influenza viruses have been studied in animal models and in humans [71,72]; however, they are often weakly immunogenic [73,74]. Various alternative approaches have been used to improve their delivery and immunogenicity, including jet injection [75], electroporation with injection [16], gene gun [76], transcutaneous DNA vaccination [77], and microneedle delivery [78]. The gene gun has received attention specifically for DNA vaccination in the skin [76]. In particular, gene gun or electroporation delivery of polyvalent DNA vaccines to the skin of pigs was effective in inducing protective immunity to influenza viruses [78].
Solid microneedle coating and delivery of DNA vaccines encoding herpes simplex virus or hepatitis virus antigens were demonstrated to enhance antibody responses probably by targeting skin dendritic cells [79,80]. Also, influenza H5 HA encoding plasmid DNA vaccines were more immunogenic when delivered to the skin using microneedles than by IM immunization with the same DNA vaccine [81]. The viscosity-enhancing ingredient carboxymethylcellulose is an essential component for effective solid microneedle coating formulation for inactivated whole virus or VLP vaccines [82]. However, the inclusion of carboxymethylcellulose in the microneedle coating formulation was found to negatively affect antigen production by DNA vaccines [81]. Because of the high molecular weight and viscous property of DNA vaccines, coating of microneedles with naked DNA vaccines in the absence of other excipients (carboxymethylcellulose, detergent) was feasible and found to be effective [83,84]. Delivery of microneedle-coated DNA vaccines to the skin also conferred dose sparing effects and induced improved protection compared to IM immunization [83]. In a novel formulation for a dual component vaccination strategy, the viscous solution property of a DNA vaccine itself enabled its use as a coating solution for inactivated whole virus vaccine [84]. Skin delivery of microneedles coated with these vaccine components and additional excipients, showed increased immunogenicity as well as cross-reactive antibodies and broader cross protection against a heterologous influenza virus [84].
A different design of microneedle was also used to deliver DNA vaccines to the skin. A solid microprojection device called a Nanopatch™ contained thousands of short (110 µm) projections (70 µm spacing) [85]. DNA vaccines encoding herpes simplex virus antigen were successfully administered to the skin of mice using the Nanopatch device [86]. Protective immune responses were induced with solid, short, densely packed arrays of microprojects coated with DNA vaccines applied to the skin [86]. In addition, the efficacy of a Nanopatch-coated DNA vaccine for West Nile virus was demonstrated [85]. Thus, various microneedle vehicles can be developed as skin delivery systems for DNA vaccines.
4.5 Microneedle-based delivery of stabilized recombinant influenza HA antigen
An alternative approach for expediting and simplifying production of influenza antigens is the use of recombinant expression systems; however, because of the inherent instability of the HA antigen, this can result in loss of antigenic properties. Therefore, it is of interest to develop approaches that would stabilize the HA antigen in the form of its native trimeric structure [87], such that HA can remain stable upon conversion to dry state either when it is incorporated in dry films of coated microneedles, or upon encapsulation in dry solid polymeric microneedles. It was observed that a modified form of soluble HA which contained a GCN4pII trimerization repeat stabilized the trimeric structure of the HA protein. Transdermal delivery of the stabilized trimeric influenza HA from the H3 virus A/Aichi/2/68 via coated microneedles was used for skin immunization [87].
As maintenance of the native trimeric structure is important for maintaining immunogenicity when used for skin immunization, it was determined if the structure of sHA. GCN4pII was disrupted by the microneedle coating process. The sHA.GCN4pII protein dissociated into dimeric and monomeric species in the absence of a stabilizer. However, the trimeric structure was maintained when 15% trehalose was added to the coating buffer. These data suggest that the trimeric sHA protein structure is disrupted during the microneedle coating and drying process; however, the structure is preserved by the addition of trehalose to the coating solution [87].
Mice vaccinated with sHA.GCN4pII had higher virus-specific serum IgG titers than mice boosted with sHA (p = 0.0032), indicating the enhanced immunogenicity of the HA stabilized in its trimeric native form. In addition, 28 days after boosting with sHA.GCN4pII, 3.7-fold higher IgG titers were observed compared with mice boosted with sHA (p < 0.0001). To determine the induction of mucosal responses by microneedle vaccination with recombinant soluble HA, sIgA levels were measured in vaginal washes by ELISA. After boosting, the sHA.GCN4pII vaccinated mice had approximately sevenfold higher sIgA titers than mice vaccinated with the purified unmodified sHA [87].
To determine the protective efficacy of microneedle vaccination with recombinant soluble HA, vaccinated mice were administered intranasally with 5 × LD50 of mouse adapted homologous A/Aichi/2/68 virus. Microneedle vaccination with sHA.GCN4pII conferred 100% protection while a similar immunization of sHA conferred only 50% protection. Mice vaccinated with sHA.GCN4pII-coated microneedles had no detectable viral lung titers at 4 days post-challenge, representing a reduction of over 106-fold compared with the unimmunized group (p < 0.001). In contrast, mice vaccinated subcutaneously with sHA.GCN4pII had a residual virus titer of about 102 pfu/g, demonstrating less efficient reduction of virus replication. These data support the conclusion that the microneedle route of vaccination induces improved clearance of replicating virus for a recombinant HA vaccine compared to the subcutaneous route, increasing the efficacy of such vaccines.
4.6 Microneedle-based delivery of adjuvanted influenza vaccine
Although a number of studies have been reported on influenza vaccine delivery using microneedles, there is little information on the effects of adjuvants using this approach for vaccination. Some types of widely used adjuvants which are lipid-based are likely to interfere with incorporation of a vaccine into the delivery system. Therefore, the use of toll-like receptor ligands as adjuvants was investigated with skin-based delivery of influenza subunit vaccine [88]. BALB/c mice received monovalent H1N1 subunit vaccine alone or with 1 µg of imiquimod or poly(I:C) individually or in combination via coated microneedles patches inserted into the skin. Whereas poly(I:C) adjuvanted subunit influenza vaccine induced similar immune responses to the non-adjuvanted vaccine, imiquimod-adjuvanted vaccine elicited higher levels of serum IgG2a antibodies and increased hemagglutination inhibition titers, suggesting enhanced induction of functional antibodies. In addition, imiquimod-adjuvanted vaccine induced a robust IFN-γ cellular response. These responses correlated with improved protection compared to influenza subunit vaccine alone, as well as reduced viral replication and cytokines in the lungs, and provide support for pursuing further studies using adjuvants to enhance skin immunization. In another recent study, saponin Quil-A was used as an adjuvant with a licensed influenza vaccine. Anti-influenza IgG antibody and HA inhibition responses induced by very low antigen doses, 6.5 ng of vaccine with 1.4 µg of Quil-A induced similar responses to conventional IM injection with 6000 ng of vaccine injected without adjuvant. The antigen dose-sparing effect demonstrates marked improvement to vaccines by co-delivery of both antigen and adjuvant directly to the skin [43]. Importantly, several hundred-fold dose sparing of influenza vaccine antigen was obtained by Nanopatch delivery of both antigen and the saponin Quil-A adjuvant [43]. Thus, co-delivery of antigen and adjuvant to the skin immune cells provided significant dose sparing effect.
4.7 Nature of immune response to microneedle-based delivery of influenza vaccines
Un-stabilized solid microneedle vaccines induced IgG1 antibody as the dominant isotype indicating T helper type 2 (Th2) immune responses [39]. However, use of stabilized microneedle vaccines maintaining hemagglutination activity shifted the pattern of antibodies to the IgG2a isotype implicating Th1 immune responses [38,39]. Also, microneedle vaccination induced higher levels of IFN-γ cytokine-secreting splenocytes compared to IM immunization upon MHC II peptide stimulation [39,40]. Enhanced MHC II-associated CD4+ T helper cell responses could be important to provide protection especially in the elderly. Microneedle vaccination in the skin increased trafficking of dendritic cells to regional lymph nodes, probably contributing to effective induction of T and B cell responses [89]. Within 12 h after microneedle vaccine delivery to the skin, increased levels of cytokines, which are important for recruitment of neutrophils, monocytes, and dendritic cells, were observed at the site of immunization [90]. These cytokines included interleukin-1β, macrophage inflammatory protein-1α and 2, tumor necrosis factor-α, monocyte chemoattractant protein-1, granulocyte colony-stimulating factor, interferon γ-induced protein-10, leukemia inhibitory factor, and neutrophil chemoattractant. Also, microneedle delivery of influenza vaccines resulted in prolonged antigen deposition, and migration of matured dendritic cells bearing influenza virus antigen from the skin [90]. The prolonged antigen deposition could contribute to the sustained antibody levels and long-term protection after microneedle skin vaccination [70,91].
Some lymphoid dendritic cells are more likely to induce Th1 type immune responses via receptor-mediated uptake of vaccine antigens, whereas macrophage cells typically induce Th2 type responses via non-receptor-mediated uptake of vaccines [92]. Therefore, maintaining the receptor binding activity of the HA protein is expected to be important for interaction with antigen-presenting cells with sialic acid receptors as well as presenting conformational epitopes for the induction of functional antibodies with hemagglutination inhibition activity. This may explain why trehalose-stabilized microneedle vaccines are more effective in inducing IgG2a isotype (Th1) immune responses than un-stabilized microneedle influenza vaccines in which a loss in hemagglutination activity occurs. After uptake of transdermally delivered antigens, skin-derived dendritic cells traffic to the local and systemic lymphoid organs, such as the lymph nodes and spleen [93,94]. A better understanding of each component in the coating formulation and further detailed immunologic study will help to develop more effective skin delivery of influenza vaccine.
It is possible that the skin’s abundant immune cell populations, such as dermal dendritic cells, would be also effective in inducing CD8 T cell responses. Delivery of a model subunit protein (ovalbumin) using a densely packed microprojection array (Nanopatch) was shown to enhance the CD8 T cell responses when compared to IM injection [95].
4.8 Microneedle delivery of influenza vaccines to human skin in vitro
Although many studies on microneedle influenza vaccines were done using the mouse model, there are significant differences in the skin architecture and immune system between mice and humans [96]. In addition, the microneedle vaccines aim to target specific layers in human skin. Therefore, it is extremely valuable to understand immune activation in the actual human skin environment after microneedle targeting of vaccine to the skin epidermis.
An ex vivo human skin organ culture system [32,97] was utilized to investigate the response of human skin to microneedles coated with influenza VLP vaccine candidates [98]. Dendritic cells are professional APCs, which are highly efficient at taking up antigens from the surrounding milieu, trafficking to peripheral lymphoid tissues, and activating naïve T cells [99]. LCs are a type of dendritic cells that migrate from the site of antigen uptake to activate T cells. LCs are spread over the entire skin surface, forming a network with many characteristic dendritic protrusions [100].
Application of microneedles coated with influenza VLP vaccines resulted in a consistent pattern of puncture of the ex vivo human skin, through the epidermis into the upper reaches of the dermis, thereby targeting the resident APCs including the LCs of the epidermis and dermal dendritic cells [98]. LCs display a highly dendritic morphology, suggesting that they have limited potential for transport between tightly packed epidermal keratinocyte layers. Delivery of microneedles coated with influenza VLP vaccines to the human skin ex vivo or intradermal delivery of influenza VLPs resulted in a significant reduction in dendrites of LCs when compared with untreated skin at 24 – 48 h [98,100]. Both microneedle delivery and intradermal delivery of influenza VLPs stimulated LCs to retract their dendrites, facilitating their physical movement through the epidermis. To obtain better insight into molecular changes at the level of gene expression, the results of microarray analysis (47,296 discrete transcripts) were determined with viable human skin samples after intradermal delivery of influenza VLPs or skin immunization using microneedles coated with influenza VLPs [101]. Host genes responsible for key immunomodulatory processes and host antiviral responses were found to be up-regulated, including genes associated with cell recruitment, activation, migration, and T cell interaction, following either intradermal or microneedle delivery of VLPs to the human skin. These results using human skin are consistent with those showing improved protection using animal models.
5. Conclusion
Microneedle technology offers multiple advantages over other methods for vaccine delivery to the skin. Microneedles are painless, minimally invasive, can be self-applied, offer convenience of use, and do not require bulky and/or expensive accessories and power to operate. In contrast, other technologies, such as gene gun, jet injectors, ultrasound electroporation, thermal abrasion or microdermabrasion, require either a continuous supply of a carrier fluid such as argon gas, or require electrical power to operate. These constraints severely limit their applicability in remote regions and the developing world. Recent findings show that improved protective immunity and longer duration of immunity can be induced by a variety of vaccines, particularly for influenza prevention, and support the development of this technology for use in annual vaccination as well as for prevention of future viral pandemics. Nonetheless, there is still a significant gap in developing affordable and marketable vaccines which are well suited for cutaneous application to humans. Future studies should be directed to developing effective devices for coating vaccines onto microneedle patches and methods for incorporation of antigens into dissolving microneedles, further improving the stability of vaccines in dry formulations, and performing more extensive preclinical studies in relevant larger animals and clinical trials in human subjects.
6. Expert opinion
Intradermal delivery of vaccines is a promising alternative to the conventional IM route because of its immunological and practical advantages. Immunological advantages may depend on several factors, such as the type and dose of vaccine, and the different ages and immune status of subjects. In particular, it is important to determine if high-risk populations, such as the elderly and children, may generate improved immune responses after intradermal vaccination. Dose sparing is another potential benefit of intradermal delivery, thus reducing the cost of vaccines and increasing vaccine availability in response to pandemics or outbreaks, when demand is high and vaccine manufacturing facility capacity is limited. A number of clinical trials have shown comparable efficacy to IM immunization using reduced intradermal dosages of vaccines. It is important to keep in mind that dose reductions might also be achievable via the IM or subcutaneous route of vaccines by the use of adjuvants.
Development of solid vaccine-coated microneedles has enabled detailed immunological and dose sparing effects by targeting vaccines to the skin using small animal models, particularly mice. Solid microneedle vaccination in the skin confers protective immunity with various forms of vaccines including whole inactivated virus, split vaccine, subunit vaccine, live attenuated virus, and DNA vaccines. These preclinical studies demonstrated dose sparing, immunological advantages, and long-term protective immunity. However, there may be a significant gap between solid microneedle vaccines used in animal studies and appropriate application devices for human use, especially if self-administration is a goal. It should be noted that mice have very different skin properties from those of humans. Thus, it is important for studies to be extended to more relevant animal models (pig, guinea pig, and monkey models) that are expected to have skin properties that more closely resemble those of humans. Certainly, testing of microneedle administration of vaccines in humans is the most important objective, and such studies are anticipated in the near future.
Substantial issues still need to be overcome to enable the development of coating and manufacturing technologies for vaccine-coated microneedles suitable for mass vaccination.
The process of coating solid microneedles with vaccines involves a phase change from a liquid to solid formulation, which could represent an issue for retention of vaccine immunogenicity. On the other hand, once a dry formulation is achieved, it is anticipated that its stability will be significantly enhanced, such that a ‘cold chain’ may no longer be required during transport and storage. Each type of vaccine preparation needs to be tested for its stability by in vitro assays as well as efficacy in animal models.
Several other future directions are anticipated to offer significant advantages for skin immunization with microneedles. Incorporation of adjuvants into the vaccine formulation offers the potential for delivery of antigen and adjuvants to the same localized site, which could further reduce the required vaccine dose and enhance the resulting immune response. This approach is of particular interest for use with antigens that have exhibited low immunogenicity, such as those of avian influenza viruses, which have recently caused outbreaks of human disease. The development of microneedles formulated from dissolvable polymers also represents an important advance, because such devices would eliminate the potential hazard resulting from residual sharp protrusions, and they will, therefore, be more appropriate for development of self-administered vaccines. It is conceivable that in pandemic situations, such devices, which also do not require refrigeration, could be rapidly distributed to the population via mail to achieve rapid mass vaccination.
Article highlights.
Preparedness for influenza pandemics could benefit from the emerging microneedle technology, which allows delivery of influenza vaccines to the skin. Microneedle-based delivery of different influenza antigens, including split influenza, virus-like particles, DNA vaccines, and recombinant antigens, has been shown to be protective in animal models against lethal influenza challenge.
Microneedle-based skin vaccination resulted in better protection and survival upon lethal influenza challenge as compared to intramuscular vaccination in mice.
Not only can microneedles enable painless delivery of influenza vaccines to the skin, but they also have potential for dose sparing, which could allow vaccination of larger population in case vaccine supplies are limited.
Stimulation of proinflammatory cytokines at the site of microneedle-based vaccination, increased deposition time of vaccine antigens in skin, and enhanced migration of dendritic cells could all contribute toward the enhanced immunogenicity observed with microneedles.
Similar to mouse skin, ex vivo viable cultures of human skin demonstrated similar migration characteristics of dendritic cells as mouse skin, suggesting a similar mechanistic expectation and immune response in humans.
In conclusion, microneedles offer a simple, easy-to-use and potent vaccination approach for influenza vaccination. To fully develop this system for clinical translation, additional studies in humans are needed to assess their effectiveness.
This box summarizes key points contained in the article.
Acknowledgements
The authors thank Erin-Joi Collins for her assistance in the preparation and submission of this manuscript.
Research by the authors has been supported by NIH/NIAID grants AI105170 (Sang-Moo Kang), AI093772 (Sang-Moo Kang) and EB012495 (Richard W. Compans).
Footnotes
Declaration of interest
HS Gill is a co-inventor of a coating technology, which has been licensed to a US company. The patent application is still pending in the US patent office. No collaboration or other financial contracts exist between HSG and the licensee. The official technology transfer and license is managed by Georgia Tech Research Corp.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Jenner E. An inquiry into the causes and effects of the variolæ vaccinæ, or cow-pox. The three original publications on vaccination against smallpox. The Harvard Classics. :1909–1914. [Google Scholar]
- 2.Foege WH, Millar JD, Henderson DA. Smallpox eradication in West and Central Africa. Bull World Health Organ. 1975;52(2):209–222. [PMC free article] [PubMed] [Google Scholar]
- 3.Sticchi L, Alberti M, Alicino C, et al. The intradermal vaccination: past experiences and current perspectives. J Prev Med Hyg. 2010;51(1):7–14. [PubMed] [Google Scholar]
- 4.Teunissen MBM. Intradermal immunization. Düsseldorf, Germany: Springer-Verlag; 2012. [Google Scholar]
- 5.Weniger BG, Papania MJ. Alternative vaccine delivery methods. In: Plotkin SA, Orenstein WA, Offi PA, editors. Vaccines. 6th edition. New York, NY: Elsevier; 2013. pp. 1200–1231. [Google Scholar]
- 6.Pearson FE, McNeilly CL, Crichton ML, et al. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One. 2013;8(7):e67888. doi: 10.1371/journal.pone.0067888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin’s barrier function. Pharm Sci Technolo Today. 2000;3(9):318–326. doi: 10.1016/s1461-5347(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 8.Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14(2):75–78. doi: 10.1016/0167-5699(93)90062-P. [DOI] [PubMed] [Google Scholar]
- 9.Glenn GM, Kenney RT, Ellingsworth LR, et al. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev Vaccines. 2003;2(2):253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234(1):120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sparber F, Tripp CH, Hermann M, et al. Langerhans cells and dermal dendritic cells capture protein antigens in the skin: possible targets for vaccination through the skin. Immunobiology. 2010;215(9–10):770–779. doi: 10.1016/j.imbio.2010.05.014. • Reports the theoretical basis for skin as a potentially improved route of vaccination.
- 12.Karande P, Arora A, Pham TK, et al. Transcutaneous immunization using common chemicals. J Control Release. 2009;138(2):134–140. doi: 10.1016/j.jconrel.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Tezel A, Paliwal S, Shen Z, et al. Low-frequency ultrasound as a transcutaneous immunization adjuvant. Vaccine. 2005;23(29):3800–3807. doi: 10.1016/j.vaccine.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Hooper JW, Golden JW, Ferro AM, et al. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babiuk S, Baca-Estrada ME, Foldvari M, et al. Needle-free topical electroporation improves gene expression from plasmids administered in porcine skin. Mol Ther. 2003;8(6):992–998. doi: 10.1016/j.ymthe.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Laddy DJ, Yan J, Khan AS, et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83(9):4624–4630. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skountzou I, Quan FS, Jacob J, et al. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24(35–36):6110–6119. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Frolov VG, Seid RC, Jr, Odutayo O, et al. Transcutaneous delivery and thermostability of a dry trivalent inactivated influenza vaccine patch. Influenza Other Respir Viruses. 2008;2(2):53–60. doi: 10.1111/j.1750-2659.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg S, Hoelscher M, Belser JA, et al. Needle-free skin patch delivery of a vaccine for a potentially pandemic influenza virus provides protection against lethal challenge in mice. Clin Vaccine Immunol. 2007;14(7):926–928. doi: 10.1128/CVI.00450-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill HS, Andrews SN, Sakthivel SK, et al. Selective removal of stratum corneum by microdermabrasion to increase skin permeability. Eur J Pharm Sci. 2009;38(2):95–103. doi: 10.1016/j.ejps.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews SN, Zarnitsyn V, Bondy B, et al. Optimization of microdermabrasion for controlled removal of stratum corneum. Int J Pharm. 2011;407(1–2):95–104. doi: 10.1016/j.ijpharm.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitragotri S. Current status and future prospects of needle-free liquid jet injectors. Nat Rev Drug Discov. 2006;5(7):543–548. doi: 10.1038/nrd2076. [DOI] [PubMed] [Google Scholar]
- 23.Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40(1):86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Jones S, Evans K, McElwaine-Johnn H, et al. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine. 2009;27(18):2506–2512. doi: 10.1016/j.vaccine.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 25.Gill HS, Denson DD, Burris BA, et al. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24(7):585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prausnitz MR, Mikszta JA, Cormier M, et al. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–393. doi: 10.1007/978-3-540-92165-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. •• Recent review of microneedles and their application for vaccine delivery.
- 28. Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–1380. doi: 10.1007/s11095-007-9286-4. •• Reports comprehensive basis for microneedle vaccine formulations.
- 29.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117(2):227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenney RT, Frech SA, Muenz LR, et al. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351(22):2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 31. La Montagne JR, Fauci AS. Intradermal influenza vaccination–can less be more? N Engl J Med. 2004;351(22):2330–2332. doi: 10.1056/NEJMe048314. •• Perspective on dose sparing by intradermal immunization.
- 32.Birchall J, Coulman S, Pearton M, et al. Cutaneous DNA delivery and gene expression in ex vivo human skin explants via wet-etch micro-fabricated micro-needles. J Drug Target. 2005;13(7):415–421. doi: 10.1080/10611860500383705. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Dean HJ. Epidermal delivery of protein and DNA vaccines. Expert Opin Drug Deliv. 2005;2(2):227–236. doi: 10.1517/17425247.2.2.227. [DOI] [PubMed] [Google Scholar]
- 35.Zaric M, Lyubomska O, Touzelet O, et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano. 2013;7(3):2042–2055. doi: 10.1021/nn304235j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Q, Zarnitsyn VG, Ye L, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106(19):7968–7973. doi: 10.1073/pnas.0812652106. •• An initial report on influenza vaccination using coated metal microneedles.
- 37. Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS One. 2009;4(3):e4773. doi: 10.1371/journal.pone.0004773. • An initial report on influenza vaccination using vaccine-coated metal microneedle arrays.
- 38.Quan FS, Kim YC, Vunnava A, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quan FS, Kim YC, Yoo DG, et al. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4(9):e7152. doi: 10.1371/journal.pone.0007152. • Reports detailed immunological benefits of using a stabilizer in microneedle vaccines.
- 40. Kim YC, Quan FS, Yoo DG, et al. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27(49):6932–6938. doi: 10.1016/j.vaccine.2009.08.108. • Reports mechanistic studies on stabilizing microneedle vaccine formulations.
- 41.Choi HJ, Yoo DG, Bondy BJ, et al. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33(14):3756–3769. doi: 10.1016/j.biomaterials.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi HJ, Bondy BJ, Yoo DG, et al. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. J Control Release. 2013;166(2):159–171. doi: 10.1016/j.jconrel.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernando GJ, Chen X, Primiero CA, et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release. 2012;159(2):215–221. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 44.Koyama S, Aoshi T, Tanimoto T, et al. Plasmacytoid dendritic cells delineate immunogenicity of influenza vaccine subtypes. Sci Transl Med. 2010;2(25):25ra24. doi: 10.1126/scitranslmed.3000759. [DOI] [PubMed] [Google Scholar]
- 45.Gross PA. Reactogenicity and immunogenicity of bivalent influenza vaccine in one- and two-dose trials in children: a summary. J Infect Dis. 1977;136(Suppl):S616–S625. doi: 10.1093/infdis/136.supplement_3.s616. [DOI] [PubMed] [Google Scholar]
- 46.Gross PA, Ennis FA, Gaerlan PF, et al. A controlled double-blind comparison of reactogenicity, immunogenicity, and protective efficacy of whole-virus and split-product influenza vaccines in children. J Infect Dis. 1977;136(5):623–632. doi: 10.1093/infdis/136.5.623. [DOI] [PubMed] [Google Scholar]
- 47.Gross PA, Ennis FA. Influenza vaccine: split-product versus whole-virus types–How do they differ. N Engl J Med. 1977;296(10):567–568. doi: 10.1056/NEJM197703102961012. [DOI] [PubMed] [Google Scholar]
- 48.Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009;27(Suppl 4):D65–D68. doi: 10.1016/j.vaccine.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcelin G, Bland HM, Negovetich NJ, et al. Inactivated seasonal influenza vaccines increase serum antibodies to the neuraminidase of pandemic influenza A (H1N1) 2009 virus in an age-dependent manner. J Infect Dis. 2010;202(11):1634–1638. doi: 10.1086/657084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10(3):379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204(4):582–591. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. • First study to demonstrate efficacy of dissolving polymer microneedles for vaccination.
- 53.Koutsonanos DG, Vassilieva EV, Stavropoulou A, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kommareddy S, Baudner BC, Bonificio A, et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine. 2013;31(34):3435–3441. doi: 10.1016/j.vaccine.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 55.Laurent PE, Bonnet S, Alchas P, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25(52):8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Atmar RL, Patel SM, Keitel WA. Intanza ((R)): a new intradermal vaccine for seasonal influenza. Expert Rev Vaccines. 2010;9(12):1399–1409. doi: 10.1586/erv.10.134. [DOI] [PubMed] [Google Scholar]
- 57. Ansaldi F, de Florentiis D, Durando P, et al. Fluzone((R)) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines. 2012;11(1):17–25. doi: 10.1586/erv.11.154. •• Comprehensive review of licensed human vaccines delivered intradermally.
- 58.Kang SM, Song JM, Quan FS, et al. Influenza vaccines based on virus-like particles. Virus Res. 2009;143(2):140–146. doi: 10.1016/j.virusres.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assad S, Francis A. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999;18(1–2):57–67. doi: 10.1016/s0264-410x(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 60.McAleer WJ, Buynak EB, Maigetter RZ, et al. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307(5947):178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 61.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 62.Moron VG, Rueda P, Sedlik C, et al. In vivo, dendritic cells can cross-present virus-like particles using an endosome-to-cytosol pathway. J Immunol. 2003;171(5):2242–2250. doi: 10.4049/jimmunol.171.5.2242. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Macias C. Virus-like particle (VLP)-based vaccines for pandemic influenza: performance of a VLP vaccine during the 2009 influenza pandemic. Hum Vaccin Immunother. 2012;8(3):411–414. doi: 10.4161/hv.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez-Macias C, Ferat-Osorio E, Tenorio-Calvo A, et al. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine. 2011;29(44):7826–7834. doi: 10.1016/j.vaccine.2011.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khurana S, Wu J, Verma N, et al. H5N1 virus-like particle vaccine elicits cross-reactive neutralizing antibodies that preferentially bind to the oligomeric form of influenza virus hemagglutinin in humans. J Virol. 2011;85(21):10945–10954. doi: 10.1128/JVI.05406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quan FS, Vunnava A, Compans RW, et al. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS One. 2010;5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim YC, Quan FS, Song JM, et al. Influenza immunization with trehalose-stabilized virus-like particle vaccine using microneedles. Procedia Vaccinol. 2010;2(1):15–19. doi: 10.1016/j.provac.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song JM, Kim YC, Barlow PG, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88(2):244–247. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quan FS, Kim YC, Compans RW, et al. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147(3):326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quan FS, Kim YC, Song JM, et al. Long-term protective immunity from an influenza virus-like particle vaccine administered with a microneedle patch. Clin Vaccine Immunol. 2013;20(9):1433–1439. doi: 10.1128/CVI.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donnelly JJ, Friedman A, Martinez D, et al. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1(6):583–587. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 72. Drape RJ, Macklin MD, Barr LJ, et al. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24(21):4475–4481. doi: 10.1016/j.vaccine.2005.08.012. • Reports delivery of influenza DNA vaccines to the skin of humans.
- 73.Babiuk LA, Pontarollo R, Babiuk S, et al. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21(7–8):649–658. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- 74.MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178(1):92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 75.Haensler J, Verdelet C, Sanchez V, et al. Intradermal DNA immunization by using jet-injectors in mice and monkeys. Vaccine. 1999;17(7–8):628–638. doi: 10.1016/s0264-410x(98)00242-4. [DOI] [PubMed] [Google Scholar]
- 76.Yager EJ, Dean HJ, Fuller DH. Prospects for developing an effective particle-mediated DNA vaccine against influenza. Expert Rev Vaccines. 2009;8(9):1205–1220. doi: 10.1586/erv.09.82. [DOI] [PubMed] [Google Scholar]
- 77.Falo LD., Jr Targeting the skin for genetic immunization. Proc Assoc Am Physicians. 1999;111(3):211–219. doi: 10.1046/j.1525-1381.1999.99227.x. [DOI] [PubMed] [Google Scholar]
- 78.Bragstad K, Vinner L, Hansen MS, et al. A polyvalent influenza A DNA vaccine induces heterologous immunity and protects pigs against pandemic A(H1N1) pdm09 virus infection. Vaccine. 2013;31(18):2281–2288. doi: 10.1016/j.vaccine.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 79.Chen X, Kask AS, Crichton ML, et al. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J Control Release. 2010;148(3):327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 80. Gill HS, Soderholm J, Prausnitz MR, et al. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17(6):811–814. doi: 10.1038/gt.2010.22. • First study to demonstrate delivery of DNA vaccines to the skin using metal coated microneedles to elicit a cytotoxic T lymphocyte cellular immune response.
- 81.Kim YC, Song JM, Lipatov AS, et al. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur J Pharm Biopharm. 2012;81(2):239–247. doi: 10.1016/j.ejpb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim YC, Quan FS, Compans RW, et al. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11(3):1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song JM, Kim YC, O E, et al. DNA vaccination in the skin using microneedles improves protection against influenza. Mol Ther. 2012;20(7):1472–1480. doi: 10.1038/mt.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim YC, Yoo DG, Compans RW, et al. Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles. J Control Release. 2013;172(2):579–588. doi: 10.1016/j.jconrel.2013.04.016. • Novel approach for using DNA in a dual role of viscosity-enhancing coating excipient and as a vaccine antigen.
- 85.Prow TW, Chen X, Prow NA, et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6(16):1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 86.Kask AS, Chen X, Marshak JO, et al. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine. 2010;28(47):7483–7491. doi: 10.1016/j.vaccine.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Weldon WC, Wang BZ, Martin MP, et al. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One. 2010;5:9. doi: 10.1371/journal.pone.0012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weldon WC, Zarnitsyn VG, Esser ES, et al. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One. 2012;7(7):e41501. doi: 10.1371/journal.pone.0041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim YC, Quan FS, Yoo DG, et al. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201(2):190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.del Pilar Martin M, Weldon WC, Zarnitsyn VG, et al. Local response to microneedle-based influenza immunization in the skin. MBio. 2012;3(2):e00012–e00012. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quan FS, Yoo DG, Song JM, et al. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83(9):4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Becker G, Sornasse T, Nabavi N, et al. Immunoglobulin isotype regulation by antigen-presenting cells in vivo. Eur J Immunol. 1994;24(7):1523–1528. doi: 10.1002/eji.1830240710. [DOI] [PubMed] [Google Scholar]
- 93.Belyakov IM, Hammond SA, Ahlers JD, et al. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113(7):998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hon H, Jacob J. Tracking dendritic cells in vivo: insights into DC biology and function. Immunol Res. 2004;29(1–3):69–80. doi: 10.1385/IR:29:1-3:069. [DOI] [PubMed] [Google Scholar]
- 95.Ng HI, Fernando GJ, Kendall MA. Induction of potent CD8(+) T cell responses through the delivery of subunit protein vaccines to skin antigen-presenting cells using densely packed microprojection arrays. J Control Release. 2012;162(3):477–484. doi: 10.1016/j.jconrel.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 96.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 97.Pearton M, Allender C, Brain K, et al. Gene delivery to the epidermal cells of human skin explants using microfabricated microneedles and hydrogel formulations. Pharm Res. 2008;25(2):407–416. doi: 10.1007/s11095-007-9360-y. [DOI] [PubMed] [Google Scholar]
- 98.Pearton M, Kang SM, Song JM, et al. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine. 2010;28(37):6104–6113. doi: 10.1016/j.vaccine.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26(26):3197–3208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 100. Pearton M, Kang SM, Song JM, et al. Changes in human Langerhans cells following intradermal injection of influenza virus-like particle vaccines. PLoS One. 2010;5(8):e12410. doi: 10.1371/journal.pone.0012410. •• Report on morphological changes in human Langerhans cells in response to microneedle vaccine delivery to the human skin.
- 101.Pearton M, Pirri D, Kang SM, et al. Host responses in human skin after conventional intradermal injection or microneedle administration of virus-like-particle influenza vaccine. Adv Healthc Mater. 2013;2(10):1401–1410. doi: 10.1002/adhm.201300006. [DOI] [PMC free article] [PubMed] [Google Scholar]