Abstract
Abdominal aortic aneurysm (AAA) is a common disease with often life-threatening consequences. This vascular disorder is responsible for 1–2% of all deaths in men aged 65 years or older. Autoimmunity may be responsible for the pathogenesis of AAA. Although it is well documented that infiltrating T cells are essentially always present in AAA lesions, little is known about their role in the initiation and/or progression of the disease. To determine whether T cells infiltrating AAA lesions contain clonally expanded populations of T cells, we amplified β-chain TCR transcripts by the nonpalindromic adaptor–PCR/Vβ-specific PCR and/or Vβ-specific PCR, followed by cloning and sequencing. We report in this article that aortic abdominal aneurysmal lesions from 8 of 10 patients with AAA contained oligoclonal populations of T cells. Multiple identical copies of β-chain TCR transcripts were identified in these patients. These clonal expansions are statistically significant. These results demonstrate that αβ TCR+ T lymphocytes infiltrating aneurysmal lesions of patients with AAA have undergone proliferation and clonal expansion in vivo at the site of the aneurysmal lesion, in response to unidentified self- or nonself Ags. This evidence supports the hypothesis that AAA is a specific Ag–driven T cell disease.
Introduction
Abdominal aortic aneurysm (AAA) is a common disease characterized by the presence of aortic dilations with diameter > 3 cm (1.5 times greater than the normal artery). As the diameter of the AAA grows beyond 5.0 cm, there is an increasing risk for rupture. The mortality associated with ruptured AAA may be as high as 80–90% (1–3). AAA is present in 3% of those aged ≥60 y and is responsible for 1–2% of all deaths in men aged 65 y or older (3). AAA is among the 10 leading causes of death among 55–74-y-olds and is the 13th leading cause of death in the United States (all ages) (3).
Although genetic and environmental factors are involved, our understanding of the etiology and pathogenesis of AAA is limited (4–6). AAA is a complex multifactorial disease (4–6). Autoimmunity may be responsible for the pathogenesis of AAA. AAA may be an autoimmune disease. This is supported by the following. i) The presence of inflammatory mononuclear cell infiltrates in AAA lesions, consisting mostly of T and B cells, NK cells, and macrophages (7–9). These inflammatory infiltrates are particularly profound in the adventitia. Also, inflammatory AAA contains numerous inflammatory cells arranged in follicles, suggesting a cell-mediated Ag response (7). ii) Mononuclear cells infiltrating AAA lesions express early (CD69), intermediate (CD25, CD38), and late (CD45RO, HLA class II) activation Ags, demonstrating an active ongoing inflammatory response in these lesions (9). iii) AAA is associated with particular HLA alleles (10, 11). iv) IgG Ab purified from the wall of AAAs is immunoreactive with proteins isolated from normal aortic tissue (12, 13). v) Putative self- and nonself AAA Ags have been identified, including elastin and elastin fragments (14–16), collagen types I and III (reviewed in Ref. 4), aortic AAA protein 40 (also known as microbial-associated glycoprotein 36) (12, 13, 17), oxidized low-density lipoprotein (18), Chlamydia pneumoniae (19, 20), Treponema palladium (21), and CMV (22). Molecular mimicry, which is defined as the sharing of antigenic epitopes between microorganisms and host Ags (23), may be responsible for inducing T cell inflammatory responses in AAA. vi) Proinflammatory Th1 cytokines play an important role in the pathogenesis of AAA; however, production of Th2 cytokines also has been reported (reviewed in Ref. 4; 24–26).
Although infiltrating T cells are essentially always present in AAA lesions (7–9), little is known about the role of T cells in the initiation and progression of AAA. The CD4+/CD8+ ratio in AAA lesions is 2–4-fold higher than in normal peripheral blood, indicating a redistribution or expansion of certain T cell subtypes in AAA (7–9). Determination of whether mononuclear cells infiltrating AAA lesions contain oligoclonal populations of T cells (i.e., clonally expanded T cells in response to specific Ag [self or nonself]), and eventually the identification of the Ag(s) that they recognize, is critical for our understanding of the pathogenesis of AAA.
We report in this article that AAA lesions contain clonally expanded T cells. Substantial proportions of identical β-chain TCR transcripts were found in these lesions, after PCR amplification followed by cloning of the amplified transcripts and sequencing. Their presence can be explained only by proliferation and clonal expansion in vivo of the corresponding T cell clones in response to specific, as yet unidentified Ag(s) (27). These results strongly suggest that AAA is a specific Ag–driven T cell disease.
Materials and Methods
Patients
AAA specimens were obtained from patients undergoing surgery for repair of infrarenal AAAs. AAA size, gender, race, age, past and recent history of associated diseases, and cardiovascular risk factors of the patients are shown in Table I. All adherent blood clots were carefully stripped away from the aneurysm walls prior to use. Grossly normal infrarenal abdominal aortic specimens from patients who died of nonvascular causes were obtained at autopsy and used as controls. Venous peripheral blood was obtained from healthy donors. These studies were reviewed and approved by the Institutional Review Boards of the Advocate Lutheran General Hospital and Temple University Hospital.
Table I. Characteristics of the patients with AAA.
| Patient | Gender | Race | Age (y) | AAA Size (cm) | HTN | COPD | TOB | CHOL | DM | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| AAA00 | Male | White | 71 | 4.8 | Yes | No | No | No | No | |
| AAA02 | Male | White | 65 | 5.8 | Yes | Yes | Yes | Yes | No | CAD |
| AAA03 | Male | White | 80 | 5.5 | Yes | No | No | Yes | No | |
| AAA04 | Male | White | 62 | 10.0 | Yes | No | No | Yes | No | CAD |
| AAA06 | Male | White | 77 | 5.5 | Yes | No | No | No | No | CAD/Lymphoma |
| AAA07 | Male | White | 71 | 5.5 | No | No | No | Yes | Yes | AOVR |
| AAA09 | Male | White | 78 | 7.4 | Yes | Yes | No | No | No | CAD/CRI |
| AAA10 | Male | White | 78 | 7.9 | No | No | No | Yes | Yes | CAD |
| AAA12 | Male | White | 77 | UN | UN | UN | UN | UN | UN | UN |
| AAA14 | UN | UN | UN | UN | UN | UN | UN | UN | UN | UN |
| Male = 9; | White = 9; | Avg.= 73.2; | Avg.=6.6; | Yes = 6; | Yes = 2; | Yes = 1; | Yes = 5; | Yes = 2; | ||
| UN = 1 | UN = 1 | n = 9; | n = 8; | No = 2; | No = 6; | No = 7 | No = 3; | No = 6; | ||
| UN = 1 | UN = 2 | UN = 2 | UN = 2 | UN = 2 | UN = 2 | UN = 2 |
AOVR, aortic valve replacement; CAD, coronary artery disease; CHOL, high cholesterol; COPD, chronic obstructive pulmonary disease; CRI, chronic renal disease; DM; diabetes mellitus; HTN, hypertension; TOB, current or former tobacco smoker; UN, unknown.
Immunohistochemistry
Each AAA specimen was divided into two fractions. One was embedded onto OCT, snap-frozen in liquid nitrogen, and stored at −70°C for immunohistochemistry. The remaining specimen was either snap-frozen in liquid nitrogen or used fresh for preparation of RNA. Immunostaining was carried out using an anti-CD3 mAb (clone NCL-CD3-PS1; Novocastra, Newcastle-upon-Tyne, U.K.), an anti-CD4 mAb (clone 4B12; Dako, Glostrup, Denmark), and an anti-CD8 mAb (clone C8/144B; Dako) by the avidin-biotin complex–immunoperoxidase method (Vector Labs, Burlingame, CA), as described (28, 29).
Preparation of single-cell suspensions from aortic specimens with AAA lesions
Fresh aortic specimens containing AAA lesions from patients with AAA were dissected into 2 mm3 blocks in a petri dish and incubated with collagenase (0.5 mg/ml) and DNase (120 U/ml) for 1 h at 37°C. The digested tissue and supernatant were passed through a cell strainer (30 μm). A single-cell suspension was obtained by incubating for an additional 10 min in trypsin-EDTA solution. Mononuclear cells were isolated using a Ficoll-Paque density cushion (30).
Isolation of CD4+ and CD8+ T cells from AAA specimens
CD4+ and CD8+ T cells were isolated from single-cell suspensions from AAA lesions using Dynabeads (Dynal Biotech, Brown Deer, WI). Single cell suspensions were divided into two aliquots, and one was used for the isolation of CD4+ T cells by employing the CD4+ Isolation Kit (Dynal Biotech), and the other aliquot was used for the isolation of CD8+ T cells by employing the CD8+ Isolation Kit (Dynal Biotech) following the manufacturer’s specifications. DETACHaBEAD was used to release CD4+ or CD8+ T cells, as recommended by the manufacturer (http://tools.lifetechnologies.com/content/sfs/manuals/DETACHaBEAD%20CD4-CD8.pdf).
Isolation of PBMCs from healthy donors
PBMCs were used as methodological control and were isolated from peripheral blood using a Ficoll-Hypaque density cushion (30).
DNA-based HLA typing for HLA-DRB1, HLA-DQA1, and HLA-DQB1
DNA was extracted from aortic specimens of patients with AAA for DNA-based typing of DRB1, DQA1, and DQB1 loci (31). Samples were typed at HLA-DRB1 (exon 2) and HLA-DQB1 (exons 2 and 3) with AlleleSEQR typing reagents (Abbott Molecular, Des Plaines, IL). Sequencing was performed using an ABI 3130 sequencer (Applied Biosystems, Foster City, CA), and the results were analyzed using Assign-SBT v3.5 Software (Conexio Genomics, Fremantle, Australia). DQA1 and any remaining ambiguities were resolved by sequence-specific primer typing (SSP UniTray; Invitrogen, Carlsbad, CA and Olerup-SSP; QIAGEN, Valencia, CA).
RNA isolation
Total RNA was prepared from fresh (cryopreserved) aorta tissue from AAA lesions from patients with AAA, PBMCs, CD4+ cells, or CD8+ cells using a solution of guanidinium thiocyanate (Stratagene, La Jolla, CA), as recommended by the manufacturer.
Synthesis of cDNA
cDNA was synthesized from total RNA and primed with oligo-(dT)15-NotI (Promega), using SuperScript II (Gibco/Brl), following the manufacturer’s instructions (32–37). Double-stranded cDNA was blunt ended for efficient adaptor ligation by adding T4 DNA polymerase.
Amplification by the nonpalindromic adaptor PCR/Vβ-specific PCR
Adaptor ligation and NotI digestion.
The blunt-ended cDNA was ligated at both the 5′ and 3′ blunt ends with an equivalent molar concentration of a nonpalindromic adaptor (NPA) (32–37) by incubation for 14 h at 16°C with T4 DNA ligase (Life Technologies-BRL). This adaptor, a modification of the one previously described (32–37), consists of two oligonucleotides (Supplemental Table I), the 5′-AATTCGAACCCCTTCGAGAATGCG-3′ and its complementary 3′-GCTTGGGGAAGCTCTTACGC-p-5′, preannealed to each other. The ligated adaptor was removed from the 3′ end of the double-stranded cDNA by digestion with NotI restriction endonuclease, as described (32–37), while it remained in the 5′ end. The digested cDNA was purified using a G-50 column (5′-3′, Boulder, CO), as recommended by the manufacturer.
First cycle of amplification by NPA-PCR.
NPA-PCR was carried out as previously described (32–37), with minor modifications. The NPA 5′-AATTCGAACCCCTTCGAGAATGCT-3′ was used as the 5′ amplification primer. The 3′ amplification primer hCβ3 was located in the Cβ region, starting at nucleotide 208 (Supplemental Table I). The purified NotI-digested cDNA was amplified by NPA-PCR in 100 μl, which contained, in addition to the primers and cDNA, 5 U native PFU DNA polymerase and 1 mM deoxynucleotide triphosphates in 1× buffer. The cDNA was denatured at 95°C for 5 min and amplified by 30 cycles of PCR at 94°C for 1 min, 45°C for 1 min, and 72°C for 2 min, with a final extension at 72°C for 7 min. The amplified transcripts were purified using a G-50 column, as described above.
Second cycle of amplification by individual Vβ-specific PCR.
Vβ-specific PCRs were carried out using, as a template, 4 μl β-chain TCR cDNA, which had been amplified previously by NPA-PCR, as described. Single oligonucleotides, each specific for 1 of 24 Vβ families (32–37) (Supplemental Table I), were used each as 5′ end amplification primer in 24 separate amplifications. A Cβ primer designated as hCβ2 was used as 3′ amplification primer, and it was located in the Cβ region starting at nucleotide 113, 5′ to the hCβ3 primer used for the first (NPA-PCR) amplification (nested design). This design eliminates the possible amplification of other members of the Ig supergene family, which may share homology with the β-chain TCR transcript, because it is unlikely that the same molecule, member of the Ig supergene family, has substantial homology with the β-chain TCR transcript at both the hCβ3 and hCβ2 sites. The reaction mixture of 50 μl contained, in addition to the primers and cDNA, 2.5 U native PFU DNA polymerase and 1 mM deoxynucleotide triphosphates in 1× buffer. It was denatured at 95°C for 5 min and amplified by 30 cycles of PCR at 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min, followed by a 7-min final extension at 72°C.
Vβ-specific PCR amplification
Vβ-specific PCR amplifications were carried out as described (32–37) to examine, in more detail, a single Vβ family or subfamily or to confirm the clonal expansions observed by NPA-PCR/Vβ-specific PCR. Vβ3, Vβ6, Vβ12, Vβ14, and Vβ24 families were amplified by Vβ-specific PCR. 5′ and 3′ (hCβ2) amplification primers are shown in Supplemental Table I. Template cDNA was synthesized from total RNA isolated from the same AAA specimen used for NPA-PCR/Vβ-specific PCR or from total RNA from the same specimen isolated separately. cDNA was denatured at 95°C for 5 min and amplified by 35 cycles of PCR, as described above.
Cloning and sequencing of PCR products
To reduce the workload for cloning and sequencing of the NPA-PCR/Vβ-specific PCR amplified TCR transcripts, 8 μl from each of the 24 NPA-PCR/Vβ-specific PCR products was mixed and incubated with Taq polymerase at 72°C for 10 min to add an adenine at the 3′ end. The mixture (192 μl) was size selected by agarose gel electrophoresis and purified with a GENECLEAN Kit (Bio101, Vista, CA). The purified NPA-PCR/Vβ-specific PCR or Vβ-specific PCR products were cloned into the TOPO-TA cloning vector (Invitrogen), transformed into Top10 One Shot Chemically Competent cells (Invitrogen), according to the manufacturer’s instructions, and subjected to blue-white screening. The competent cells were incubated for 30–45 min with the vector on ice, submitted to heat shock for 30–45 s at 42°C, incubated for 2 min on ice, added to 250 μl SOC medium (32–36), incubated for 1 h at 37°C, and plated onto X-gal–containing agar plates. White colonies were picked out using the PerfectPrep Plasmid Mini Kit (Eppendorf, Westbury, NY), as recommended by the manufacturer. Plasmids were sequenced by the dideoxy chain termination method on 6% polyacrylamide DNA sequencing gels using an ABI373A DNA Sequencer (Applied Biosystems). Large numbers of white colonies were obtained. Comparable numbers of TCR clones were obtained after NPA-PCR/Vβ-specific PCR and cloning and after each Vβ-specific PCR and cloning. The maximum theoretical number of unique β-chain TCR transcripts is estimated to be 1012 (38). Because this number is so large, the probability of finding, by chance, two identical copies of a single β-chain TCR transcript in an independent sample of T cells is negligible. However, during transformation of Escherichia coli, the plasmid/cell mixture was subjected to heat shock, followed by growth for 1 h in SOC medium at 37°C before plating the colonies. Under log-phase growth (ideal growth conditions), E. coli can undergo a division in 20 min, which could result in two doublings within 60 min (39). However, because of the heat shock, E. coli does not immediately enter the log phase, although the unlikely possibility for a few E. coli–transformed cells to double before plating does exist. Therefore, a doublet (i.e., identical TCR transcript sequences from two different colonies) may be the result of a single transfected E. coli that doubled before plating, or it may reveal a clonal expansion. In the statistical analysis, we addressed this issue. However, doubling of singly transfected E. coli before plating is infrequent. We amplified (by NPA-PCR, NPA-PCR/Vβ-specific PCR, or Vβ-specific PCR), cloned, and sequenced 488 β-chain TCR transcripts from PBMCs from healthy donors. All β-chain TCR transcripts were unique compared with each other, with the exception of seven β-chain TCR clones that appeared in duplicate (1.4%) (32–37).
Computer analysis and comparison of sequences
The nucleic acid sequence of TCR transcripts encoding for the V, D, J, and C region was compared with those in the National Center for Biotechnology Information databases using the standard nucleotide–nucleotide basic local alignment search tool (BLAST) program, as described (32–37). The nucleotide sequence of the N-D-N region of each β-chain TCR transcript was identified as the sequence between the last discernible Vβ nucleotide and the first discernible Jβ nucleotide. The deduced amino acid sequences in the CDR3 regions were compared with those in the National Center for Biotechnology Information nr database, using the BLAST program to search for short, nearly exact matches. There is no information on the maximum number of CDR3 amino acid differences that permit substantial CDR3 homology. Differences of two conservative and one nonconservative amino acids were chosen arbitrarily as the maximum number of differences allowed between CDR3 motifs from different T cell clones to define substantial CDR3 homology.
Statistical analysis
We used the binomial distribution (34, 36) to calculate the probability of the number (x) of the multiple copies of the identical transcripts that have been identified (x/n; n = total number of transcripts sequenced) against the alternative hypothesis that each transcript is expressed only once [i.e., all transcripts are unique when compared with each other (1/n)] or against a second alternative hypothesis that a single β-chain TCR transcript is only expressed twice, and all other transcripts sequenced are expressed only once (2/n). The alternative and the second alternative hypotheses were developed using results obtained by NPA-PCR/Vβ-specific PCR, NPA-PCR, or Vβ-specific PCR amplification of β-chain TCR transcripts from PBMCs from healthy donors, cloning, and sequencing (32–37). Each β-chain TCR transcript from these PBMC is expressed only once (all transcripts are unique when compared with each other; 1/n) or a single transcript is expressed twice, and all other β-chain TCR transcripts sequenced are expressed only once (all transcripts except one are unique when compared with each other; 2/n).
Results
T cells are infiltrating AAA lesions
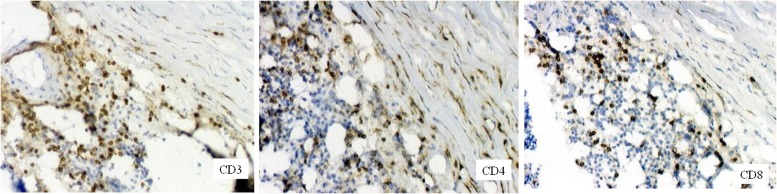
Representative pathology and immunohistochemical staining with anti-CD3, anti-CD4, or anti-CD8 mAbs of AAA tissue is shown in Fig. 1. Substantial proportions of infiltrating CD3+ T cells are present in AAA lesions and, in particular, in the adventitia and media, and they are comprised of CD4+ and CD8+ cells, as described (9–11). Grossly normal infrarenal abdominal aortic specimens obtained at autopsy from patients who died of nonvascular causes rarely showed, if any, mononuclear cell infiltrates (data not shown).
FIGURE 1.
Immunohistochemical staining of AAA tissue using anti-CD3, anti-CD4, or anti-CD8 mAbs revealed CD3+ T cell infiltrates primarily in the arterial wall, particularly in the adventitia (left panel). CD4+ (middle panel) and CD8+ (right panel) T cell infiltrates are also shown. Original magnification ×100.
Oligoclonal T cells are infiltrating AAA lesions from patients with AAA
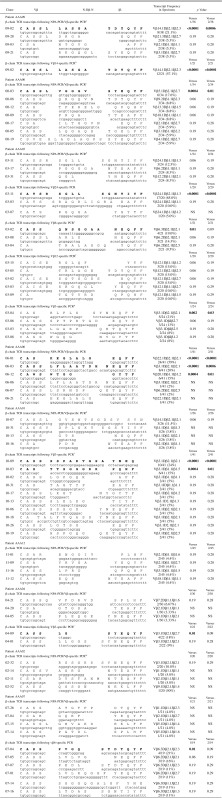
To determine whether fresh (not expanded in culture) mononuclear cells infiltrating AAA lesions contain oligoclonal T cell populations, β-chain TCR transcripts were amplified, cloned, and sequenced from 10 AAA specimens (Table I). All sequences obtained were compared with those from the GenBank database using the BLAST program. More than 97% of the sequences obtained were typical of productively rearranged human β-chain TCR transcripts, were novel, and were not reported in the GenBank database. The remaining <3% of these sequences were unproductively rearranged and were not included in the analysis. Sequence analysis revealed substantial proportions of identical β-chain TCR transcripts in AAA specimens from 8 of 10 patients, demonstrating the presence of oligoclonal populations of T cells in these aneurysmal lesions.
Sequence analysis of β-chain TCR transcripts from AAA lesions from patient AAA09 after NPA-PCR/Vβ-specific PCR revealed 8 of 38 transcripts (21%) (clone 09-02) (Vβ14.1Dβ2.1Jβ2.3) (CDR3:LASGA) (Table II). These results were statistically significant by the bimodal distribution. The probability of the appearance of the 8/38 observed identical transcripts against the alternative hypothesis, that each β-chain TCR transcript is expressed only once (1/n = 1/38), was p < 0.0001 or against the alternative hypothesis, that a single transcript is only expressed twice and all other TCR transcripts sequenced are expressed only once (2/n = 2/38), was p < 0.0006. Three other TCR clones appeared in duplicate. The remaining transcripts were unique when compared with each other (Table II). The Vβ14.1Dβ2.1Jβ2.3 clonal expansion was confirmed by Vβ14-specific PCR, followed by cloning and sequencing; 12 of 21 (57%; p < 0.0001) Vβ14 transcripts were identical to the clonally expanded 09-02 clone found by NPA-PCR/Vβ-specific PCR (Table II). The remaining transcripts were unique when compared with each other.
Table II. β-chain TCR transcripts (CDR3 region) expressed in AAA lesions of patients with AAA.
 |
The remaining 24 sequences are unique when compared with each other and are not listed here.
The remaining 9 sequences are unique when compared with each other and are not listed here.
The remaining 13 sequences are unique when compared with each other and are not listed here.
The remaining 17 sequences are unique when compared with each other and are not listed here.
The remaining 12 sequences are unique when compared with each other and are not listed here.
The remaining 7 sequences are unique when compared with each other and are not listed here.
The remaining 12 sequences are unique when compared with each other and are not listed here.
The remaining 20 sequences are unique when compared with each other and are not listed here.
The remaining 9 sequences are unique when compared with each other and are not listed here.
The remaining 37 sequences are unique when compared with each other and are not listed here.
The remaining 26 sequences are unique when compared with each other and are not listed here.
The remaining 16 sequences are unique when compared with each other and are not listed here.
The remaining 15 sequences are unique when compared with each other and are not listed here.
The remaining 17 sequences are unique when compared with each other and are not listed here.
Sequence analysis of β-chain TCR transcripts from AAA lesions from patient AAA00 after NPA-PCR/Vβ-specific PCR revealed 6 of 34 identical transcripts (18%; p = 0.0004) (clone 00-03) (Vββ.1Dβ2.1Jβ2.7) (CDR3:VGGGV) (Table II). Three clones were expressed in triplicate, three were expressed in duplicate, and the remaining 13 were unique when compared with each other.
NPA-PCR/Vβ-specific PCR of β-chain TCR transcripts from AAA lesions from patient AAA03, followed by cloning and sequencing, revealed that clone 03-11 was expressed in three copies (Vβ24.1Dβ1.1Jβ1.3) (CDR3:RSGLL) (p = 0.06), and two clones were expressed in duplicate (Table II). The remaining 17 clones were unique when compared with each other. Separate Vβ24-, Vβ6-, Vβ12-, and Vβ3-specific PCR amplifications, followed by cloning and sequencing, revealed the following, respectively, a Vβ24 clone (#03-11) accounting for 17 of 20 identical transcripts (85%; p < 0.0001) that was identical to clone 03-11 (Vβ24.1Dβ1.1Jβ1.3) (CDR3:RSGLL), previously identified by NPA-PCR/Vβ-specific PCR; a Vβ6 clone (#03-02) accounting for 4 of 21 identical transcripts (19%; p = 0.01); three Vβ12 clones expressed in triplicate (p = 0.06); and a Vβ3 clone (#03-04) accounting for 5 of 24 identical transcripts (21%; p < 0.002) (Table II).
Sequence analysis of β-chain TCR transcripts from AAA lesions from patient AAA06 after NPA-PCR/Vβ-specific PCR and cloning revealed that clone 06-01 had 24 of 41 identical transcripts (59%; p < 0.0001) (Vβ22.1Dβ2.1Jβ2.1) (CDR3:KEGLA LG); clone 06-03 had 8 of 41 identical transcripts (20%; p < 0.0001) (Vβ3.1Dβ2.1Jβ2.1) (CDR3:LFLA ATDH); and clone 06-12 had 6 of 41 identical transcripts (15%; p = 0.0004) (Vβ20.1Dβ1.1Jβ1.2) (CDR3:WTGG) (Table II).
Sequence analysis of β-chain TCR transcripts from AAA lesions from patient AAA10 after NPA-PCR/Vβ-specific PCR and cloning revealed 3 of 26 identical transcripts (11.5%; p = 0.06) (clone 10-07) (Vβ14.1Dβ2.1Jβ2.1) (CDR3:LGVSWTSGDSV) (Table II). The remaining 23 transcripts were unique when compared with each other. Vβ3-specific PCR amplification, followed by cloning and sequencing, showed 10 of 41 identical transcripts (24%; p < 0.0001) (clone 10-09) (Vβ3.1Dβ2.1Jβ2.1) (CDR3:SSPARTGSA) and 6 of 41 identical transcripts (15%; p = 0.0004) (clone 10-03) (Vβ3.1Dβ1.1Jβ1.5) (CDR3:TTSGGGRR) (Table II). Other clones were expressed in duplicate or were unique when compared with each other. Vβ14-specific PCR revealed that 3 of 21 transcripts were identical (14%; p = 0.06) (clone 10-07) (Vβ14.1Dβ2.1Jβ2.1) (CDR3:LGVSWTSG DSV) (data not shown). This clone was first identified by NPA-PCR/Vβ-specific PCR (Table II).
NPA-PCR/Vβ-specific PCR amplification of β-chain TCR transcripts from patients AAA12, AAA04, AAA02, and AAA07, followed by cloning and sequencing, did not reveal statistically significant clonal expansions (Table II). However, Vβ3-specific PCR, followed by cloning and sequencing from AAA lesions from patient AAA04, revealed 4 of 22 identical transcripts (18.2%; p = 0.014) (clone 04-09) (Vβ3.1Dβ2.1Jβ2.7) (CDR3:CASSLGSYEQYF). Vβ6-specific PCR, followed by cloning and sequencing, showed that clone 07-04 (patient AAA07) accounted for 4 of 19 identical transcripts (21%; p = 0.013) (Vβ6.3Dβ2.1Jβ2.3) (CDR3:PVGQST DTQYF). Clone 07-05 was present in triplicate (p = 0.06) (Vβ6.2Dβ2.1Jβ2.1) (VLVG NEQFF).
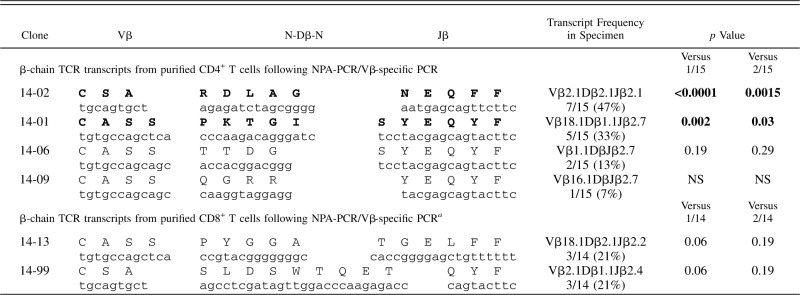
β-chain TCR transcripts from purified CD4+ and CD8+ T cells from AAA lesions from patient AAA14 were amplified by NPA-PCR/Vβ-specific PCR, followed by cloning and sequencing. Two clonal expansions were found in purified CD4+ T cells: clone 14-02 accounted for 7 of 15 identical transcripts (47%; p < 0.0001) (Vβ2.1Dβ2.1Jβ2.1) (CDR3:CSARDLAGNEQFF), and clone 14-01 accounted for 5 of 15 identical transcripts (33%; p = 0.002) (Vβ18.1Dβ1.1Jβ2.7) (CDR3:CASSPKTGISYEQYF) (Table III). These results suggest the presence of strong clonal expansions of CD4+ T cells infiltrating AAA lesions. Sequence analysis of β-chain TCR transcripts from purified CD8+ T cells from AAA lesions from patient AAA14 after NPA-PCR/Vβ-specific PCR revealed two TCR clones: clone 14-13 (Vβ18.1Dβ2.1Jβ2.2) (CDR3:CASSPYGGA) and clone 14-99 (Vβ2.1Dβ1.1Jβ2.4) (CDR3:CSASLDSWTQET). Each accounted for 3 of 14 identical transcripts (21%; p = 0.06). The remaining eight transcripts were unique (Table IV).
Table III. β-chain TCR transcripts (CDR3 region) expressed in purified CD4+ and CD8+ T cells from AAA lesions of patient AAA14.
 |
The remaining eight sequences are unique when compared with each other and are not listed here.
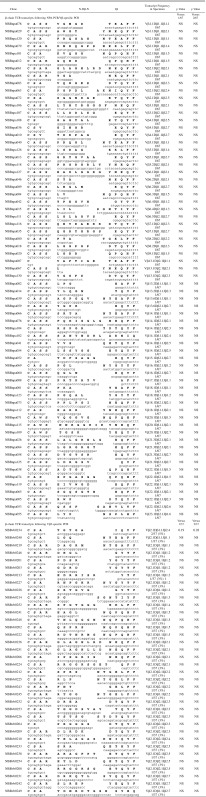
Table IV. β-chain TCR transcripts (CDR3 region) expressed in PBMCs from a healthy donor.
 |
RNA from grossly normal infrarenal abdominal aortic specimens from three patients who died of nonvascular causes were obtained at autopsy and used as controls. NPA-PCR/Vβ-specific PCR amplification did not reveal any β-chain TCR transcripts (data not shown). These results are in agreement with those of other investigators (7, 40, 41) who reported the absence of CD45+ cells or T cells from nonaneurysmal aortic tissue. We (35) reported similar results with control epicardial arteries.
PBMCs from normal donors contain polyclonal populations of T cells
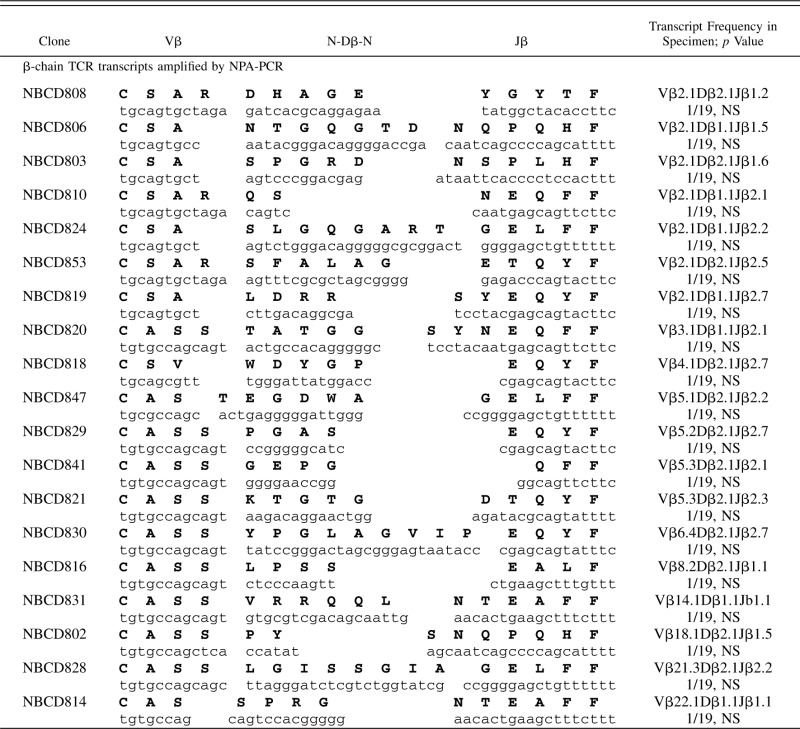
PBMCs from normal donors were used as methodological controls to make certain that all PCR amplification, cloning, and sequencing protocols were working well. Representative results of 104 β-chain TCR transcripts are shown after NPA-PCR/Vβ-specific PCR (67 β-chain TCR transcripts) or Vβ-specific PCR (37 β-chain TCR transcripts), followed by cloning and sequencing (Table IV). These sequences were unique when compared with each other, except for one that appeared in duplicate, and are typical of polyclonal populations of T cells, in agreement with previous reports (32–37). Similar results were obtained with a few hundred T cell clones (32–37). β-chain TCR transcripts from purified peripheral blood CD4+ cells (Table V) and CD8+ cells (Table VI) from a normal donor were amplified by NPA-PCR. Sequence analysis revealed unique transcripts when compared with each other, with the exception of one clone that appeared in duplicate (Table V).
Table V. β-chain TCR transcripts (CDR3 region) expressed in purified CD4+ T cells from PBMCs of a healthy donor.
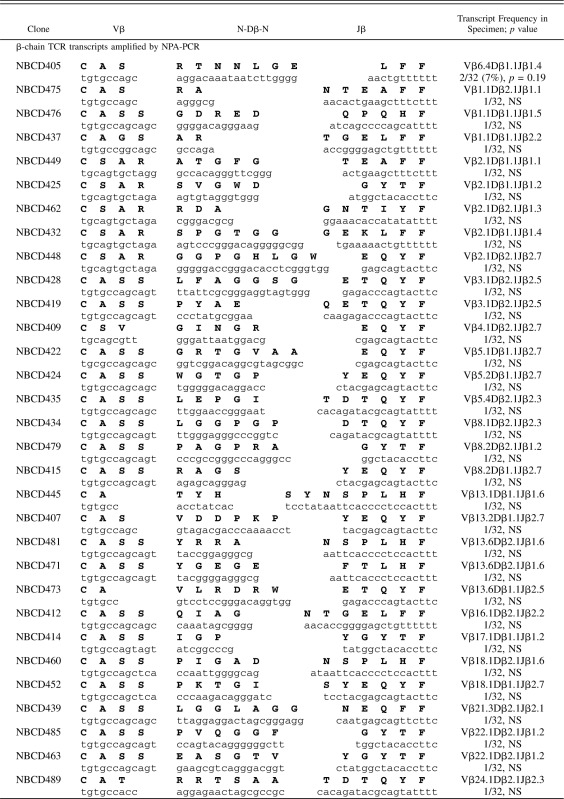 |
Table VI. β-chain TCR transcripts (CDR3 region) expressed in purified CD8+ T cells from PBMCs of a healthy donor.
 |
Control studies reveal that the results obtained represent true clonal expansions of T lymphocytes
It could be argued that if TCR transcript amplification by two PCR cycles is carried out from very few T cells, it is possible that each pair of primers will amplify signals from only a few T cells, providing results that may resemble those in Table II. We demonstrated that this is not the case; our results represent true clonal expansions of T cells and are not due to amplification of TCR transcripts from just a few T cells.
First, each aorta biopsy from patients with AAA was divided into two fractions; one was used for histology and immunohistochemistry, and the other was used for RNA preparation. RNA was isolated with a yield of ∼10 μg/preparation, which represents ∼1.0 × 107 cells. From this RNA we used 50 ng for PCR amplification, which represents ∼5.0 × 104 cells. It should be emphasized that the representation of the TCR clonotypes does not change between the sample of 10 μg of RNA and the sample of 50 ng that we used for PCR amplification, cloning, and sequencing. The TCR clonotypes, particularly the expanded ones present in 10 μg of RNA, are also present in the 50 ng of RNA. The ratio of the various TCR clonotypes to each other does not change. What does change when different amounts of RNA are used is the absolute number of the TCR copies present. Second, we determined the number of T cells present in aorta specimens used for RNA preparations from two patients with AAA (AAA09 and AAA10) by immunohistochemical staining using an anti-CD3 mAb. The number of CD3+ T cells varies significantly, ranging from 0 to 155 for each high-power field. Twenty high-power fields were counted in each specimen by two persons independently. The average number of CD3+ T cells was ∼780/section in specimen AAA09 and ∼660/section in specimen AAA10. Given the thickness of aorta biopsy specimens of ∼5 mm and that the cryostat sections of aorta biopsies used for the immunohistochemical determinations were 6-μm-thick each, the total number of CD3+ T cells used for RNA isolation in specimens AAA09 and AAA10 was estimated to be 6.5 × 105 and 5.5 × 105, respectively. Because 10 μg of RNA (representing ∼1 × 107 cells) was recovered per preparation, CD3+ T cells in these specimens accounted for 6.5 and 5.5%, respectively (mean 6.0%), of the total cells used for RNA isolation. Fifty nanograms of RNA (representing ∼5 × 104 cells) was used for PCR amplification. Approximately 6.0% of these cells (i.e., ∼3000 cells) were CD3+ T lymphocytes in these amplification reactions.
Control experiments were conducted to detect the threshold of starting T cell numbers that will give polyclonal sequencing results after two PCR cycles. Sequence analysis, after NPA-PCR amplification and cloning with various amounts of template, revealed the presence of unique transcripts when compared with each other, with the exception of two doublets (not statistically significant), which is typical of polyclonal T cells, when the starting T cell number was as low as 300 (data not shown). These cells were 10 times lower than those present in AAA specimens used in the experiments reported in this study (i.e., 3000 T cells).
A similar strategy was used for Vβ-specific amplification, followed by cloning and sequencing (data not shown). Sequence analysis, from the mixture that contained as few as 1200 T cells, corresponding to 100 Vβ2+ T cells in 50 ng of RNA, revealed the presence of unique Vβ2+ TCR transcripts when compared with each other, with the exception of two doublets (not statistically significant), which is typical of polyclonal populations of T cells. The T cell number (1200) was lower than that present (3000 cells) in 50 ng of RNA from AAA specimens. From the mixture, which contained only 300 T cells or 24 Vβ2+ T cells, a more restricted pattern was observed (not statistically significant), consisting of one Vβ2+ transcript that appeared in triplet, four transcripts that appeared in doublets, and eight other transcripts that appeared as a single copy (data not shown). These results confirmed that the clonality of T cells in AAA lesions was due to real clonal expansions and not to amplifications of TCR transcripts from just a few T cells.
DNA-based HLA typing for HLA-DRB1, HLA-DQA1, and HLA-DQB1
Six of 10 patients with AAA were typed by DNA-based HLA typing for HLA-DRB1, HLA-DQA1, and HLA-DQB1 (Table VII). Five of these six patients—AAA02, AAA03, AAA04, AAA09, and AAA10—expressed DRB1 alleles positive for the DRβGln70 amino acid residue (Table VII), which was reported to be associated with AAA (13). Clonally expanded T cells in AAA lesions were found in five (AAA03, AAA04, AAA06, AAA09, and AAA10) of these six patients (Table II). These included four patients who expressed DRβGln70 (AAA03, AAA04, AAA09, and AAA10) and one who did not (AAA06) (Table VII).
Table VII. DNA-based typing for HLA-DRB1, HLA-DQA1, and HLA-DQB1 loci of patients with AAA.
| Sample | DRB1-1 | DRB1-2 | DQA1-1 | DQA1-2 | DQB1-1 | DQB1-2 | DRβQ70 |
|---|---|---|---|---|---|---|---|
| AAA02 | 03:01 | 11:04 | 05:01 | 05:05 | 02:01 | 03:01 | + |
| AAA03 | 03:01 | 01:01 | 01:01 | 05:01 | 02:01 | 05:01 | + |
| AAA04 | 04:01 | 07:01 | 02:01 | 03:01 | 02:02 | 03:02 | + |
| AAA06 | 07:01 | 12:01 | 02:01 | 05:05 | 03:01 | 03:03 | − |
| AAA09 | 01:01 | 07:01 | 01:01 | 02:01 | 02:02 | 05:01 | + |
| AAA10 | 03:01 | 15:01 | 01:02 | 05:01 | 02:01 | 06:02 | + |
Conserved CDR3 amino acid motifs
In addition to clonally expanded TCR transcripts, several conserved CDR3 amino acid motifs (GA, GL, LA, SG, and SR) were expressed in statistically significantly higher proportions in CDR3s of patients with AAA than in CDR3s of PBMCs from healthy donors (Table VIII). Sequences from 333 β-chain TCR transcripts from PBMCs of seven healthy donors (this study and 32–36) were used as normal controls.
Table VIII. CDR3 TCR-conserved amino acid motifs found in AAA lesions of patients with AAA.
| CDR3 Motif | Patient | Normal PBMCs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AAA00 | AAA02 | AAA03 | AAA04 | AAA06 | AAA07 | AAA09 | AAA10 | AAA12 | AAA14 | ||
| GA | 3/34 (8.8%) | 0/20 (0%) | 16/109 (14.7%) | 7/73 (9.6%) | 0/41 (0%) | 4/40 (10.0%) | 25/59 (42.4%) | 7/88 (8.0%) | 8/45 (17.8%) | 0/29 (0%) | 20/333 (6.0%) |
| GG | 10/34 (29.4%) | 3/20 (15.0%) | 16/109 (14.7%) | 11/73 (15.1%) | 6/41 (14.6%) | 11/40 (27.5%) | 4/59 (6.8%) | 16/88 (18.2%) | 12/45 (26.7%) | 2/29 (6.9%) | 48/333 (14.4%) |
| GL | 4/34 (11.8%) | 3/20 (15.0%) | 29/109 (26.6%) | 7/73 (9.6%) | 25/41 (61.0%) | 0/40 (0%) | 2/59 (3.4%) | 2/88 (2.3%) | 2/45 (4.4%) | 0/29 (0%) | 22/333 (6.6%) |
| GV | 8/34 (23.5%) | 0/20 (0%) | 3/109 (2.8%) | 4/73 (5.5%) | 0/41 (0%) | 0/40 (0%) | 4/59 (6.8%) | 7/88 (8.0%) | 4/45 (8.9%) | 0/29 (0%) | 13/333 (3.9%) |
| LA | 2/34 (5.9%) | 1/20 (5.0%) | 13/109 (11.9%) | 4/73 (5.5%) | 34/41 (82.9%) | 1/40 (2.5%) | 23/59 (39.0%) | 2/88 (2.3%) | 0/45 (0%) | 7/29 (24.1%) | 20/333 (6.0%) |
| RG | 7/34 (20.6%) | 6/20 (30.0%) | 13/109 (11.9%) | 9/73 (12.3%) | 0/41 (0%) | 2/40 (5.0%) | 8/59 (13.6%) | 6/88 (6.9%) | 3/45 (6.7%) | 0/29 (0%) | 26/333 (7.8%) |
| SG | 4/34 (11.8%) | 4/20 (20.0%) | 34/109 (31.2%) | 4/73 (5.5%) | 0/41 (0%) | 7/40 (17.5%) | 26/59 (44.1%) | 27/88 (30.7%) | 6/45 (13.3%) | 1/29 (3.8%) | 34/333 (10.2%) |
| SP | 2/34 (5.9%) | 3/20 (15.0%) | 3/109 (2.8%) | 10/73 (13.7%) | 0/41 (0%) | 10/40 (25.0%) | 3/59 (5.1%) | 17/88 (19.3%) | 6/45(13.3%) | 9/29 (31.0%) | 29/333 (8.7%) |
| SR | 3/34 (8.8%) | 6/20 (30.0%) | 32/109 (29.4%) | 9/73 (12.3%) | 1/41 (2.4%) | 5/40 (12.5%) | 7/59 (11.9%) | 4/88 (4.5%) | 1/45 (2.2%) | 2/29 (6.9%) | 21/333 (6.3%) |
Statistical analysis was performed for AAA versus healthy PBMC. GA, p = 0.0006; GG, p = 0.3192; GL, p = 0.00007; GV, p = 0.2585; LA, p ≤ 0.0001; RG, p = 0.2600; SG, p ≤ 0.0001; SP, p = 0.1565; and SR, p =0.0012.
Comparison of the nucleic acid and deduced amino acid sequences with those in the GenBank/European Molecular Biology Laboratory database
Comparison of all sequences obtained in this study with those in the GenBank/European Molecular Biology Laboratory database using BLAST software revealed that they are novel. There were no cases of identical β-chain TCR transcripts appearing in different AAA patients. However, analysis of the CDR3 motifs in the clonally expanded sequences, using the gapped BLAST and PSI-BLAST protein database search programs, revealed substantial homologies between the β-chain TCR transcripts in AAA patients and those in the GenBank/European Molecular Biology Laboratory database. The clonally expanded clone 09-02 (CDR3:CASSLLASGATDTQYF) from patient AAA09 shared similar CDR3 sequences with an alloreactive human T cell clone (GenBank GI no. 930056; CDR3:CASSFLAAGVADTQYF) and a human T cell clone of unknown specificity (GenBank GI no. 16566875; CDR3:CASSEVASGTDTQYF). Clone 09-25 (CDR3:CASTPLAAGSGNTIYF) exhibited substantial CDR3 homology to a T cell clone (GenBank GI no. 3859306; CDR3:CASRGGQGASYEQYF) derived from synovial fluid from patients with rheumatoid arthritis (RA). Clone 02-32 (CDR3:CASRSSGKSSYNEQFF) from patient AAA02 was homologous to a human T cell clone (GenBank GI no. 10304701; CDR3:CASRRSRSSYNEQFF) reported from our laboratory (35), indicating that they may recognize similar antigenic determinants located in vascular tissue. The CDR3 of clone 03-31 (CDR3:CASSQGLYNEQFF) from patient AAA03 was substantially homologous to the CDR3 of a T cell clone (GenBank GI no. 12751193; CDR3: CASSQGGYNEQFF) isolated from active psoriatic arthritis joint fluid. Clone 03-17 (CDR3:CASSPGGGGANTEAFF) shared substantial homology with a T cell clone (GenBank GI no. 13249236; CDR3:CAWSRGGIGLNTEAFF) that has anti-snRNP activity. The clonally expanded clone 00-03 (CDR3:CASSLVGGGVSYEQYF) from AAA00 shared substantial homology with a T cell clone (GenBank GI no. 241751; CDR3:CASSLTTGGGYEQYF) reactive to superantigen staphylococcal enterotoxins. CDR3s of the remaining clonally expanded TCR transcripts were not highly homologous to T cell clones retrieved from the database.
Discussion
We report the presence of statistically significant clonal expansions of T cells infiltrating AAA lesions of patients with AAA. PCR amplification, followed by cloning and sequencing, revealed the presence of multiple identical copies of β-chain TCR transcripts in AAA lesions from 8 of 10 patients, demonstrating the presence of monoclonal or oligoclonal populations of T cells. Additional studies need to be carried out to determine whether clonally expanded T cell populations are present in the peripheral blood of patients with AAA, in addition to their presence in AAA lesions.
T cells are comprised of many different T cell clones. Each one of them expresses a unique TCR on the cell surface that serves as a unique fingerprint of that particular T cell clone (38). Each T cell clone recognizes a different antigenic epitope through its unique TCR. The TCR repertoire is very large. The maximum number of different T cell clones expressing αβ TCR was estimated to be on the order of 1018 (38). The maximum theoretical number of different β-chain TCR transcripts is 1012 (38). However, only a small portion of these cells survive thymic selection and became mature T lymphocytes. For this reason, the size of the T cell repertoire in the peripheral blood was estimated to be 106 different β-chain TCR polypeptides, and each one of them pairs with ≥25 different α-chain TCR polypeptides (reviewed in Ref. 27). The number of T cell clones is very large and sufficient to recognize all possible antigenic epitopes. Because of the large size of the T cell repertoire, the probability of finding, by chance, multiple identical copies of α- or β-chain TCR transcripts in an independent sample of T cells is negligible. Therefore, the presence of multiple identical copies of α- or β-chain TCR transcripts must be the result of specific Ag–driven proliferation and clonal expansion in response to unidentified self- or nonself Ag(s).
Our studies support the hypothesis that AAA is a specific Ag–driven T cell disease. We used sequencing analysis of TCR transcripts after PCR amplification and cloning to test the hypothesis that mononuclear cells infiltrating AAA lesions contain monoclonal or oligoclonal populations of T cells. Clonal expansions of β-chain TCR transcripts were identified using NPA-PCR/Vβ-specific PCR, followed by cloning and sequencing. These results were confirmed by an independent amplification method, two-sided Vβ-specific PCR, followed by cloning and sequencing, and identical clonal expansions were obtained (Table II). Also, α-chain TCR transcripts in AAA lesions were clonally expanded (unpublished results). Preferential usage of Vβ22 and Vβ25 was reported (42) in aneurysmic lesions from 10 of 14 patients with Marfan syndrome or familial thoracic aortic aneurysms, as well as in patients with sporadic thoracic aortic aneurysms.
Several lines of evidence suggest that AAA is an autoimmune disease. Mononuclear cell infiltrates, comprised primarily of T cells and monocytes, have been reported in AAA lesions (7–9). At this time, it is not known whether more infiltrating T cells are present in AAA lesions containing clonally expanded T cells (8 of 10 patients) versus in AAA lesions containing polyclonal T cells (2 of 10 patients). We have previously shown that higher numbers of T cells infiltrating in vivo ovarian carcinoma human tumors correlate well with HLA class I expression on ovarian tumor cells and the ability of these T cells to expand in vitro in culture with rIL-2 to T cell lines with potential antitumor activity (43). These results suggest the presence of higher numbers of infiltrating T cells in these tumors in the presence of an immune response under conditions of chronic inflammation. Many of these infiltrates express early (CD69), intermediate (CD25, CD38), and late (CD45RO, HLA class II) activation Ags, demonstrating the presence of an ongoing immune response in AAA lesions (9) where the production of Th1 and Th2 cytokines has been reported (24–26). The role of Th1 and Th2 cytokines in the pathogenesis of AAA remains to be elucidated (reviewed in Ref. 6). γδ TCR+ T cells also were clonally expanded in AAA lesions, and this includes the various forms of γδ TCR+ T cells previously reported (9, 44). The frequency and the suppressor activity of CD4+CD25+FOXP3+ T cells in the peripheral blood of patients with AAA were significantly lower versus with those in patients with abdominal aortic atherosclerotic occlusive disease or healthy donors (45). These results demonstrate impaired immunoregulation in AAA, which may play a role in the pathogenesis of the disease. Elevated levels of CD4+CD28− cells were found in the peripheral blood and AAA lesions of patients with AAA (46). These T cells produce high levels of IFN-γ and perforin and may be responsible, among other cell populations, for causing injury to the aorta. APCs, such as dendritic cells, monocytes, and B cells, are present (7–9) in this chronic inflammatory environment and play a critical role in the propagation of the disease. This chronic inflammation is typical of that observed in autoimmune diseases (47, 48) and the immune response to tumors (49).
Along the same lines and of considerable significance is the association of particular HLA class I (HLA-A2, HLA-B61) and class II (HLA-DRB1*02, HLA-DRB1*04) alleles with AAA (10, 11, 50). Six of 10 patients with AAA who were included in this study were typed for HLA-DRB1, HLA-DQA1, and HLA-DQB1 loci using DNA-based approaches. Five of these six patients expressed DRB1 alleles positive for the DRβGln[70] amino acid residue, which is associated with inflammatory AAA (11). Amino acid residue 70, together with amino acids in positions 67, 71, and 74, form an important peptide-binding pocket (#4) in HLA-DRB1 (51, 52). The shared epitope sequence at position 70–74 of HLA-DRB1 alleles is strongly associated with susceptibility to RA (53–56). Specifically, the shared epitope Arg-Ala-Ala sequence at positions 72–74 of the HLA-DRB1 alleles is associated with a high risk for developing RA, and this risk is modulated by amino acids in positions 70 and 71. Gln or Arg in position 70 and Lys in position 71 confer the highest risk (53–56). The association of amino acid Gln in position 70 with susceptibility to RA, and the presence of Gln in position 70 of HLA-DRB1 alleles in patients with AAA, provides an additional argument that AAA is an autoimmune disease. Amino acid 70 is located in a critical position at the entrance of pocket 4, affecting the binding of the αβ TCR to the HLA-DRB1:peptide complex. Replacement of the negatively charged Asp, which is usually present in position 70 in many HLA-DRB1 alleles and favors interactions with peptide side chains that are positively charged, by the noncharged amino acid Gln radically changes the binding characteristics of the HLA-DRB1 site. The studies reported in this article may provide the basis for the identification of the three molecular entities responsible for eliciting immune responses in AAA: namely, the αβ TCR, HLA-DRβGln[70], and the AAA-associated peptides.
As mentioned earlier, a number of putative AAA Ags of self origin (reviewed in Refs. 4, 12–18) and nonself origin (19–22) have been identified. Initiation and propagation of AAA are the two phases of the disease that may be driven by different, although cross-reactive, antigenic determinants. Production of IFN-γ by CD4+ T cells in a murine model of AAA (25) and our results support a critical role for a specific Ag–driven immune response in the pathogenesis of AAA. It is possible that AAA is initiated by an immune response against a nonself antigenic epitope of a microorganism, such as those mentioned above, which cross-reacts with an epitope of a self-Ag (12, 13, 17, 21, 57, 58). After the microorganism is cleared, the immune response is propagated by molecular mimicry in response to cross-reacting antigenic epitope(s) of self-Ag(s) of the host. Molecular mimicry is defined as the sharing of cross-reactive antigenic epitopes between microorganisms and host Ags (23), and it is responsible for a number of diseases.
Although the clonal expansions of T cells in AAA lesions that we report in this article may not provide the necessary proof that AAA is initiated by a specific Ag–driven T cell response, they do provide strong evidence suggesting that a specific Ag–driven T cell response is critical for the propagation of the disease. Again, this type of immune response may involve molecular mimicry. It could be argued that the clonal expansions of T cells that we found in AAA lesions may represent an immune response to matrix degradation products present in AAA lesions and that this immune response may be a less important epiphenomenon in the pathogenesis of AAA. This possibility appears unlikely. T cells recognize peptides in association with self-MHC, and self-antigenic determinants do not necessarily require degradation of the matrix to be presented to self T cells. T cell clones that could recognize self-determinants are eliminated from the T cell repertoire during thymic selection. It is more likely that molecular mimicry mechanisms (reviewed in Ref. 23) are responsible for the T cell clonal expansions that we report in this article. Because these T cell clones may recognize cross-reactive antigenic epitopes between microorganisms and host Ags, they may escape elimination during thymic selection.
The presence of monoclonal/oligoclonal T cells infiltrating AAA lesions provides strong evidence that AAA is likely a specific Ag–driven autoimmune T cell disease. The identification of the clonally expanded TCR transcripts in these AAA lesions may permit the identification of the self or nonself Ags recognized by these clonally expanded T cells in AAA. Critical evidence for increasing our understanding of the pathogenesis of the disease may be provided by the identification of the Ag(s) recognized by the clonally expanded T cells in AAA lesions. These Ags may play a key role in the initiation and/or the propagation of the disease.
Supplementary Material
This work was supported in part by National Institutes of Health Grant R01 HL64340 and by the Ralph and Marian Falk Foundation.
The online version of this article contains supplemental material.
- AAA
- abdominal aortic aneurysm
- BLAST
- basic local alignment search tool
- NPA
- nonpalindromic adaptor
- RA
- rheumatoid arthritis.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.van der Vliet J. A., Boll A. P. 1997. Abdominal aortic aneurysm. Lancet 349: 863–866. [DOI] [PubMed] [Google Scholar]
- 2.Beckman J. A. 2006. Aortic aneurysms: pathophysiology, epidemiology, and prognosis. In Vascular Medicine. Creager M. A., Dzau V. J., Loscalzo J., eds. Saunders, Elsevier, Philadelphia, p. 543–559. [Google Scholar]
- 3.Stanley J. C., Barnes R. W., Ernst C. B., Hertzer N. R., Mannick J. A., Moore W. S. 1996. Vascular surgery in the United States: workforce issues. Report of the Society for Vascular Surgery and the International Society for Cardiovascular Surgery, North American Chapter, Committee on Workforce Issues. J. Vasc. Surg. 23: 172–181. [DOI] [PubMed] [Google Scholar]
- 4.Kuivaniemi H., Platsoucas C. D., Tilson M. D., III 2008. Aortic aneurysms: an immune disease with a strong genetic component. Circulation 117: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wassef M., Baxter B. T., Chisholm R. L., Dalman R. L., Fillinger M. F., Heinecke J., Humphrey J. D., Kuivaniemi H., Parks W. C., Pearce W. H., et al. 2001. Pathogenesis of abdominal aortic aneurysms: a multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. J. Vasc. Surg. 34: 730–738. [DOI] [PubMed] [Google Scholar]
- 6.White J. V., Ryjewski C., Trinidad M., Rosenblum J., Platsoucas C. D. 2007. Aortic aneurysm: search for the trigger. Ann. Vasc. Surg. 21: 292–295. [DOI] [PubMed] [Google Scholar]
- 7.Koch A. E., Haines G. K., Rizzo R. J., Radosevich J. A., Pope R. M., Robinson P. G., Pearce W. H. 1990. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am. J. Pathol. 137: 1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce W. H., Koch A. E. 1996. Cellular components and features of immune response in abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 800: 175–185. [DOI] [PubMed] [Google Scholar]
- 9.Platsoucas C. D., Lu S., Nwaneshiudu I., Solomides C., Agelan A., Ntaoula N., Purev E., Li L. P., Kratsios P., Mylonas E., et al. 2006. Abdominal aortic aneurysm is a specific antigen-driven T cell disease. Ann. N. Y. Acad. Sci. 1085: 224–235. [DOI] [PubMed] [Google Scholar]
- 10.Tilson M. D., Ozsvath K. J., Hirose H., Xia S. 1996. A genetic basis for autoimmune manifestations in the abdominal aortic aneurysm resides in the MHC class II locus DR-beta-1. Ann. N. Y. Acad. Sci. 800: 208–215. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen T. E., Hallett J. W., Jr., Metzger R. L., Richardson D. M., Harmsen W. S., Goronzy J. J., Weyand C. M. 1997. Genetic risk factors in inflammatory abdominal aortic aneurysms: polymorphic residue 70 in the HLA-DR B1 gene as a key genetic element. J. Vasc. Surg. 25: 356–364. [DOI] [PubMed] [Google Scholar]
- 12.Gregory A. K., Yin N. X., Capella J., Xia S., Newman K. M., Tilson M. D. 1996. Features of autoimmunity in the abdominal aortic aneurysm. Arch. Surg. 131: 85–88. [DOI] [PubMed] [Google Scholar]
- 13.Xia S., Ozsvath K., Hirose H., Tilson M. D. 1996. Partial amino acid sequence of a novel 40-kDa human aortic protein, with vitronectin-like, fibrinogen-like, and calcium binding domains: aortic aneurysm-associated protein-40 (AAAP-40) [human MAGP-3, proposed]. Biochem. Biophys. Res. Commun. 219: 36–39. [DOI] [PubMed] [Google Scholar]
- 14.Haas K. S., Phillips S. J., Comerota A. J., White J. V. 1991. The architecture of adventitial elastin in the canine infrarenal aorta. Anat. Rec. 230: 86–96. [DOI] [PubMed] [Google Scholar]
- 15.White J. V., Haas K., Phillips S., Comerota A. J. 1993. Adventitial elastolysis is a primary event in aneurysm formation. J. Vasc. Surg. 17: 371–380, discussion 380–381. [DOI] [PubMed] [Google Scholar]
- 16.Reilly J. M., Brophy C. M., Tilson M. D. 1992. Characterization of an elastase from aneurysmal aorta which degrades intact aortic elastin. Ann. Vasc. Surg. 6: 499–502. [DOI] [PubMed] [Google Scholar]
- 17.Tilson M. D. 1995. Similarities of an autoantigen in aneurysmal disease of the human abdominal aorta to a 36-kDa microfibril-associated bovine aortic glycoprotein. Biochem. Biophys. Res. Commun. 213: 40–43. [DOI] [PubMed] [Google Scholar]
- 18.Stemme S., Faber B., Holm J., Wiklund O., Witztum J. L., Hansson G. K. 1995. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 92: 3893–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juvonen J., Juvonen T., Laurila A., Alakärppä H., Lounatmaa K., Surcel H. M., Leinonen M., Kairaluoma M. I., Saikku P. 1997. Demonstration of Chlamydia pneumoniae in the walls of abdominal aortic aneurysms. J. Vasc. Surg. 25: 499–505. [DOI] [PubMed] [Google Scholar]
- 20.Halme S., Juvonen T., Laurila A., Juvonen J., Mosorin M., Saikku P., Surcel H. M. 1999. Chlamydia pneumoniae reactive T lymphocytes in the walls of abdominal aortic aneurysms. Eur. J. Clin. Invest. 29: 546–552. [DOI] [PubMed] [Google Scholar]
- 21.Ozsvath K. J., Hirose H., Xia S., Tilson M. D. 1996. Molecular mimicry in human aortic aneurysmal diseases. Ann. N. Y. Acad. Sci. 800: 288–293. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka S., Komori K., Okadome K., Sugimachi K., Mori R. 1994. Detection of active cytomegalovirus infection in inflammatory aortic aneurysms with RNA polymerase chain reaction. J. Vasc. Surg. 20: 235–243. [DOI] [PubMed] [Google Scholar]
- 23.Oleszak E. L., Chang J. R., Jr., Friedman H., Katsetos C. D., Platsoucas C. D. 2004. Theiler’s virus infection: a model for multiple sclerosis. Clin. Microbiol. Rev. 17: 174–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönbeck U., Sukhova G. K., Gerdes N., Libby P. 2002. T(H)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am. J. Pathol. 161: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong W., Zhao Y., Prall A., Greiner T. C., Baxter B. T. 2004. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J. Immunol. 172: 2607–2612. [DOI] [PubMed] [Google Scholar]
- 26.Galle C., Schandené L., Stordeur P., Peignois Y., Ferreira J., Wautrecht J. C., Dereume J. P., Goldman M. 2005. Predominance of type 1 CD4+ T cells in human abdominal aortic aneurysm. Clin. Exp. Immunol. 142: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Platsoucas C. D., Oleszak E. L. 2007. Human autoimmune diseases are specific antigen-driven T-cell diseases: identification of the antigens. Immunol. Res. 38: 359–372. [DOI] [PubMed] [Google Scholar]
- 28.Sakkas L. I., Johanson N. A., Scanzello C. R., Platsoucas C. D. 1998. Interleukin-12 is expressed by infiltrating macrophages and synovial lining cells in rheumatoid arthritis and osteoarthritis. Cell. Immunol. 188: 105–110. [DOI] [PubMed] [Google Scholar]
- 29.Xu B., Sakkas L. I., Slachta C. A., Goldman B. I., Jeevanandam V., Oleszak E. L., Platsoucas C. D. 2001. Apoptosis in chronic rejection of human cardiac allografts. Transplantation 71: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 30.Kunicka J. E., Platsoucas C. D. 1988. Defective helper function of purified T4 cells and excessive suppressor activity of purified T8 cells in patients with B-cell chronic lymphocytic leukemia. T4 suppressor effector cells are present in certain patients. Blood 71: 1551–1560. [PubMed] [Google Scholar]
- 31.Bunin N., Aplenc R., Iannone R., Leahey A., Grupp S., Monos D., Pierson G. 2005. Unrelated donor bone marrow transplantation for children with severe aplastic anemia: minimal GVHD and durable engraftment with partial T cell depletion. Bone Marrow Transplant. 35: 369–373. [DOI] [PubMed] [Google Scholar]
- 32.Oleszak E. L., Lin W. L., Legido A., Melvin J., Hardison H., Hoffman B. E., Katsetos C. D., Platsoucas C. D. 2001. Presence of oligoclonal T cells in cerebrospinal fluid of a child with multiphasic disseminated encephalomyelitis following hepatitis A virus infection. Clin. Diagn. Lab. Immunol. 8: 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P. F., Platsoucas C. D. 1992. Development of the non-palindromic adaptor polymerase chain reaction (NPA-PCR) for the amplification of alpha- and beta-chain T-cell receptor cDNAs. Scand. J. Immunol. 35: 539–549. [DOI] [PubMed] [Google Scholar]
- 34.Lin W. L., Fincke J. E., Sharer L. R., Monos D. S., Lu S., Gaughan J., Platsoucas C. D., Oleszak E. L. 2005. Oligoclonal T cells are infiltrating the brains of children with AIDS: sequence analysis reveals high proportions of identical β-chain T-cell receptor transcripts. Clin. Exp. Immunol. 141: 338–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slachta C. A., Jeevanandam V., Goldman B., Lin W. L., Platsoucas C. D. 2000. Coronary arteries from human cardiac allografts with chronic rejection contain oligoclonal T cells: persistence of identical clonally expanded TCR transcripts from the early post-transplantation period (endomyocardial biopsies) to chronic rejection (coronary arteries). J. Immunol. 165: 3469–3483. [DOI] [PubMed] [Google Scholar]
- 36.Pappas J., Jung W. J., Barda A. K., Lin W. L., Fincke J. E., Purev E., Radu M., Gaughan J., Helm C. W., Hernandez E., et al. 2005. Substantial proportions of identical βετα-chain T-cell receptor transcripts are present in epithelial ovarian carcinoma tumors. Cell. Immunol. 234: 81–101. [DOI] [PubMed] [Google Scholar]
- 37.Sakkas L. I., Xu B., Artlett C. M., Lu S., Jimenez S. A., Platsoucas C. D. 2002. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J. Immunol. 168: 3649–3659. [DOI] [PubMed] [Google Scholar]
- 38.Boehm T., Rabbitts T. H. 1989. The human T cell receptor genes are targets for chromosomal abnormalities in T cell tumors. FASEB J. 3: 2344–2359. [DOI] [PubMed] [Google Scholar]
- 39.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166: 557–580. [DOI] [PubMed] [Google Scholar]
- 40.Forester N. D., S. M. Cruickshank, D.J. Scott, and S.R. Carding. 2005. Functional characterization of T cells in abdominal aortic aneurysms. Immunology 115: 262–270. [DOI] [PMC free article] [PubMed]
- 41.Henderson E. L., Geng Y. J., Sukhova G. K., Whittemore A. D., Knox J., Libby P. 1999. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation 99: 96–104. [DOI] [PubMed] [Google Scholar]
- 42.He R., Guo D. C., Sun W., Papke C. L., Duraisamy S., Estrera A. L., Safi H. J., Ahn C., Buja L. M., Arnett F. C., et al. 2008. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J. Thorac. Cardiovasc. Surg. 136: 922–929, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kooi S., Zhang H. Z., Patenia R., Edwards C. L., Platsoucas C. D., Freedman R. S. 1996. HLA class I expression on human ovarian carcinoma cells correlates with T-cell infiltration in vivo and T-cell expansion in vitro in low concentrations of recombinant interleukin-2. Cell. Immunol. 174: 116–128. [DOI] [PubMed] [Google Scholar]
- 44.Seki H., Nanno M., Chen P.-F., Itoh K., Ioannides C., Good R. A., Platsoucas C. D. 1989. Molecular heterogeneity of gamma delta T-cell antigen receptors expressed by CD4− CD8− T-cell clones from normal donors: both disulfide- and non-disulfide-linked receptors are delta TCS1+. Proc. Natl. Acad. Sci. USA 86: 2326–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin M., Zhang J., Wang Y., Wang S., Böckler D., Duan Z., Xin S. 2010. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 30: 1825–1831. [DOI] [PubMed] [Google Scholar]
- 46.Duftner C., Seiler R., Klein-Weigel P., Göbel H., Goldberger C., Ihling C., Fraedrich G., Schirmer M. 2005. High prevalence of circulating CD4+CD28− T-cells in patients with small abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 25: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 47.Sakkas L. I., Chikanza I. C., Platsoucas C. D. 2006. Mechanisms of Disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat. Clin. Pract. Rheumatol. 2: 679–685. [DOI] [PubMed] [Google Scholar]
- 48.Sakkas L. I., Platsoucas C. D. 2007. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum. 56: 409–424. [DOI] [PubMed] [Google Scholar]
- 49.Platsoucas C. D., Fincke J. E., Pappas J., Jung W. J., Heckel M., Schwarting R., Magira E., Monos D., Freedman R. S. 2003. Immune responses to human tumors: development of tumor vaccines. Anticancer Res. 23(3A): 1969–1996. [PubMed] [Google Scholar]
- 50.Rasmussen T. E., Hallett J. W., Jr., Schulte S., Harmsen W. S., O’Fallon W. M., Weyand C. M. 2001. Genetic similarity in inflammatory and degenerative abdominal aortic aneurysms: a study of human leukocyte antigen class II disease risk genes. J. Vasc. Surg. 34: 84–89. [DOI] [PubMed] [Google Scholar]
- 51.Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. 1994. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature 368: 215–221. [DOI] [PubMed] [Google Scholar]
- 52.Zerva L., Cizman B., Mehra N. K., Alahari S. K., Murali R., Zmijewski C. M., Kamoun M., Monos D. S. 1996. Arginine at positions 13 or 70-71 in pocket 4 of HLA-DRB1 alleles is associated with susceptibility to tuberculoid leprosy. J. Exp. Med. 183: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe Y., Tokunaga K., Matsuki K., Takeuchi F., Matsuta K., Maeda H., Omoto K., Juji T. 1989. Putative amino acid sequence of HLA-DRB chain contributing to rheumatoid arthritis susceptibility. J. Exp. Med. 169: 2263–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.du Montcel S. T., Michou L., Petit-Teixeira E., Osorio J., Lemaire I., Lasbleiz S., Pierlot C., Quillet P., Bardin T., Prum B., et al. 2005. New classification of HLA-DRB1 alleles supports the shared epitope hypothesis of rheumatoid arthritis susceptibility. Arthritis Rheum. 52: 1063–1068. [DOI] [PubMed] [Google Scholar]
- 55.Michou L., Croiseau P., Petit-Teixeira E., du Montcel S. T., Lemaire I., Pierlot C., Osorio J., Frigui W., Lasbleiz S., Quillet P., et al. European Consortium on Rheumatoid Arthritis Families 2006. Validation of the reshaped shared epitope HLA-DRB1 classification in rheumatoid arthritis. Arthritis Res. Ther. 8: R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prahalad S., Thompson S. D., Conneely K. N., Jiang Y., Leong T., Prozonic J., Brown M. R., Ponder L. A., Angeles-Han S. T., Vogler L. B., et al. 2012. Hierarchy of risk of childhood-onset rheumatoid arthritis conferred by HLA-DRB1 alleles encoding the shared epitope. Arthritis Rheum. 64: 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bachmaier K., Neu N., de la Maza L. M., Pal S., Hessel A., Penninger J. M. 1999. Chlamydia infections and heart disease linked through antigenic mimicry. Science 283: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 58.Lindholt J. S., Støvring J., Østergaard L., Urbonavicius S., Henneberg E. W., Honoré B., Vorum H. 2004. Serum antibodies against Chlamydia pneumoniae outer membrane protein cross-react with the heavy chain of immunoglobulin in the wall of abdominal aortic aneurysms. Circulation 109: 2097–2102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.