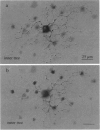
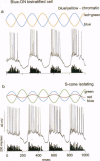
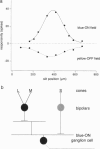
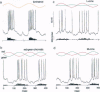
Abstract
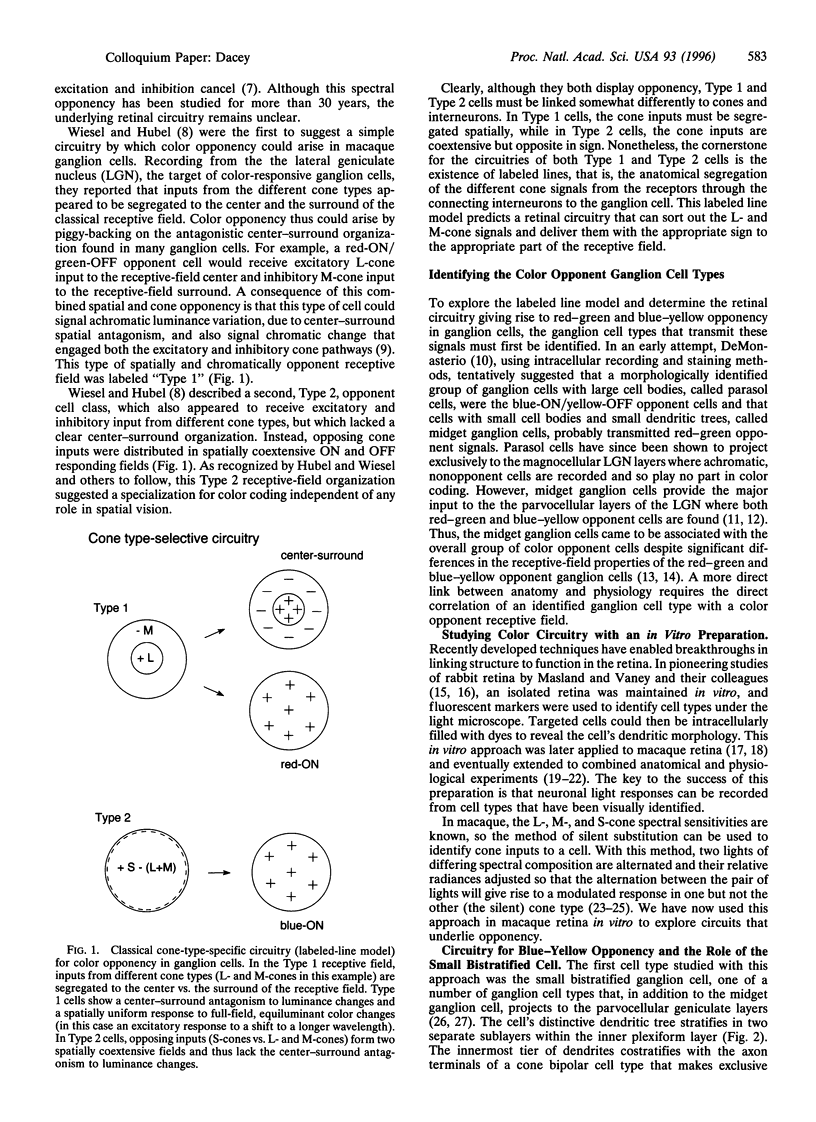
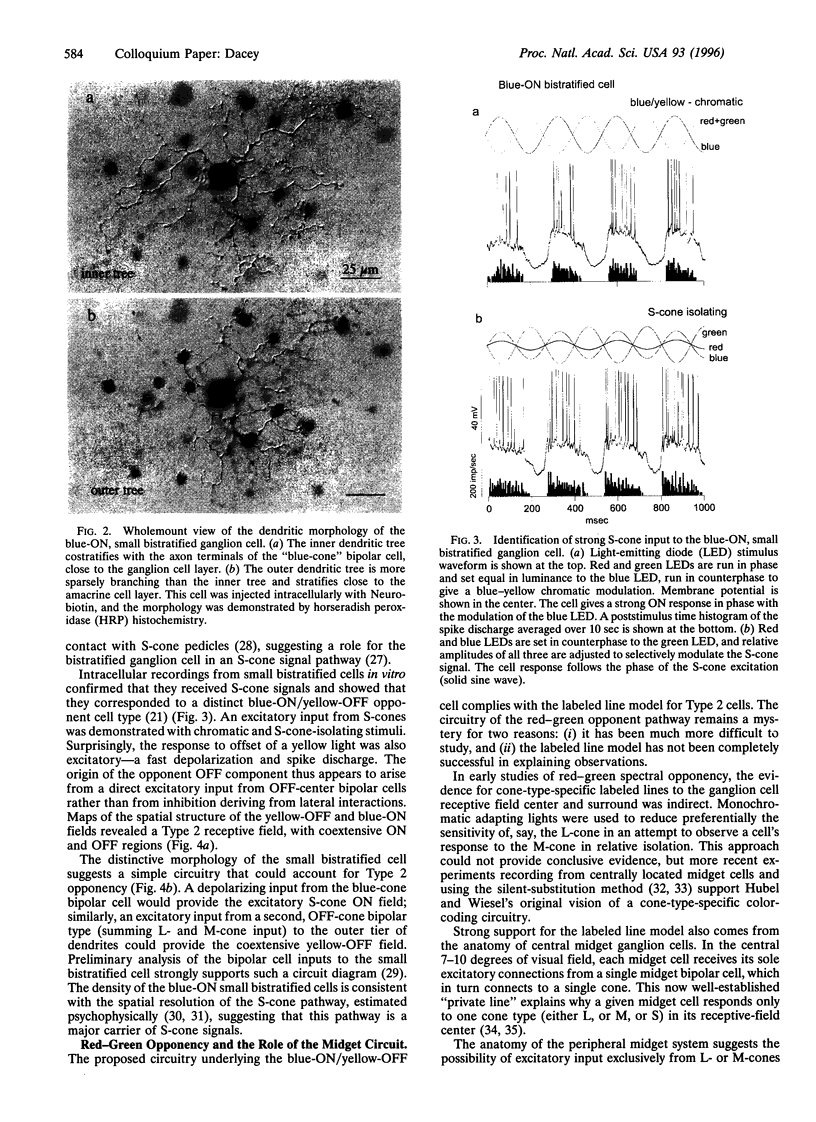
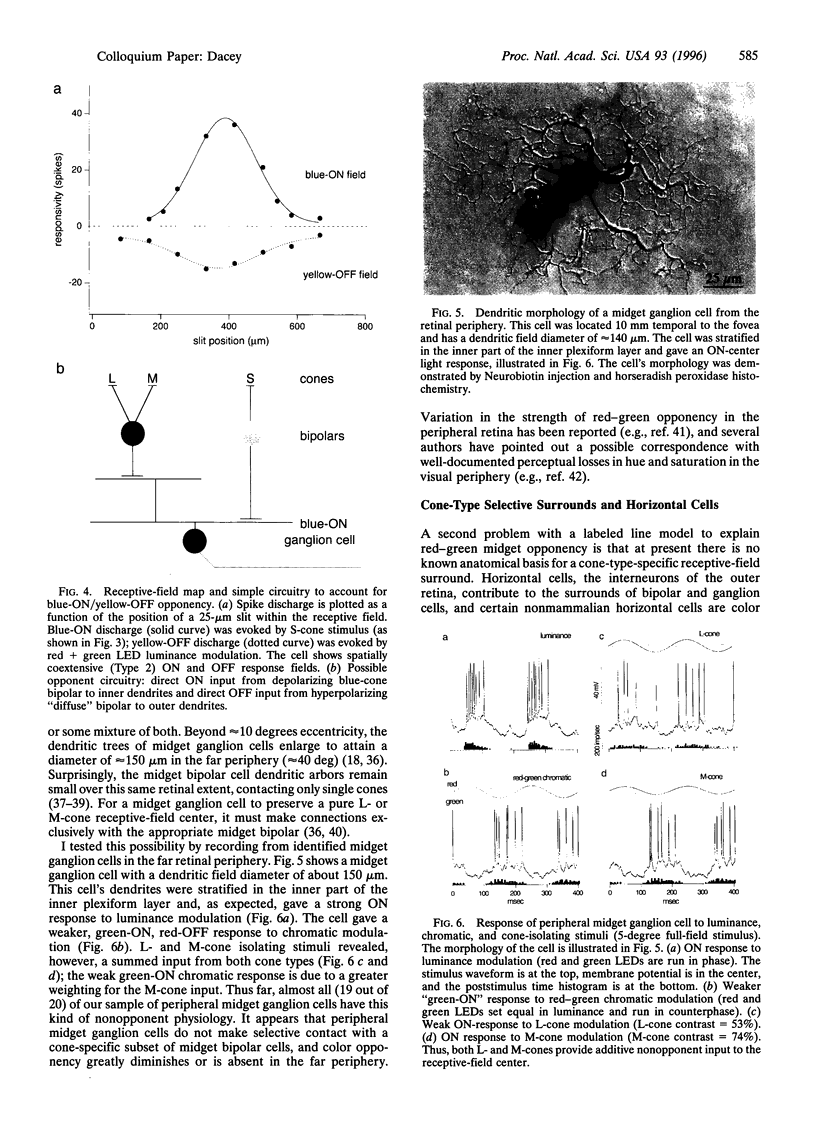
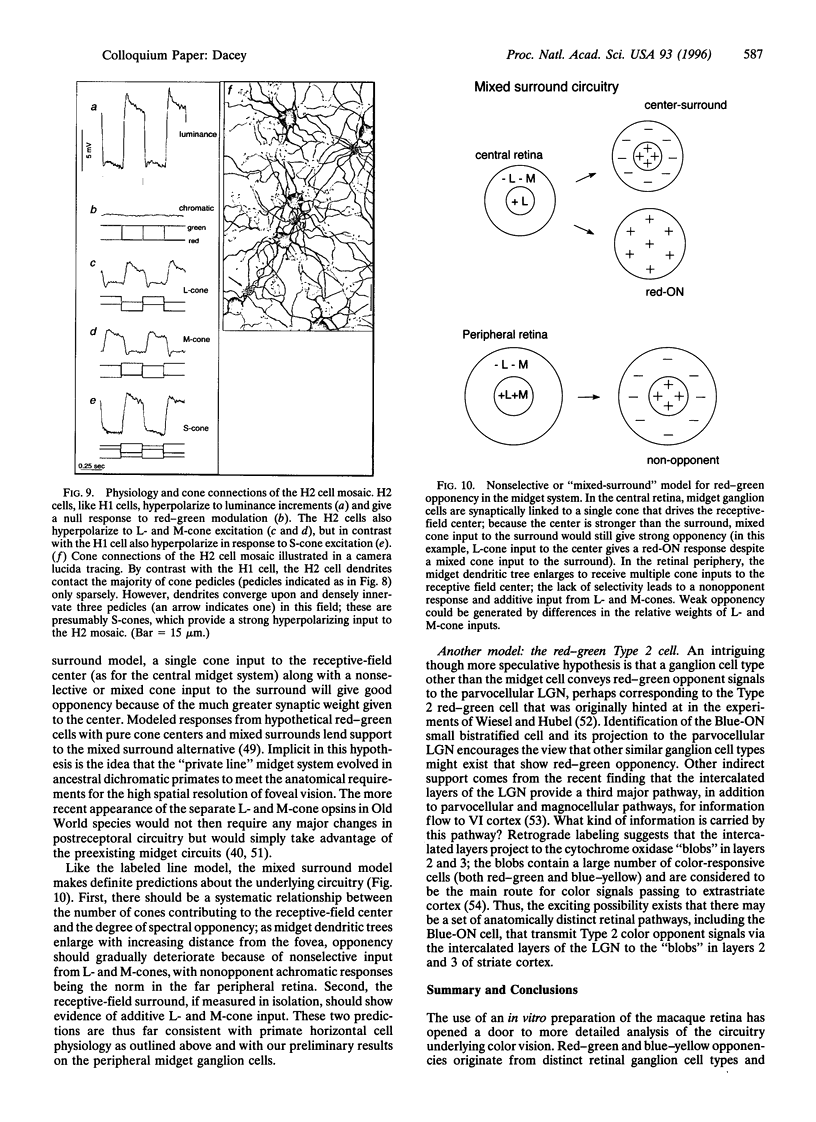
Human color vision starts with the signals from three cone photoreceptor types, maximally sensitive to long (L-cone), middle (M-cone), and short (S-cone) wavelengths. Within the retina these signals combine in an antagonistic way to form red-green and blue-yellow spectral opponent pathways. In the classical model this antagonism is thought to arise from the convergence of cone type-specific excitatory and inhibitory inputs to retinal ganglion cells. The circuitry for spectral opponency is now being investigated using an in vitro preparation of the macaque monkey retina. Intracellular recording and staining has shown that blue-ON/yellow-OFF opponent responses arise from a distinctive bistratified ganglion cell type. Surprisingly, this cone opponency appears to arise by dual excitatory cone bipolar cell inputs: an ON bipolar cell that contacts only S-cones and an OFF bipolar cell that contacts L- and M-cones. Red-green spectral opponency has long been linked to the midget ganglion cells, but an underlying mechanism remains unclear. For example, receptive field mapping argues for segregation of L-and M-cone signals to the midget cell center and surround, but horizontal cell interneurons, believed to generate the inhibitory surround, lack opponency and cannot contribute selective L- or M-cone input to the midget cell surround. The solution to this color puzzle no doubt lies in the great diversity of cell types in the primate retina that still await discovery and analysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramov I., Gordon J., Chan H. Color appearance in the peripheral retina: effects of stimulus size. J Opt Soc Am A. 1991 Feb;8(2):404–414. doi: 10.1364/josaa.8.000404. [DOI] [PubMed] [Google Scholar]
- Ahnelt P., Kolb H. Horizontal cells and cone photoreceptors in human retina: a Golgi-electron microscopic study of spectral connectivity. J Comp Neurol. 1994 May 15;343(3):406–427. doi: 10.1002/cne.903430306. [DOI] [PubMed] [Google Scholar]
- Ahnelt P., Kolb H. Horizontal cells and cone photoreceptors in primate retina: a Golgi-light microscopic study of spectral connectivity. J Comp Neurol. 1994 May 15;343(3):387–405. doi: 10.1002/cne.903430305. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. Morphological Classification of Bipolar Cells of the Primate Retina. Eur J Neurosci. 1991 Oct;3(11):1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Calkins D. J., Schein S. J., Tsukamoto Y., Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994 Sep 1;371(6492):70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Dacey D. M. Dopamine-accumulating retinal neurons revealed by in vitro fluorescence display a unique morphology. Science. 1988 May 27;240(4856):1196–1198. doi: 10.1126/science.3375811. [DOI] [PubMed] [Google Scholar]
- Dacey D. M., Lee B. B. The 'blue-on' opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994 Feb 24;367(6465):731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Dacey D. M. Morphology of a small-field bistratified ganglion cell type in the macaque and human retina. Vis Neurosci. 1993 Nov-Dec;10(6):1081–1098. doi: 10.1017/s0952523800010191. [DOI] [PubMed] [Google Scholar]
- Dacey D. M. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993 Dec;13(12):5334–5355. doi: 10.1523/JNEUROSCI.13-12-05334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux R. F., Raviola E. Physiology of HI horizontal cells in the primate retina. Proc R Soc Lond B Biol Sci. 1990 Mar 22;239(1295):213–230. doi: 10.1098/rspb.1990.0014. [DOI] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., De Valois K. K. A multi-stage color model. Vision Res. 1993 May;33(8):1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- Estévez O., Spekreijse H. The "silent substitution" method in visual research. Vision Res. 1982;22(6):681–691. doi: 10.1016/0042-6989(82)90104-3. [DOI] [PubMed] [Google Scholar]
- Estévez O., Spekreuse H. A spectral compensation method for determining the flicker characteristics of the human colour mechanisms. Vision Res. 1974 Sep;14(9):823–830. doi: 10.1016/0042-6989(74)90147-3. [DOI] [PubMed] [Google Scholar]
- Green D. G. Visual acuity in the blue cone monochromat. J Physiol. 1972 Apr;222(2):419–426. doi: 10.1113/jphysiol.1972.sp009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S. H., Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994 Apr 22;264(5158):575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Jensen R. J. Intracellular recording of light responses from visually identified ganglion cells in the rabbit retina. J Neurosci Methods. 1991 Dec;40(2-3):101–112. doi: 10.1016/0165-0270(91)90058-8. [DOI] [PubMed] [Google Scholar]
- Kolb H., Dekorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J Comp Neurol. 1991 Jan 22;303(4):617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kouyama N., Marshak D. W. Bipolar cells specific for blue cones in the macaque retina. J Neurosci. 1992 Apr;12(4):1233–1252. doi: 10.1523/JNEUROSCI.12-04-01233.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P., D'Zmura M. Mechanisms of color vision. Crit Rev Neurobiol. 1988;3(4):333–400. [PubMed] [Google Scholar]
- Leventhal A. G., Rodieck R. W., Dreher B. Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science. 1981 Sep 4;213(4512):1139–1142. doi: 10.1126/science.7268423. [DOI] [PubMed] [Google Scholar]
- Merigan W. H., Maunsell J. H. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Milam A. H., Dacey D. M., Dizhoor A. M. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci. 1993 Jan-Feb;10(1):1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- Mollon J. D. "Tho' she kneel'd in that place where they grew..." The uses and origins of primate colour vision. J Exp Biol. 1989 Sep;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- Paulus W., Kröger-Paulus A. A new concept of retinal colour coding. Vision Res. 1983;23(5):529–540. doi: 10.1016/0042-6989(83)90128-1. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Pu M., Berson D. M., Pan T. Structure and function of retinal ganglion cells innervating the cat's geniculate wing: an in vitro study. J Neurosci. 1994 Jul;14(7):4338–4358. doi: 10.1523/JNEUROSCI.14-07-04338.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R. C., Shapley R. M. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992 Apr 23;356(6371):716–718. doi: 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Watanabe M. Survey of the morphology of macaque retinal ganglion cells that project to the pretectum, superior colliculus, and parvicellular laminae of the lateral geniculate nucleus. J Comp Neurol. 1993 Dec 8;338(2):289–303. doi: 10.1002/cne.903380211. [DOI] [PubMed] [Google Scholar]
- Smith V. C., Pokorny J., Davis M., Yeh T. Mechanisms subserving temporal modulation sensitivity in silent-cone substitution. J Opt Soc Am A Opt Image Sci Vis. 1995 Feb;12(2):241–249. doi: 10.1364/josaa.12.000241. [DOI] [PubMed] [Google Scholar]
- Tauchi M., Masland R. H. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Vaney D. I. The morphology and topographic distribution of AII amacrine cells in the cat retina. Proc R Soc Lond B Biol Sci. 1985 Jun 22;224(1237):475–488. doi: 10.1098/rspb.1985.0045. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Rodieck R. W. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989 Nov 15;289(3):434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Williams D. R., Collier R. Consequences of spatial sampling by a human photoreceptor mosaic. Science. 1983 Jul 22;221(4608):385–387. doi: 10.1126/science.6867717. [DOI] [PubMed] [Google Scholar]
- Wässle H., Boycott B. B. Functional architecture of the mammalian retina. Physiol Rev. 1991 Apr;71(2):447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H., Boycott B. B., Röhrenbeck J. Horizontal Cells in the Monkey Retina: Cone connections and dendritic network. Eur J Neurosci. 1989 Sep;1(5):421–435. doi: 10.1111/j.1460-9568.1989.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Wässle H., Grünert U., Martin P. R., Boycott B. B. Immunocytochemical characterization and spatial distribution of midget bipolar cells in the macaque monkey retina. Vision Res. 1994 Mar;34(5):561–579. doi: 10.1016/0042-6989(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Yang G., Masland R. H. Direct visualization of the dendritic and receptive fields of directionally selective retinal ganglion cells. Science. 1992 Dec 18;258(5090):1949–1952. doi: 10.1126/science.1470920. [DOI] [PubMed] [Google Scholar]
- Yeh T., Lee B. B., Kremers J. Temporal response of ganglion cells of the macaque retina to cone-specific modulation. J Opt Soc Am A Opt Image Sci Vis. 1995 Mar;12(3):456–464. doi: 10.1364/josaa.12.000456. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Asymmetry of on- and off-pathways of blue-sensitive cones of the retina of macaques. Brain Res. 1979 Apr 20;166(1):39–48. doi: 10.1016/0006-8993(79)90647-4. [DOI] [PubMed] [Google Scholar]