Abstract
Electrical stimulation has been used clinically to promote bone regeneration in cases of fractures with delayed union or nonunion, with several in vitro and in vivo reports suggesting its beneficial effects on bone formation. However, the use of electrical stimulation of titanium (Ti) implants to enhance osseointegration is less understood, in part because of the few in vitro models that attempt to represent the in vivo environment. In this article, the design of a new in vitro system that allows direct electrical stimulation of osteoblasts through their Ti substrates without the flow of exogenous currents through the media is presented, and the effect of applied electrical polarization on osteoblast differentiation and local factor production was evaluated. A custom-made polycarbonate tissue culture plate was designed to allow electrical connections directly underneath Ti disks placed inside the wells, which were supplied with electrical polarization ranging from 100 to 500 mV to stimulate MG63 osteoblasts. Our results show that electrical polarization applied directly through Ti substrates on which the cells are growing in the absence of applied electrical currents may increase osteoblast differentiation and local factor production in a voltage-dependent manner.
Keywords: electrical stimulation, current, osseointegration of metal implants, bone, Ti surface properties, polarization
INTRODUCTION
The role of endogenous electrical signals in normal bone growth and development has prompted the study of bone repair using external electrical stimulation of cells and tissues in vitro [Wang et al., 1998; Kim et al., 2006] and in vivo [Becker et al., 1977; Spadaro, 1982]. Electrical stimulation can be supplied using different setups such as direct current (DC), capacitive or electromagnetic stimulation, of which DC stimulation offers great promise because it can be incorporated in implantable devices in order to minimize issues related to patient compliance with treatment. Most commonly, DC stimulation, also known as faradic stimulation, uses an electrode close to the injury site to apply a DC current and associated electric field to nearby cells and tissues [Becker et al., 1977]. Electrical stimulation has been used clinically to promote bone regeneration in cases of fractures with delayed union or nonunion, but its widespread application has been hindered by inconclusive effectiveness results due to the small sample size in a few randomized trials and differences in the electrical signals used in limited in vitro studies [Kooistra et al., 2009; Goldstein et al., 2010].
Other fields that could potentially benefit from the use of electrical stimulation are those of implantology and osseointegration of metallic implants. Titanium (Ti) and its alloys are widely used in dental and orthopaedic applications due to their favorable mechanical properties and good biological performance. The modification of Ti surface properties, such as surface roughness and chemistry, has been used to enhance the interactions between the bone and implant [Buser et al., 2004; Giavaresi et al., 2008]. However, success rates are still not satisfactory for certain populations of compromised patients [Granstrom, 2005]. The idea of enhancing bone formation by electrically stimulating implanted metallic electrodes has been previously explored using wires to supply the stimulation to the bone [Becker et al., 1977; Spadaro, 1982]. More recently, the concept of incorporating electrical stimulation to dental and orthopaedic implants has also been explored [Cook et al., 2004; Song et al., 2009], with some in vitro studies attempting to explain the complex in vivo conditions of supplying electrical currents and potentials through the surface in direct contact with the cells and tissue [Ehrensberger and Gilbert, 2010; Ehrensberger et al., 2010].
In vitro DC stimulation models usually use electrodes submerged in the tissue culture medium to establish fixed DC currents between the anode and cathode in order to influence the growing cells [Curtze et al., 2004; Ercan and Webster, 2008]. DC currents are treated as a drug, with electrons representing an actual physical entity that can be measured and administered [Black, 1987]. Several reports confirm the beneficial effects that supplying these electrical signals have on bone formation [Becker et al., 1977; Wang et al., 1998; Kim et al., 2006]. However, the role of fixed DC currents on osteoblast maturation remains controversial. The flow of faradic currents in the culture medium is highly difficult to model and it is unclear if these currents directly interact with the cells growing on the substrates or act on them indirectly through the resulting electric fields. Finally, the electrochemical products generated on the surface of both negative and positive electrodes differ widely, with hydrogen peroxide and hydroxyl ions forming around the cathode, and hydrogen and metallic ions forming around the anode [Bodamyali et al., 1999; Gittens et al., 2011b], possibly obscuring the results of DC electrical stimulation. Fixed potentials have been less studied [Ehrensberger et al., 2010] but could mimic the endogenous injury potentials more effectively and potentially control bone growth and regeneration successfully.
In this study we present a new electrical stimulation in vitro system that allows stimulation of osteoblasts directly through the electrical polarization of their Ti substrates without the flow of exogenous currents in the media, having a similar working principle as in capacitive coupling systems. Our results show that applied electrical polarization of Ti substrates, in the absence of applied electrical currents, can enhance osteoblast differentiation and local factor production. Our hypothesis is that direct electrical stimulation through cathodically polarized surfaces can affect osteoblast differentiation and that this effect is voltage dependent.
MATERIALS AND METHODS
Titanium Specimens
Ti was chosen as the substrate/electrode material as this is the most widely used metal for dental implant applications. Specimens with a diameter of 15 mm and thickness of 1 mm (ASTM F67 unalloyed Ti, Grade 2, for surgical implant applications) were supplied by Institut Straumann (Basel, Switzerland), and treated as described previously [Zhao et al., 2005]. Briefly, after being punched from metal sheets, the specimens were degreased in acetone and later exposed to an aqueous solution consisting of 2% ammonium fluoride, 2% hydrofluoric acid and 10% nitric acid at 55 °C for 30 s to generate “pre-treatment” (PT) Ti disks. Clinically relevant, microrough Ti specimens were used as positive controls and were generated by sandblasting PT specimens with corundum grit (0.25–0.50 mm) at 5 bars of pressure, followed by etching in a solution of hydrochloric and sulfuric acids heated above 100 °C for several minutes (proprietary process of Institut Straumann) to produce “sandblasted, large grit, acid-etched” (SLA) disks. Relatively smooth PT specimens have been previously characterized to have a microscale roughness average (Sa) of 0.43 ± 0.02 μm, while microrough SLA surfaces have an Sa of 3.29 ± 0.18 μm [Gittens et al., 2011a]. For surface characterization studies by scanning electron microscopy (SEM), some of the PT disks were laser etched with distinct geometric figures (i.e., triangle, square, pentagon and hexagon) to be used as coordinates for specific locations on the surface. The samples were then rinsed with water and sterilized by gamma irradiation at 25 kGy overnight (≥12 h).
Electrical Stimulation System
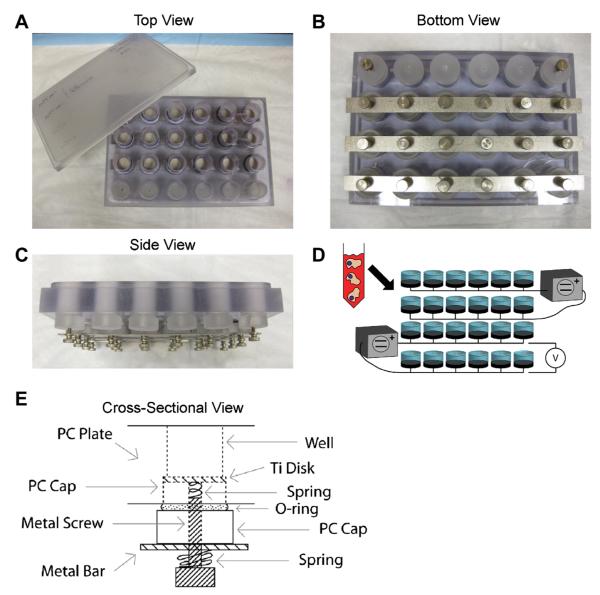
An electrical stimulation system was designed to resemble a standard 24-well tissue culture polystyrene (TCPS) plate and was used to expose cells growing on Ti substrates to different electrical polarizations (Fig. 1). Custom-made polycarbonate screw caps, which tightly fit a 15 mm Ti disk on the top, sealed the threaded wells of the polycarbonate plates. The polycarbonate caps had a threaded hole through the middle for a small stainless steel spring, and a stainless steel metal screw to establish a secure electrical connection with the bottom side of Ti substrates. Leaks in the system were prevented by using O-rings (McMaster-Carr, Atlanta, GA) for the polycarbonate caps and pipe thread sealant tape (McMaster-Carr) for the metal screws. Stainless steel bars were used to connect all the screws in one row to ensure that all the samples in the group were being stimulated with the same potential. All the components of the electrical stimulation system were thoroughly cleaned with microsoap (Micro-90; International Products, Burlington, NJ), and ultrapure water (Advantage A10, Millipore, Billerica, MA) with water resistivity of 18.2 MΩ/cm at 25 °C. The polycarbonate and metallic parts of the system were sterilized with an autoclave (Model 2540E; Tuttnauer, Hauppauge, NY) for 20 min at 121 °C and 15 PSI and left to dry in air inside the autoclave bags. The O-rings and sealant tape were sprayed with ethanol and sterilized under UV light for 12 h before use in cell experiments.
Fig.1.
Optical images and schematics of a custom-made system used to apply electrical polarization to cells growing on Ti substrates. A: Top, (B) bottom, and (C) side views of the electrical stimulation system. D: Schematic of the electrical stimulation setup for cell experiments. E: Schematic of the cross-sectional view of an individual culture well and related electrical hardware.
Electrical polarization was provided with a dual source DC power supply (6302D, Topward, Taipei Hsien, Taiwan) in fixed potential mode. The anode was connected to one row of the plate and the cathode to the following row in order to establish positive and negative polarities, respectively (Fig. 1D). The different wells in the plate were not connected with salt bridges or any other ionic paths when applying the electrical polarization to avoid the flow of current through the medium, constraining the system to behave similarly to a capacitively coupled system. The flow of current between two rows was negligible (Fig. 2B) in wells with media that had been incubated at 37 °C overnight, as confirmed with a source meter (SMU 2400, Keithley, Cleveland, OH). For voltage-dependent experiments, additional dual source (MPS 620M; Kepco, Flushing, NY) and single source (ZUP 10–20, TDK-Lambda, Tokyo, Japan) DC power supplies were used. For one of the experiments, a potentiostat (WaveNow, Pine, Durham, NC) was used in a 2-electrode open circuit potential (OCP) configuration and connected in parallel to a custom-made polycarbonate plate to record the voltage supplied during electrical stimulation experiments. The potentiostat was not used in its standard 3-electrode setup. Instead, the potentiostat was used in a 2-electrode mode to measure OCP, where the reference and counter electrodes were connected together to the anodically polarized row of wells, and the working electrode was connected to the cathodically polarized row of cells. For all experiments, the supplied signals were monitored every 30 min with a multimeter (80 series V, Fluke, Everett, WA) connected in parallel to measure voltage.
Fig. 2.
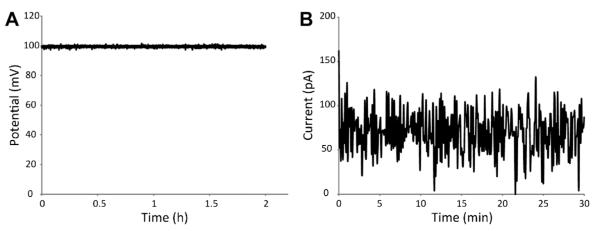
Routine characterization of voltage and currents in the electrical stimulation system. A: Voltage supplied by a power supply during the 2 h window used for cell experiments showed good stability. B: Current measurements between two adjacent electrodes/wells were below 100 pA and considered negligible.
Electrochemical Measurements
PT disks were characterized electrochemically by cyclic voltammetry using the WaveNow potentiostat. A three-electrode electrochemical cell, with a Pt wire as a counter electrode and an Ag/AgCl reference electrode, was used to evaluate the charging currents associated with each type of disk in an acidic environment, and to check the susceptibility of the culture medium to break down with respect to voltage. For these types of cyclic voltammetry tests, regions where the curve is flat represent the capacitive charging of the electrode-electrolyte interface where the constant current value should be proportional to surface area; whereas regions where the curve rises correspond to oxidation or reduction reactions occurring in the electrolyte. Cyclic voltammetry experiments in the potential range from −100 to 1000 mV were performed at a scan rate of 50 mV/s in either a 1 M sulfuric acid solution or a full cell culture medium consisting of Dulbecco’s modified Eagle medium (DMEM; Cellgro-Mediatech, Manassas, VA) containing 10% fetal bovine serum (FBS; Gibco-Life Technologies, Grand Island, NY) and 1% penicillin–streptomycin (P/S).
Surface Analysis of Ti Specimens
The Ti specimen surface topography was qualitatively evaluated using a cold field-emission scanning electron microscope (FE-SEM, Model S-4700, Hitachi, Tokyo, Japan) before and after electrical stimulation. PT specimens were imaged at specific locations using laser-etched coordination markings. The same specimens were then placed in the custom polycarbonate plates and electrically stimulated for 2 h inside an incubator at 37 °C with 5% CO2 and 100% humidity. Immediately after stimulation, samples were rinsed in ultrapure water and dried overnight. Finally, electrically stimulated specimens were imaged in the same locations to check for changes at the micro- and nanoscale. Secondary electron (SE) images were recorded using a 5 kV accelerating voltage and 30 μm aperture.
Cell Culture Model and Assays
MG63 cells were obtained from the American Type Culture Collection (Rockville, MD) and were cultured in DMEM with 10% FBS and 1% P/S at 37 °C in an atmosphere of 5% CO2 and 100% humidity. The MG63 human osteoblast-like cell line was chosen for these experiments because it exhibits many characteristics of pre-mature osteoblasts, making it an attractive model for in vitro studies [Franceschi et al., 1985; Lajeunesse et al., 1991], and previous experiments by our group have shown that these cells are sensitive to electrical signals [Lohmann et al., 2000; Schwartz et al., 2009]. Cells were grown at a density of 10000 cells/cm2 on TCPS plates to check for confluence on PT and SLA controls that were not electrically polarized or on the experimental PT surfaces that were stimulated with the anode (positive lead) or cathode (negative lead) of the different applied electrical polarizations, depending on the particular study design (Table 1).
TABLE 1.
Nomenclature for the Different Experimental Groups
| Group name | Description |
|---|---|
| PTctrl | Pre-treatment (PT) titanium surfaces on TCPS plate |
| SLActrl | Sand-blasted with large grit and acid-etched (SLA) surfaces in TCPS plate |
| PT0 | PT surfaces on polycarbonate custom-made plate not connected to power supply (non-polarized) |
| PT100+ | PT surfaces on polycarbonate custom-made plate anodically polarized with 100 mV |
| PT100− | PT surfaces on polycarbonate custom-made plate cathodically polarized with 100 mV |
| PT100+ w/potentiostat | PT surfaces on polycarbonate custom-made plate anodically polarized with 100 mV and connected to potentiostat |
| PT100− w/potentiostat | PT surfaces on polycarbonate custom-made plate cathodically polarized with 100 mV and connected to potentiostat |
| PT200− | PT surfaces on polycarbonate custom-made plate cathodically polarized with 200 mV |
| PT300− | PT surfaces on polycarbonate custom-made plate cathodically polarized with 300 mV |
| PT400− | PT surfaces on polycarbonate custom-made plate cathodically polarized with 400 mV |
| PT500− | PT surfaces on polycarbonate custom-made plate cathodically polarized with 500 mV |
To compare the effect of anodically and cathodically polarized surfaces on the MG63 response, cells were plated on standard tissue culture plates using PT surfaces (PTctrl) as a negative control and SLA surfaces (SLActrl) as a positive control, considering that osteoblasts are known to differentiate on rougher surfaces. In addition, using the custom-made plates, cells were exposed to surfaces polarized with 100 mV using PT surfaces as the anode (PT100+) or cathode (PT100−). Another set of anodically and cathodically polarized groups were connected to a potentiostat to monitor the potential that was being supplied (PT100+, PT100− with potentiostat).
The PTctrl group, in which Ti specimens are placed in standard TCPS plates, provides a good negative control for electrical stimulations experiments. However, the possibility exists that just by establishing electrical connections with the bottom of PT specimens in the custom-made plates, even without electrical stimulation, the surface polarization of the specimens could be affected and cause an effect on the MG63 cell response. To evaluate this phenomenon, additional experiments were performed to analyze PTctrl and PT surfaces on the polycarbonate custom-made plates that were subjected to no (zero) polarization (PT0) and compared to cathodically polarized PT100− surfaces.
Finally, voltage-dependent effects on osteoblast maturation were also evaluated on PT surfaces. MG63 cells were plated on non-polarized PT0 surfaces or on surfaces that were polarized with 100, 200, 300, 400, or 500 mV in the custom-made polycarbonate plate in order to demonstrate that a voltage-dependent stimulus had a biologically relevant consequence. From preliminary results, only the cells grown on the cathodically polarized surfaces were assayed.
MG63 cells were fed 24 h after they were plated on the different surfaces and every 48 h until confluent, as evaluated using the TCPS substrate. At confluence, cells were treated with fresh medium and experimental groups were electrically stimulated with their respective applied electrical polarizations for 2 h. The duration of the stimulation was selected arbitrarily, knowing that endogenous injury potentials are long-lasting signals that can persist for several hours [McCaig et al., 2005]. After stimulation, the cells were incubated for an additional 22 h and harvested for assays. Conditioned media were collected, and cell layers were washed twice with serum-free medium, released from their substrate by two sequential incubations in 500 μl of 0.25% trypsin for 10 min at 37 °C, and counted with a Z1 Coulter particle counter (Beckman Coulter, Brea, CA). Subsequently, cells were resuspended in 500 μl 0.05% Triton-X-100 (Sigma–Aldrich, St. Louis, MO) and lysed by sonication for further analyses.
Osteocalcin content in the conditioned media, used as a late differentiation marker because of the high levels found right before the onset of bone mineralization, was measured with a commercially available radioimmunoassay kit (Human Osteocalcin RIA Kit; Biomedical Technologies, Stoughton, MA), as described previously [Boyan et al., 1998], using a LS1500 gamma counter (Perkin Elmer, Waltham, CA).
The conditioned media were also assayed for protein levels of local factors important for bone development. Osteoprotegerin (OPG), a cytokine that works as a decoy receptor for “receptor activator for nuclear factor κ B ligand” (RANKL) to inhibit osteoclastogenesis, was measured using enzyme-linked immunosorbent assay (ELISA) kits (DY805 Osteoprotegerin DuoSet, R&D Systems, Minneapolis, MN). Vascular endothelial growth factor (VEGF), a potent growth factor involved in vasculogenesis and angiogenesis important for the initiation of bone formation, was also measured using ELISA kits (DY293B VEGF DuoSet, R&D Systems).
Statistical Analysis
Data from experiments examining cell response are presented as the mean ± standard error for six cultures per variable (n = 6). All experiments were independently repeated at least twice to ensure the validity of the observations and results from individual representative experiments. Data for each experiment were evaluated by analysis of variance, and significant differences between groups were determined using Tukey’s modification of the Student’s t-test for independent studies. In Figure 6, data are presented as treatment over control (PT control) using three different independent experiments (n = 3), and evaluated with a regular Student’s t-test. A P-value below 0.05 was considered to indicate a statistically significant difference.
Fig. 6.
Evaluation of different titanium control surfaces on the response of osteoblast-like MG63 cells. Cells were plated for PT controls on TCPS plates (PTctrl), and compared to non-polarized PT surfaces on custom-made plates (PT0) and 100 mV cathodically polarized PT surfaces (PT100−). At confluence, (A) cell number, (B) osteocalcin, (C) osteoprotegerin, and (D) VEGF levels were measured. Data represented as treatment over control analyses ± standard error of three independent experiments with six different samples per group. PT ctrl group was used as the control for treatment over control analyses. *P-value < 0.05 versus PTctrl. No statistical differences were found between PT0 and PT100−.
RESULTS
Characterization of Electrical Stimulation System
Routine validation measurements on the custom-made electrical stimulation plates confirmed that the potential supplied by the power supplies was stable over the 2 h period used for cell experiments (Fig. 2A), and that the current between electrodes/wells in different rows was negligible (below 100 pA, shown in Fig. 2B). Electrochemical characterization of the PT specimens showed that in 1 M sulfuric acid, the PT surface had constant current values close to 1 μA, with stable hysteresis maintained throughout the test (Fig. 3A). When using cell culture media as the electrolyte (Fig. 3B), the current rose sharply at around 0.8 V. The curve also showed small peaks between 0.1 and 0.2 V that were transient and only present in the first cycle. No media breakdown was evident at the potentials used for the cell experiments. Additionally, qualitative evaluation of PT surfaces by SE images showed that there were no topographical changes due to the applied potentials (Fig. 4). Some surface charging distortion was evident on the SE images of PT surfaces after electrical stimulation (Fig. 4D), probably due to adsorbed proteins from the media during treatment.
Fig. 3.

Cyclic voltammetry curves for PT surfaces in (A) 1 M sulfuric acid or (B) cell culture media. In the acidic environment, the PT surfaces showed relatively low current values with constant hysteresis around zero (horizontal dashed line), suggesting low reactivity to the parameters used. In cell culture media, the curve revealed a sharp increase at higher voltages, which corresponded to the breakdown of the media. None of the samples exhibited any media breakdown at or below the potentials used for cell experiments (vertical dashedline).
Fig. 4.
SE images of a laser-etched PT surface before and after electrical stimulation. A: Laser etched markings were used to find the same location on the surface before and after stimulation. B,C:Low and high magnification images of the surface before stimulation showed the presence of microscale machining marks and no appreciable nanostructures on the surface. D,E: Low and high magnification images after 2 h of electrical stimulation in the custom-made polycarbonate plates with culture media revealed no visible differences on the surface except for some electron charging, probably due to protein adsorption from the media.
Cell Assays
Cathodic versus anodic effect
Osteoblast-like MG63s were sensitive to the applied electrical potentials supplied directly through their Ti substrates. MG63 cell numbers (Fig. 5A) were lower in the electrically polarized groups when compared to both PTctrl and SLActrl groups on TCPS plates, with the lowest levels found on the cathodically polarized PT100− group when compared to the anodically polarized PT100+ surfaces. The effect of the electrical stimulation, both anodic and cathodic, on cell number was diminished when connected to the potentiostat, but still had lower levels than the PTctrl group. Production of osteocalcin was sensitive to the electrical stimulation and had the highest levels in the cathodically polarized PT100− group (Fig. 5B). The increase in osteocalcin levels by the electrical stimulation was completely lost when connecting the potentiostat to measure the voltage. Production of the anti-osteoclastogenic factor (osteoprotegerin) was greatly enhanced by the electrical stimulation, especially on the cathodically polarized surfaces, and this effect was again diminished when monitoring with the potentiostat (Fig. 5C). Although OPG levels were enhanced by both anodic and cathodic stimulation, the levels in the cathodically polarized PT100− group were significantly higher than in the anodically polarized PT100+ group. In addition, VEGF levels increased in the cathodically polarized PT100− group compared to the PTctrl and SLActrl groups and the cathodic group monitored by the potentiostat, but not when compared to the anodically polarized PT100+ group (Fig. 5D).
Fig. 5.
Effects of 100 mV anodically and cathodically polarized surfaces on osteoblast-like MG63 cells. A: Cell number, (B) osteocalcin, (C) osteoprotegerin, and (D) VEGF levels measured on PT (PTctrl) and SLA (SLActrl) surfaces on TCPS plates, as well as on surfaces connected to the a node (PT100+) and cathode (PT100−) of a power supply, and two additional anodically and cathodically stimulated groups connected to a potentiostat (PT100+, PT100− w/potentiostat) to measure the applied potential. Introducing monitoring instrumentation seemed to disturb the effect of surface polarization. Data represented are the mean standard error of six independent samples per group. *P-value < 0.05 versus PT ctrl; #P-value < 0.05 versus SLA ctrl; $P-value < 0.05 versus PT100+; %P-value <0.05 versus PT100−.
Titanium controls in TCPS plates versus nonpolarized surfaces in custom-made plates
Significant enhancements in cell differentiation and local factor production were found on the cathodically polarized PT100− surfaces stimulated with 100 mV when compared to the PTctrl surfaces in the TCPS plates and, interestingly, similar enhancements were found just by establishing electrical connections, without polarization, on the PT0 surfaces in the custom-made plates. Cell number decreased in both the PT0 and PT100− groups when compared to the PTctrl group, with the lowest levels found in the PT100− group (Fig. 6A). However, the difference between the non-polarized PT0 group and the cathodically polarized PT100− group was not significant. At the same time, the highest levels of the late differentiation marker osteocalcin were found in the PT100− group, which were significantly different compared to the PTctrl but not to PT0 surfaces (Fig. 6B). A similar response was found for OPG, with the levels found in PT100− being significantly higher than PTctrl but not statistically different than PT0 (Fig. 6C). In addition, comparable levels of VEGF were found in PT0 and PT100− groups, which were slightly lower than the PTctrl group (Fig. 6D).
Voltage-dependent effect of polarized PT surfaces on the MG63 response
Initial studies, described above, suggested that PT surfaces connected to the 100 mV cathode elicited the strongest maturation response from MG63s, compared to the anode. Thus, subsequent voltage-dependent studies focused on cathodically polarized surfaces. When additional potentials of 200, 300, 400, and 500 mV were evaluated, the cell number decreased with an increase in the supplied potential, with the PT500− group having 40% lower numbers than non-polarized PT0 surfaces (Fig. 7A). Osteocalcin production was highest in the PT500− group, which had levels 70% higher than the PT0 and PT100− groups (Fig. 7B). Production of OPG (Fig. 7C) and VEGF (Fig. 7D) also responded to the higher applied electrical potentials. OPG production was 100% higher and 70% higher in the PT400− and in PT500− groups, respectively, when compared to the non-polarized PT0, whereas VEGF had 50% higher levels in the PT300− and PT500− groups compared to the PT0 surfaces.
Fig.7.
Voltage-dependent effects of cathodically polarized PT surfaces on osteoblast-like MG63 cells. Cells were plated on non-polarized PT0 surfaces, as well as PT surfaces stimulated with cathodical polarizations of 100, 200, 300, 400, and 500 mV. At confluence, checked on TCPS, (A) cell number, (B) osteocalcin, (C) osteoprotegerin, and (D) VEGF levels were measured on the cathodically polarized surfaces. Data represented are the mean ± standard error of six independent samples per group. *P-value < 0.05 versus PT0; #P-value < 0.05 versus PT100−; $P-value < 0.05 versus PT 200−; %P-value < 0.05 versus PT 300−.
DISCUSSION
Recently, implantable devices that supply DC stimulation directly to the surrounding tissue have been used to provide localized treatment and improve osseointegration [Yonemori et al., 1996; Song et al., 2009]. However, in vitro models that represent these conditions need to be developed, as most available systems provide electrical stimulation to cells indirectly through the tissue culture medium [Curtze et al., 2004; Ercan and Webster, 2008]. In this study, a new in vitro electrical stimulation system was designed to provide stimulation directly through electrically polarized Ti substrates used to culture cells. The results suggest that osteoblast maturation responds strongly to applied electrical potentials supplied through anodically and cathodically polarized Ti surfaces and the response is voltage-dependent.
We successfully designed and implemented an in vitro system that allowed direct electrical connections underneath the Ti substrates used to culture the cells. In this way, the surfaces where the cells are growing could be used as the electrodes that supply the electrical stimulation, similar to a DC stimulation system. Most in vitro systems for DC stimulation have been modeled after in vivo conditions to treat nonunions, where electrodes are placed near the healing ends of the injured bone [Hagiwara and Bell, 2000]. Thus, in vitro setups commonly submerge electrodes in the culture media to supply fixed DC currents and associated potentials to cells growing on standard TCPS plates [Hammerick et al., 2010]. However, such a setup does not necessarily represent a situation where the actual osseointegrating implant is providing the electrical stimulation [Song et al., 2009]. Additionally, the flow of current through the media/electrodes promotes electrochemical reactions on the anode and cathode that could confound the results [Bodamyali et al., 1999], and it is not clear if both the currents and the potentials are needed for the beneficial effects of the electrical stimulation. The system designed in this study also sought to avoid the flow of exogenous current through the medium, constraining the system to behave as a capacitively coupled system and effectively isolating the effects of the applied electrical polarizations.
The electrochemical stability of the electrolyte is an important factor to consider when applying electrical stimulation. Although no currents were detected in the system, which minimizes the possibility of electrochemical reactions occurring around the electrodes, cyclic voltammetry tests were performed on PT surfaces using a standard three-electrode electrochemical cell to ensure that the voltages used would not elicit oxidation of the surface or breakdown of the media. In the acidic environment, specimens showed relatively low current values with constant hysteresis throughout the test, indicating good electrochemical stability. Sudden rises in the current curve are indicative of the onset of oxidation, and it appears that relatively smooth PT surfaces were not reactive under these conditions. When cyclic voltammetry tests were performed on the cell culture media, the current increased sharply at higher voltages. The sharp increase in the curve corresponds to the breakdown of media, and the PT surfaces seem to facilitate this breakdown at higher potentials. However, media breakdown was not exhibited until at least 0.6 V, which was higher than the potentials used for the cell experiments. Small current peaks were found before 0.5 V during the first voltammetry cycle in media, possibly due to surface passivation reactions, but these were considered negligible because of their transient nature. In addition, no topographical changes were detected on the Ti surfaces after simulated runs in the in vitro electrical stimulation system, which is important to ensure that the responses obtained during cell experiments are strictly caused by the applied electrical polarizations.
MG63 cell numbers were sensitive to electrical stimulation with 100 mV, and this effect was more pronounced on the surfaces that were cathodically polarized. Cell number is directly related to proliferation and inversely related to differentiation, which means that osteoblasts generally have to choose between dividing and proliferating or differentiating and maturing to produce proteins necessary for bone formation. MG63 numbers were lower in the electrically polarized groups, with the lowest levels on the negatively charged surface. However, we did not directly evaluate cell death, so this cannot be ruled out. Conversely, other studies evaluating the effects of electrical stimulation, albeit with a different experimental setup where both an anode and cathode are submerged in the media of each well, have found an increase in proliferation upon DC stimulation through the culture media [Kim et al., 2006; Ercan and Webster, 2008]. However, these studies have failed to correlate the increase in proliferation with a corresponding increase in cell differentiation.
Additionally, several studies on microrough surfaces, which are well documented to promote osteoblast differentiation, confirm that the transcriptionally regulated transition from a proliferative to a differentiated state leads to lower cell numbers on the rougher surfaces [Stein et al., 1996; Olivares-Navarrete et al., 2008]. This is in agreement with our results showing lower cell numbers coupled to higher levels of the late differentiation marker (osteocalcin) on the electrically polarized groups, even when compared to clinically relevant, microrough control surfaces. The highest levels of osteocalcin were found in the negatively charged group, which is in accord with a number of other reports associating negative polarities with the natural process of bone healing [Friedenberg and Brighton, 1966] and the beneficial effects of electrical stimulation [Cook et al., 2004]. Interestingly, anodic polarization also seemed to promote osteoblast differentiation but to a lesser extent, which has not been commonly shown in the literature possibly due to the detrimental effects of electrochemical products produced around the anode in common in vitro setups [Bodamyali et al., 1999]. Production of the local factors OPG, an inhibitor of osteoclastogenesis, and VEGF, a potent angiogenic factor important for bone development, were also significantly higher on the cathodically polarized surfaces. Remarkably, the results differed when connecting a monitoring device to measure the potentials supplied to the experimental groups. The electrical stimulation effect was lost in all the cell assays performed on the groups connected to a potentiostat used as a voltmeter, underscoring the susceptibility of the system to instrumentation disturbances. The disruption of the stimulation effect when connecting a measuring device suggests that the reported result on osteoblast maturation could be due to the double-layer phenomena on the surface of the Ti electrodes, but this was not evaluated in this study.
Our initial studies assessing the effects of electrical stimulation on osteoblast responses were performed using titanium control surfaces that were placed in standard TCPS plates. However, to rule out the possibility that the response of osteoblasts could be affected just by establishing electrical connections with the substrates, non-polarized titanium control surfaces on the custom-made plates were also evaluated. Indeed, osteoblast maturation and OPG production were significantly enhanced on the cathodically polarized titanium surfaces compared to the titanium surfaces in TCPS plates, as seen in the initial studies. Interestingly, a similar enhancement was achieved just by establishing electrical connections with the Ti specimens with no polarization applied, resulting in no statistical differences between the cathodically polarized surfaces and the non-polarized titanium surfaces in the custom-made plates. VEGF levels were not greatly influenced by electrical stimulation with 100 mV. The non-polarized specimens were not connected to the power supply but were connected to the stainless steel bar, which could have affected the double-layer phenomena and the native distribution of surface charges. Another possibility is that the chemistry of the polycarbonate custom-made plates could have exerted an influence on the growth of the cells.
Cell experiments were also performed using different potentials on the cathodically polarized PT surfaces, and we found that the response of osteoblast-like cells was voltage-dependent. The cell number was lower in the groups stimulated with the higher potentials and these data, in combination with higher production of differentiation markers, suggest that the cells growing on cathodically polarized PT surfaces exhibited a more mature phenotype with an increase in potentials. The effects of higher potentials on osteoblast differentiation were also accompanied by a higher production of the local factors OPG and VEGF, which have been shown to enhance osseointegration in vivo [Kim et al., 2006; Schwartz et al., 2009]. Our results indicate that the maturation of osteoblasts growing on relatively smooth surfaces can be accelerated by increasing the strength of the applied electrical polarization. Our hypothesis when designing these experiments was that electrical stimulation at lower potentials would promote osteoblast differentiation but higher potentials would inhibit differentiation, as had been shown previously in the case of the extreme signals found during corrosion events [Gilbert et al., 1998]. The range of potentials evaluated in this study did not show any detrimental effect on the development and maturation of osteoblasts on relatively smooth surfaces even at the highest levels, which instead promoted osteoblast maturation. The possibility exists that even higher potentials than the ones provided could inhibit the maturation of osteoblasts but this was not explored in this study.
The electrical stimulation system described in the present work is not representative of a conventional electrochemical cell because there is no ionic path connecting the wells and the media to close the electrochemical loop. Still, the system can be envisioned as a standard cell in which no charge passes across the electrode-electrolyte interface over a given range of potentials, dealing with what is known as an ideally polarizable electrode [Landolt, 2007]. Under these conditions, a change in the potential at the working electrode from its equilibrium value necessarily causes a charge imbalance across the interface that must be neutralized by the rearrangement of charged species in the solution near the electrode surface. This interfacial region, where a separation of charges is observed, forms the well-known electric double layer that has been extensively evaluated in the field of electrochemistry, and, in the particular case of an ideally polarizable electrode, can be represented by the two plates of a capacitor.
The relevance of such a model is to mimic more closely the real scenario of a titanium implant in direct contact with the surrounding tissue, being electrically stimulated in vivo or clinically. However, a thorough evaluation of the electrostatically influenced interface is required to fully understand the results obtained in the present study. Additionally, most surface characteristics, such as surface preparation, history, metal composition and surface charge, must be systematically assessed as they could influence the behavior of this interface.
During corrosion events, electric potentials are generated in association with extreme electrochemical currents that result in products injurious to the cells, such as metal and hydrogen ions [Black et al., 1984; Ramp et al., 1994; Bodamyali et al., 1999]. Corrosion related electrochemical products and wear debris have been implicated in complications surrounding orthopaedic implants, such as aseptic loosening [Jacobs et al., 2001; Lohmann et al., 2002]. However, the electrical currents and potentials present during corrosion events could also have an effect on cell response. The in vitro system described in this study is connected in such a way as to avoid the flow of currents through the culture medium while supplying cathodic polarization to the surfaces where the cells are growing. Our results suggest that electrochemical potentials by themselves may not contribute to the detrimental effects of corrosion events around implants, leaving currents and associated electrochemical products as possible causes for the negative impact of corrosion.
The success of dental and orthopedic implants is dependent the osseointegration of the implant with the surrounding bone and this, in turn, is greatly dependent on the surface properties of the device. Faster and better osseointegration has become the driving force of many research efforts to satisfy the demands of an increasing aging population. Properties such as surface roughness [Olivares-Navarrete et al., 2010; Gittens et al., 2011a], surface chemistry [Sul et al., 2005] and surface energy [Liu et al., 2007] have been found to affect cell response and, if tailored appropriately, can enhance cell differentiation, local factor production and, consequently, bone growth and osseointegration [Schwartz and Boyan, 1994]. In the same way, electrical stimulation of implants should be considered as an additional tool for the enhancement of bone growth and repair in patients with compromised or diseased bone, and new strategies should focus on the effective translation of successful in vitro models to clinical settings.
CONCLUSIONS
In this study, we present an in vitro system that provides electrical stimulation of cells with applied electrical polarization in the absence of exogenous electrical currents. Electrical stimulation was supplied directly through the Ti substrates used to culture cells, with the surfaces either anodically or cathodically polarized. MG63 differentiation and local factor production was enhanced on cathodically polarized surfaces, and this effect could be disturbed by introducing monitoring instrumentation. The effect of the applied electrical polarization also seemed to be voltage-dependent, with higher potentials promoting a greater enhancement of osteoblast differentiation.
ACKNOWLEDGMENTS
The PT and SLA specimens were provided by Institut Straumann (Basel, Switzerland). We would also like to thank Rosemary Song, Alice Cheng, and Min Lai for their help assembling and disassembling the electrical stimulation system, Marcus Walker and Henry Mei for their help setting up the LabView program for the source meter measurements, and Sharon Hyzy for her assistance during cell culture.
Grant sponsor: National Institutes of Health – US Public Health Services (NIH-USPHS) (Grant No. AR052102) and International Team for Implantology (ITI) Foundation. The Government of Panama (IFARHU-SENACYT) supported a fellowship to RAG.
REFERENCES
- Becker RO, Spadaro JA, Marino AA. Clinical experiences with low intensity direct current stimulation of bone growth. Clin Orthop. 1977;124:75–83. [PubMed] [Google Scholar]
- Black J. Electrical stimulation: Its role in growth, repair, and remodeling of the musculoskeletal system. Praeger Publishers; New York: 1987. [Google Scholar]
- Black J, Baranowski TJ, Brighton CT. Electrochemical aspects of dc stimulation of osteogenesis. Bioelectrochem Bioenerg. 1984;12:323–327. [Google Scholar]
- Bodamyali T, Kanczler JM, Simon B, Blake DR, Stevens CR. Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem Biophys Res Commun. 1999;264:657–661. doi: 10.1006/bbrc.1999.1355. [DOI] [PubMed] [Google Scholar]
- Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, Szmuckler-Moncler S, Dean DD, Schwartz Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1 alpha,25-(OH)2D3. J Biomed Mater Res. 1998;39:77–85. doi: 10.1002/(sici)1097-4636(199801)39:1<77::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–533. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- Cook SD, Patron LP, Christakis PM, Bailey KJ, Banta C, Glazer PA. Direct current stimulation of titanium interbody fusion devices in primates. Spine J. 2004;4:300–311. doi: 10.1016/j.spinee.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Curtze S, Dembo M, Miron M, Jones DB. Dynamic changes in traction forces with DC electric field in osteoblast-like cells. J Cell Sci. 2004;117:2721–2729. doi: 10.1242/jcs.01119. [DOI] [PubMed] [Google Scholar]
- Ehrensberger MT, Gilbert JL. The effect of static applied potential on the 24-hour impedance behavior of commercially pure titanium in simulated biological conditions. J Biomed Mater Res B Appl Biomater. 2010;93:106–112. doi: 10.1002/jbm.b.31564. [DOI] [PubMed] [Google Scholar]
- Ehrensberger MT, Sivan S, Gilbert JL. Titanium is not “the most biocompatible metal” under cathodic potential: The relationship between voltage and MC3T3 preosteoblast behavior on electrically polarized cpTi surfaces. J Biomed Mater Res A. 2010;93:1500–1509. doi: 10.1002/jbm.a.32622. [DOI] [PubMed] [Google Scholar]
- Ercan B, Webster TJ. Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int J Nanomed. 2008;3:477–485. doi: 10.2147/ijn.s3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi RT, James WM, Zerlauth G. 1-alpha,25-dihydroxyvitamin D3 specific regulation of growth, morphology, and fibronectin in a human osteosarcoma cell-line. J Cell Physiol. 1985;123:401–409. doi: 10.1002/jcp.1041230316. [DOI] [PubMed] [Google Scholar]
- Friedenberg ZB, Brighton CT. Bioelectric potentials in bone. J Bone Jt Surg (Am) 1966;48:915–923. [PubMed] [Google Scholar]
- Giavaresi G, Fini M, Chiesa R, Giordano C, Sandrini E, Bianchi AE, Ceribelli P, Giardino R. A novel multiphase anodic spark deposition coating for the improvement of orthopedic implant osseointegration: An experimental study in cortical bone of sheep. J Biomed Mater Res A. 2008;85:1022–1031. doi: 10.1002/jbm.a.31566. [DOI] [PubMed] [Google Scholar]
- Gilbert JL, Zarka L, Chang EB, Thomas CH. The reduction half cell in biomaterials corrosion: Oxygen diffusion profiles near and cell response to polarized titanium surfaces. J Biomed Mater Res. 1998;42:321–330. doi: 10.1002/(sici)1097-4636(199811)42:2<321::aid-jbm18>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gittens RA, McLachlan T, Olivares-Navarrete R, Cai Y, Berner S, Tannenbaum R, Schwartz Z, Sandhage KH, Boyan BD. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011a;32:3395–3403. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittens RA, Olivares-Navarrete R, Tannenbaum R, Boyan BD, Schwartz Z. Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res. 2011b;90:1389–1397. doi: 10.1177/0022034511408428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein C, Sprague S, Petrisor BA. Electrical stimulation for fracture healing: Current evidence. J Orthop Trauma. 2010;24:S62–S65. doi: 10.1097/BOT.0b013e3181cdde1b. [DOI] [PubMed] [Google Scholar]
- Granstrom G. Osseointegration in irradiated cancer patients: An analysis with respect to implant failures. J Oral Maxillofac Surg. 2005;63:579–585. doi: 10.1016/j.joms.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Hagiwara T, Bell WH. Effect of electrical stimulation on mandibular distraction osteogenesis. J Craniomaxillofac Surg. 2000;28:12–19. doi: 10.1054/jcms.1999.0104. [DOI] [PubMed] [Google Scholar]
- Hammerick KE, James AW, Huang ZB, Prinz FB, Longaker MT. Pulsed direct current electric fields enhance osteogenesis in adipose-derived stromal cells. Tissue Eng Part A. 2010;16:917–931. doi: 10.1089/ten.tea.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: Basic science. Clin Orthop. 2001;393:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Kim IS, Song JK, Zhang YL, Lee TH, Cho TH, Song YM, Kim DK, Kim SJ, Hwang SJ. Biphasic electric current stimulates proliferation and induces VEGF production in osteoblasts. Biochim Biophys Acta. 2006;1763:907–916. doi: 10.1016/j.bbamcr.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Kooistra BW, Jain A, Hanson BP. Electrical stimulation: Nonunions. Indian J Orthop. 2009;43:149–155. doi: 10.4103/0019-5413.50849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse D, Kiebzak GM, Frondoza C, Sacktor B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Miner. 1991;14:237–250. doi: 10.1016/0169-6009(91)90025-u. [DOI] [PubMed] [Google Scholar]
- Landolt D. Corrosion and surface chemistry of metals. EPFL Press; Lausanne, Switzerland: 2007. [Google Scholar]
- Liu X, Lim JY, Donahue HJ, Dhurjati R, Mastro AM, Vogler EA. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials. 2007;28:4535–4550. doi: 10.1016/j.biomaterials.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann CH, Schwartz Z, Liu Y, Guerkov H, Dean DD, Simon B, Boyan BD. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J Orthop Res. 2000;18:637–646. doi: 10.1002/jor.1100180417. [DOI] [PubMed] [Google Scholar]
- Lohmann CH, Dean DD, Koster G, Casasola D, Buchhorn GH, Fink U, Schwartz Z, Boyan BD. Ceramic and PMMA particles differentially affect osteoblast phenotype. Biomaterials. 2002;23:1855–1863. doi: 10.1016/s0142-9612(01)00312-x. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: Current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, Chaudhri RA, Ornoy A, Boyan BD, Schwartz Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci USA. 2008;105:15767–15772. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, Schwartz Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–2735. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramp WK, Lenz LG, Kaysinger KK. Medium pH modulates matrix, mineral, and energy-metabolism in cultured chick bones and osteoblast-like cells. Bone Miner. 1994;24:59–73. doi: 10.1016/s0169-6009(08)80131-6. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Boyan BD. Underlying mechanisms at the bone-biomaterial interface. J Cell Biochem. 1994;56:340–347. doi: 10.1002/jcb.240560310. [DOI] [PubMed] [Google Scholar]
- Schwartz Z, Fisher M, Lohmann CH, Simon BJ, Boyan BD. Osteoprotegerin (OPG) production by cells in the osteoblast lineage is regulated by pulsed electromagnetic fields in cultures grown on calcium phosphate substrates. Ann Biomed Eng. 2009;37:437–444. doi: 10.1007/s10439-008-9628-3. [DOI] [PubMed] [Google Scholar]
- Song JK, Cho TH, Pan H, Song YM, Kim IS, Lee TH, Hwang SJ, Kim SJ. An electronic device for accelerating bone formation in tissues surrounding a dental implant. Bioelectromagnetics. 2009;30:374–384. doi: 10.1002/bem.20482. [DOI] [PubMed] [Google Scholar]
- Spadaro JA. Electrically enhanced osteogenesis at various metal cathodes. J Biomed Mater Res. 1982;16:861–873. doi: 10.1002/jbm.820160611. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, VanWijnen AJ, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996;76:593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- Sul YT, Johansson C, Wennerberg P, Cho LR, Chang BS, Albrektsson P. Optimum surface properties of oxidized implants for reinforcement of osseointegration: Surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005;20:349–359. [PubMed] [Google Scholar]
- Wang Q, Zhong SZ, Jun OY, Jiang LX, Zhang ZK, Xie Y, Luo SQ. Osteogenesis of electrically stimulated bone cells mediated in part by calcium ions. Clin Orthop. 1998;348:259–268. [PubMed] [Google Scholar]
- Yonemori K, Matsunaga S, Ishidou Y, Maeda S, Yoshida H. Early effects of electrical stimulation on osteogenesis. Bone. 1996;19:173–180. doi: 10.1016/8756-3282(96)00169-x. [DOI] [PubMed] [Google Scholar]
- Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res. 2005;74A:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]