Abstract
AIM: To explore patient interest in a potential multi-organ stool-DNA test (MUST) for pan-digestive cancer screening.
METHODS: A questionnaire was designed and mailed to 1200 randomly-selected patients from the Mayo Clinic registry. The 29-item survey questionnaire included items related to demographics, knowledge of digestive cancers, personal and family history of cancer, personal concern of cancer, colorectal cancer (CRC) screening behavior, interest in MUST, importance of test features in a cancer screening tool, and comparison of MUST with available CRC screening tests. All responses were summarized descriptively. χ2 and Rank Sum Test were used for categorical and continuous variables, respectively.
RESULTS: Completed surveys were returned by 434 (29% aged 50-59, 37% 60-69, 34% 70-79, 52% women). Most participants (98%) responded they would use MUST. In order of importance, respondents rated multi-cancer detection, absence of bowel preparation, safety and noninvasiveness as most attractive characteristics. For CRC screening, MUST was preferred over colorectal-only stool-DNA testing (53%), occult blood testing (75%), colonoscopy (84%), sigmoidoscopy (91%), and barium enema (95%), P < 0.0001 for each. Among those not previously screened, most (96%) indicated they would use MUST if available. Respondents were confident in their ability to follow instructions to perform MUST (98%). Only 9% of respondents indicated that fear of finding cancer was a concern with MUST, and only 3% indicated unpleasantness of stool sampling as a potential barrier.
CONCLUSION: Patients are receptive to the concept of MUST, preferred MUST over conventional CRC screening modalities and valued its potential feature of multi-cancer detection.
Keywords: Stool-DNA testing, Colorectal cancer screening, Gastrointestinal cancer screening, Patient perceptions
Core tip: The value of stool DNA testing could be expanded beyond colorectal cancer screening by simultaneously targeting gastrointestinal cancers above the colon. Early data suggest technical feasibility for such pan-cancer detection. However, while multi-organ stool DNA testing (MUST) would seem intuitively to have broad appeal; patient perceptions have not been evaluated. In this exploratory study, we demonstrate that patients were interested in using MUST if it was available to them. The potential unique ability to detect multiple cancers was its most distinguishing and attractive feature. General population surveys are warranted to corroborate these early findings.
INTRODUCTION
In aggregate, malignancies in the digestive track account for roughly 1/4 of all cancer deaths in the United States[1] and worldwide[2]. Although early stage detection and resection lead to a favorable prognosis with tumors at each gastrointestinal site, only colorectal cancer (CRC) is currently screened at the population level in most countries. It is remarkable that the common cancers above the colon remain unscreened despite the reality that their collective mortality substantially exceeds that of CRC alone[1].
Early studies suggest that supra-colonic gastrointestinal cancers can be detected noninvasively by stool DNA testing. In 2009[3], our research group evaluated the feasibility of stool-DNA testing for detection of common neoplasms throughout the gastrointrstinal GI tract. We were able to detect specific mutations (TP53, KRAS, APC, CDH1, CTNNB1, BRAF, SMAD4, and P16) present in primary tumor tissue from matched stools of patients with diverse supra-colonic gastrointestinal malignancies. Target mutations were detected in stools from 71% (36/51) of patients with cancer overall [40% (2/5) with oropharyngeal, 65% (11/17) with esophageal, 100% (4/4) with gastric, 55% (6/11) with pancreatic, 75% (3/4) with biliary or gallbladder, and 100% (4/4) with colorectal], while none were detected in the matched-control groups. In the same year, a group from Japan[4] used a novel fecal DNA methylation assay to detect increased methylation of gene promoters in patients with gastric and colorectal tumors (57%-75%) as opposed to only 10% of subjects without neoplasms. More recently, using a similar approach, we evaluated aberrantly methylated genes as non-invasive markers by stool DNA testing for the detection of pancreatic cancer[5]. The results from this study demonstrated that at 90% specificity, methylated BMP3 detected 51% of pancreatic cancer, while a combined stool assay of methylated BMP3 and mutant KRAS increased pancreatic cancer detection to 67%. Overall, these early findings support the potential and feasibility of a non-invasive multi-organ gastrointestinal stool-DNA test for cancer screening.
Ideally, such multi-organ stool DNA testing (MUST) would have the potential to expand the value of stool screening beyond that of CRC detection alone and address the existing gap in screening for upper gastrointestinal cancers. While the potential availability of MUST would seem intuitively to have broad patient appeal, there are no data on patient acceptability or perceptions of such an approach.
Endorsed by the American Cancer Society, the US Multi-society Task Force, and the American College of Radiology, stool DNA testing has emerged as an approach to CRC screening[6]. Stool DNA testing offers user-friendly features of noninvasiveness, avoidance of unpleasant bowel preparation associated with other approaches[7-12], ease of access via off-site sample collection and shipping, single rather than multiple stool sampling per screen, no diet or medication restriction, and possibly reduced screen frequency because of its capacity to detect precursor lesions[13,14]. With advanced next generation technology, stool-DNA testing has proven highly accurate for detection of both CRC and advanced precancer[15,16], and an automated test is currently under review by the FDA following evaluation in a general population[17]. In prior surveys, patients showed interest in using stool-DNA testing for CRC screening and appeared to prefer it over both fecal occult blood testing (FOBT) and colonoscopy[9,18-22]. However, it is not clear if an expanded capacity of stool-DNA testing for multi-cancer detection would enhance or impede participation in a CRC screening application.
Knowledge of patient perceptions and preferences regarding screening tools is important to understand compliance to screening[22-26]. For example, patient concern about pain, potential injury and discomfort with cathartic preparations are recognized barriers to routine screening with colonoscopy, flexible sigmoidoscopy, or barium enema[27,28]. While FOBT is a low risk and noninvasive screening alternative, the variability in cancer detection rates, inconvenient stool sampling, dietary restrictions, and poor sensitivity for precursor lesions, may limit its acceptance by some[29-32]. If MUST is to be further considered for a potential future pan-digestive cancer screening application, an early appraisal of patient attitudes would be instructive.
In this exploratory study, we designed a questionnaire to assess interest in and preferences for using MUST. We examined and compared perceptions and preferences for MUST against available CRC screening options.
MATERIALS AND METHODS
Study population and data collection
A total of 1200 patients were randomly selected within age and gender groups from the Mayo Clinic registry. Questionnaires were mailed to 400 candidates (200 men, 200 women) in each of 3 average-risk sub-groups between 50-79 years of age (50-59, 60-69, and 70-79 years).
Sample size considerations: In this exploratory study, we targeted a sample large enough to provide a 95% confidence interval within ± 10 percentage points; and 100 respondents would yield such confidence. Based on 1200 candidates, we assumed that 1000 would have a current address, 500 would respond to the survey, and 100 respondents would not have undergone routine CRC screening.
Questionnaire survey
Questionnaire mailing from the Survey Research Center included a cover letter explaining the nature and purpose of the study and inviting the subject to complete the survey and return it in the stamped, pre-addressed envelope provided. A waiver consent form was included with the mailing and required signature for participation. Only one mailing was sent per participant with no follow-ups attempted.
Survey instrument: The questionnaire was designed in collaboration with the Mayo Clinic Survey Research Center (Appendix). Question format was modeled after those developed in the Health Information National Trends Survey (HINTS) 2007 on perceived risk, screening behavior, knowledge and concern about cancer.
The 29-item survey questionnaire included items related to demographics, knowledge of airway and digestive cancers, personal and family history of cancer, personal concern of cancer, CRC screening behavior, interest in MUST, importance of test features in a cancer screening tool, and comparison of MUST with available CRC screening tests.
Respondents’ general knowledge of cancer was assessed by their ability to associate common risk factors (i.e., age, smoking, obesity, alcohol consumption) with cancer development. Patients who specified a personal and/or family diagnosis of cancer (lung, breast, prostate, colon or rectal, esophageal, stomach, pancreatic, melanoma, and/or other) were considered to have a positive history of cancer. Personal concern of cancer was evaluated by asking how often (i.e., all the time, often, sometimes, rarely or never) patients worried about developing any of the following cancers: lung, breast, colon or rectal, esophagus, stomach, pancreas, prostate.
Patients were asked about their likelihood of using MUST if it was available to them on a 5-point Likert-like scale with the following response options: very likely, likely, unlikely, and not sure. Seven items were also included describing possible reasons patients might choose MUST. Patients were again asked to rate these test features in terms of importance to them on a 5-point Likert-like scale.
Patients were asked to rank order their preferences for CRC screening tests among the following options: MUST, FOBT, colorectal-only stool-DNA testing, colonoscopy, flexible sigmoidoscopy, and barium enema. Patients were asked to rate the importance of test features (i.e., ability to detect pre-cancerous lesions, accuracy, risk of injury, degree of discomfort, need for bowel preparation, cost) when choosing a regular CRC screening test on a 5-point Likert-like scale.
Statistical analysis
All responses were included for analysis when possible. If there was any confusion over the intent of an answer, the response was not included. All responses to surveys were summarized descriptively. χ2 tests were performed to test for differences in baseline characteristics for all categorical characteristics. The Rank Sum Test was used to test for differences for all continuous characteristics. Since only a small subset of items were available for non-respondents, we also explored differences between early and later respondents in order to better understand the impact of potential non-response bias. χ2 tests were used for these comparisons. In addition, the Wilcoxon Sign Rank Test was performed to compare the preference rank for MUST when compared to each of the other 5 colorectal screening tests. A P value of < 0.05 was considered statistically significant.
RESULTS
Sample characteristics
Thirty-six percent (434 of 1200) of mailed out surveys were completed and returned between November 14th, 2008 and January 16th, 2009. When respondents were compared to non-respondents, there was no difference in median age (66.0 years vs 64.4 years, P = 0.64), or in the number of days since the patient was last seen at the Mayo Clinic (82.5 vs 89.0, P = 0.16). Women accounted for 52% of respondents compared to 49% of non-respondents (P = 0.34).
Demographic and baseline characteristics of the sample population are summarized (Table 1). The majority of respondents were white, from Minnesota, and with the equivalent of a college degree or higher. A personal history of cancer was reported by 44%, with 9% originating from the airway or digestive tract; and 67% indicated a history of cancer in a first-degree relative. Most respondents acknowledged a personal concern with cancer (74%).
Table 1.
Demographics and baseline characteristics of sample population n (%)
| Characteristics | Value |
| Age (yr) | |
| 50-59 | 122 (28.6) |
| 60-69 | 159 (37.2) |
| 70-79 | 146 (34.2) |
| Sex | |
| Female | 225 (51.8) |
| Male | 209 (48.6) |
| Race/ethnicity | |
| White | 424 (98.4) |
| Non-white | 7 (1.6) |
| Education | |
| Some high school | 11 (2.6) |
| High school graduate or GED | 108 (25.2) |
| Vocational, technical or business school | 40 (9.3) |
| Some college or associate’s degree | 98 (22.9) |
| 4-yr college graduate or Bachelor’s degree | 76 (18.8) |
| Graduate or professional school | 95 (22.2) |
| Region | |
| Minnesota | 251 (57.8) |
| Other | 183 (42.2) |
| Positive personal history of cancer | |
| Aero-digestive cancer1 | 38 (8.8) |
| Other2 | 152 (35.0) |
| No | 244 (56.2) |
| Positive familial history of cancer | |
| Yes | 278 (66.8) |
| No | 125 (30.0) |
| Not sure | 13 (3.1) |
| CRC screening history | |
| Yes | 355 (84.9) |
| No | 50 (12.0) |
| Not sure | 13 (3.1) |
| Personal concern with cancer3 | |
| Yes | 311 (73.5) |
| No | 112 (26.5) |
Includes responses from subjects who specified they had been diagnosed with cancer from any of the following: lung, esophagus, stomach, pancreas, colon or rectum;
Includes responses from subjects who specified they had been diagnosed with cancer from any of the following: breast, prostate, skin (melanoma only), or specified as other.
Defined as subjects who responded ''all the time” or “often” when asked how often they worry about getting one or all of the following cancers: lung, breast, colon or rectal, esophagus, stomach, pancreas, prostate. GED: General equivalent diploma; CRC: Colorectal cancer.
Knowledge about digestive and airway cancers
Most subjects correctly identified age over 50 years (85%), smoking (99%), alcohol consumption (74%) and obesity (76%) as factors that can increase a person’s risk of developing cancer. Many understood that pain or other symptoms are generally absent at early curative stages of lung (65%), pancreas (60%), colorectal (63%), esophageal (47%) and stomach (50%) cancers.
Perceptions of and interest in MUST
Responses regarding the likelihood of using MUST are summarized (Table 2). Overall, most (98%) were interested in MUST and would likely use it, irrespective of physician recommendation. Subgroup comparisons were performed to assess whether likelihood of using MUST varied by age, gender, prior CRC screening, or personal concern with cancer. “Very likely” and “likely” categories were combined and considered a positive response towards likelihood of using MUST. Interest in using MUST was high across all subgroups, and no statistically significant differences were observed.
Table 2.
Likelihood of using multi-organ stool-DNA test
| Characteristic (n) | Very likely | Likely | Unlikely | Very unlikely | Not sure | P value1 |
| Age (yr) | ||||||
| 50-59 (121) | 69.4% | 25.6% | 3.3% | 1.7% | 0.0% | 0.56 |
| 60-69 (157) | 82.2% | 15.3% | 1.3% | 0.0% | 1.2% | |
| 70-79 (145) | 75.2% | 20.7% | 2.1% | 0.0% | 2.0% | |
| Sex | ||||||
| Female (219) | 76.2% | 20.1% | 1.8% | 0.5% | 1.4% | 0.89 |
| Male (204) | 76.0% | 20.0% | 2.5% | 0.5% | 1.0% | |
| Prior CRC screening | ||||||
| Yes (352) | 75.6% | 20.5% | 2.6% | 0.6% | 0.9% | 0.66 |
| No (49) | 75.5% | 20.4% | 0.0% | 0.0% | 4.1% | |
| Do not know (13) | 76.9% | 23.1% | 0.0% | 0.0% | 0.0% | |
| Personal concern with cancer | ||||||
| Yes (311) | 79.1% | 16.7% | 2.6% | 0.3% | 1.3% | 0.48 |
| No (112) | 67.9% | 29.4% | 0.9% | 0.9% | 0.9% | |
| Respondents2 | ||||||
| Early (303) | 76.6% | 19.1% | 3.0% | 0.0% | 1.3% | 0.38 |
| Late (120) | 75.0% | 22.5% | 0.0% | 1.7% | 0.8% | |
CRC: Colorectal cancer.
χ2 test;
Comparing the likelihood of using a multi-organ stool-DNA test between early and late respondents.
MUST features rated as very important included its multi-cancer detection (95%), noninvasiveness (85%), avoidance of bowel preparation (81%), ability to perform the test at home (74%), and other features (Table 3). Subjects were provided with a description of the steps required to complete MUST. Most (98%) were confident in their ability to follow the instructions to complete the test. Reasons for not choosing MUST included uncertainty about physician recommendation (21%), not enough information on MUST (12%), and fear of finding cancer (9%). Only 3% responded that the unpleasantness of stool sampling represented a barrier to using MUST.
Table 3.
Respondents’ rating of test features in multi-organ stool-DNA test and routine screening tool
| Very important | Somewhat important | Not at all important | Not sure | |
| Stool-DNA test | ||||
| Detects multiple cancers with single test | 95.1% | 4.2 | 0.0 | 0.7 |
| Safe noninvasive test | 85.0% | 13.3 | 0.7 | 1.0 |
| No need for bowel preparation | 80.8% | 15.7 | 3.0 | 0.5 |
| No need for sedation | 77.8% | 18.7 | 3.0 | 0.5 |
| No need to change diet or medications | 75.4% | 22.0 | 1.9 | 0.7 |
| Performed in the privacy of home | 73.8% | 21.5 | 4.2 | 0.5 |
| No need to take time off from work | 56.8% | 15.2 | 27.3 | 0.7 |
| A routine screening tool | ||||
| Ability of test to detect pre-cancer or change in body before it becomes cancer | 96.5% | 3.0 | 0.2 | 0.2 |
| Accuracy of the test to say there is a cancer when there really is a cancer | 94.7% | 4.6 | 0.0 | 0.7 |
| Accuracy of the test to say there really is no cancer when there is no cancer | 93.3% | 5.3 | 0.5 | 0.9 |
| Whether test is covered by insurance | 62.2% | 27.7 | 8.5 | 1.6 |
| Risk of injury with test | 55.6% | 31.4 | 10.2 | 2.8 |
| How often the test has to be done | 34.9% | 43.1 | 19.2 | 2.8 |
| The cost of the test | 34.0% | 44.4 | 17.8 | 3.8 |
| Use of laxatives and/or enemas for bowel preparation | 27.8% | 46.5 | 22.9 | 2.8 |
| Discomfort associated with the test | 24.9% | 48.2 | 24.2 | 2.6 |
Perceptions and preferences regarding colorectal cancer screening
Most respondents (85%) indicated that they had previously undergone CRC screening, including by colonoscopy (79%), FOBT (41%), flexible sigmoidoscopy (38%), barium enema (28%), and/or stool-DNA testing (3%). Among the respondent subset without prior CRC screening, most (> 95%) indicated that they would likely use MUST if it was available. The most commonly cited barriers against CRC screening by those who had no prior CRC screening and those who had been screened, but did not intend to do so again, included the perceived low risk of cancer in the absence of symptoms (57%), lack of physician recommendation (56%), bowel preparation (38%), unpleasant or embarrassing elements of the test (29%), and concern about complications such as bleeding, perforation, or injury (22%).
Respondents were asked to rank different tests for regular CRC screening, irrespective of cost or insurance coverage in their decision-making process, by assigning a number from 1 to 7 (1 representing the least preferred and 7 the most preferred). Median preference score was highest for MUST (7.0) and lowest for barium enema (2.0), as shown in Figure 1. MUST was preferred over colorectal-only stool-DNA testing by 53% of respondents, over occult blood testing by 75%, over colonoscopy by 84%, over sigmoidoscopy by 91%, and over barium enema by 95%, P < 0.0001 for each. Most indicated the ability of a test to detect pre-cancerous lesions (97%), test sensitivity (95%), test specificity (94%), insurance coverage (62%) and risk of injury (56%) as very important test features when choosing the type of screening test (Table 3).
Figure 1.
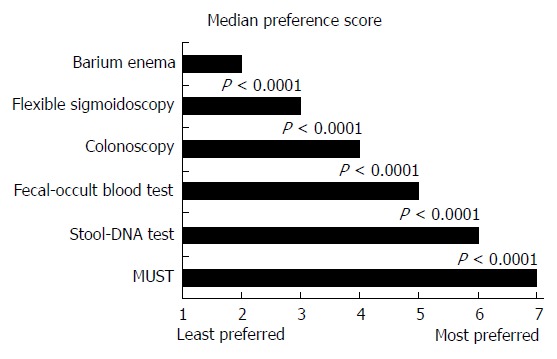
Median preference score for colorectal cancer screening. Scores were assigned from 1 (least preferred) to 7 (most preferred) for currently used screening approaches and for multi-organ stool-DNA test (MUST). P < 0.0001 using Wilcoxon sign rank test.
Assessment of potential response bias
To evaluate the potential for response bias, participants were stratified into early respondents (returned the survey in < 3 mo) and late respondents (returned survey > 3 mo). Early respondents were predominantly women (55% vs 44%, P = 0.04) and from Minnesota (63% vs 46%, P = 0.001) when compared to late respondents (Table 4). There was no difference in race/ethnicity or educational background between early and late respondents. Interest in using MUST was high in both early and late respondents, and no statistical significant difference was observed (Table 2).
Table 4.
Demographics of early versus late respondents
| Characteristics | Early respondents | Late respondents | P value1 |
| Sex | 0.040 | ||
| Female | 55.0% | 44.0% | |
| Male | 45.0% | 56.0% | |
| Race/ethnicity | 0.400 | ||
| White | 98.7% | 97.6% | |
| Non-white | 1.3% | 2.4% | |
| Education | 0.340 | ||
| Some high school | 2.9% | 1.6% | |
| High school graduate or GED | 25.2% | 25.4% | |
| Vocational, technical or business school | 10.8% | 5.7% | |
| Some college or associate’s degree | 23.5% | 21.3% | |
| 4-year college graduate or Bachelor’s degree | 17.7% | 18.0% | |
| Graduate or professional school | 19.9% | 27.9% | |
| Region | 0.001 | ||
| Minnesota | 62.8% | 45.6% | |
| Other | 37.2% | 54.4% |
χ2 test. GED: General equivalent diploma.
DISCUSSION
This study found that most respondents to a survey questionnaire were interested in using MUST if it was available to them. The likelihood of using MUST did not vary significantly on the basis of age, gender, prior history of CRC screening, or personal concern with cancer. The potential to simultaneously screen cancer at multiple organ sites was the most attractive feature of MUST. Our results suggest that the concept of screening for multiple digestive cancers with a stool test is an incentive to its potential use, and stool sampling per se was not perceived as a barrier
Of note, MUST was perceived as the preferred test for CRC screening, including a subset of respondents who had not previously undergone routine CRC screening. The concept of a stool test with capacity to detect both supra-colonic cancers and colorectal cancer was highly rated by respondents when asked to choose a CRC screening method. The majority of respondents identified accuracy, low risk of injury, and avoidance of bowel preparation and sedation as very important features when choosing a screening test. In this survey, noninvasive tests (MUST, colorectal-only stool-DNA testing, and FOBT) were preferred over invasive tests (colonoscopy, flexible sigmoidoscopy, and barium enema). However, it was the feature of multi-cancer detection that was most distinguishing in favoring MUST. These results suggest that multi-cancer detection is perceived as a value-add and that implementation of MUST has the potential to enhance patient participation in CRC screening.
Barriers to screening must be considered with the application of any new methods. Previous studies have identified lack of physician recommendation, lack of awareness of cancer, absence of symptoms and fear of finding cancer as common barriers to screening[7,9,18,19,21-28,33-35]. In our study, absence of provider recommendation was cited by some as a potential reason for not choosing MUST, highlighting the influential role of physicians in patient adherence to cancer screening. Whether personal concern with cancer would negatively impact patients’ attitudes towards multi-cancer screening has not been previously assessed. In this study, fear of finding cancer did not appear to be an obstacle to using MUST, and the concept of multi-cancer detection was positively perceived. Furthermore, nearly all respondents indicated that stool sampling and collection per se was not a barrier.
In this study, respondents identified other specific test attributes, such as the ability to detect precancerous lesions and accuracy for cancer detection as key features when choosing a CRC screening tool. Recent studies have demonstrated that next-generation stool DNA testing can detect curable stage CRC and large precancerous lesions with high sensitivity, irrespective of neoplasm site in the colorectum[15,16]. In light of these advances in stool DNA technology, recent studies have evaluated the possibility of detecting supra-colonic gastrointestinal cancers in the stool[3-5]. Clearly, more clinical studies will be required to further develop and validate stool DNA testing for pan-digestive cancer detection. The results of our survey suggest that this expanded detection capacity of stool-DNA testing appeals to patients and that there are no obvious perceptual barriers to pan-cancer screening.
This exploratory survey study has several limitations and the findings may not be generalizable. First, as a majority of those contacted did not participate, response bias may have influenced our results. However, the similarity in demographics between respondents and non-respondents as well as the striking similarities in baseline characteristics, perceptions, and preferences between early and late respondents may be evidence against a major response bias. Second, the study population of this exploratory survey questionnaire lacked the demographic diversity reflective of the general population. Third, while this study was adequately powered for its objectives, the small sample size did not allow definitive co-variate analyses by demographic subsets. Fourth, our study population was well-informed. Their educational level and knowledge of cancer characteristics may have contributed to the overall positive response to using MUST. Fifth, this study was designed as an exploratory questionnaire survey and thus, the survey tool was not piloted and reliability analysis was not performed. Last, MUST is a hypothetical rather than an actual product at this point. Further research and development are needed before it can be offered for screening. Our survey can only assess perceptions, attitudes and likelihood of using a hypothetical MUST in comparison to already available CRC screening modalities. As such, respondents’ perceptions of MUST may have been affected by its conceptual appeal and the lack of definite information on actual performance on cancer screening. Whether the overall positive response to MUST will translate to utilization once it is available remains to be determined; however, these results encourage further development and testing of MUST.
In conclusion, this study found that our population was interested in using MUST if it was available to them. The potential unique ability to detect multiple cancers was its most distinguishing and attractive feature. Other highly valued test characteristics included its noninvasiveness, absence of bowel preparation and sedation, avoidance of medication or dietary changes, and convenience of performing the test at home. MUST was preferred over conventional screening tools for routine CRC testing. Further studies are needed to determine whether a more diverse ethnic and socioeconomic population would express similar perceptions and preferences for MUST and CRC screening options.
COMMENTS
Background
The value of stool DNA testing could be expanded beyond colorectal cancer (CRC) screening by simultaneously targeting gastrointestinal cancers above the colon. Early data suggest technical feasibility for such pan-cancer detection.
Research frontiers
Knowledge of patient perceptions and preferences regarding screening tools is important to understand compliance to screening. While a multi-organ stool DNA test (MUST) would seem intuitively to have broad appeal; patient perceptions have not been evaluated. In this exploratory study, the authors demonstrate that patients are interested in using MUST if it was available to them.
Innovations and breakthroughs
Prior studies have shown patients’ interest and preference in using stool DNA testing over both fecal occult blood testing and colonoscopy for colorectal cancer screening. This is the first study to evaluate patients’ perceptions and preference for a MUST if it was available to them. The potential to simultaneously screen cancer at multiple organ sites was highly regarded by patients. MUST was preferred over conventional screening tools for routine CRC testing.
Applications
This study highlights the potential ability to detect multiple cancers by MUST as its most distinguishing and attractive feature. Patients valued the noninvasive test characteristics of MUST, and stool sampling was not considered a barrier for screening. Further studies are needed to corroborate these initial findings and to determine receptiveness of such a test in the general population.
Terminology
Stool-DNA testing: biological rationale of targeting DNA alterations (tumor markers, mutations) exfoliated from cancer cells arising in the gastrointestinal tract into stool. A MUST represents a potential noninvasive test that can detect different neoplasms in the GI tract based on multiple target DNA alterations. The concept of a MUST is based on the feasibility of stool-DNA testing for the detection of common supracolonic GI malignancies.
Peer review
This paper evaluates stool DNA testing for pan-digestive cancer screening. This is a well-designed study of survey questionnaire. This manuscript is interesting and most parts of the paper are clearly detailed.
Footnotes
Supported by Research grant from the Oswald Foundation
P- Reviewers: Cao WB, Fujino Y, Kietzmann T, Wong LS S- Editor: Qi Y L- Editor: Webster JR E- Editor: Wang CH
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Zou H, Harrington JJ, Taylor WR, Devens ME, Cao X, Heigh RI, Romero Y, Chari ST, Petersen GM, Roberts LR, et al. T2036 Pan-Detection of Gastrointestinal Neoplasms By Stool DNA Testing: Establishment of Feasibility. Gastroenterology. 2009;136:Abstract T2036. [Google Scholar]
- 4.Kisiel JB, Yab TC, Taylor WR, Chari ST, Petersen GM, Mahoney DW, Ahlquist DA. Stool DNA testing for the detection of pancreatic cancer: assessment of methylation marker candidates. Cancer. 2012;118:2623–2631. doi: 10.1002/cncr.26558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagasaka T, Tanaka N, Cullings HM, Sun DS, Sasamoto H, Uchida T, Koi M, Nishida N, Naomoto Y, Boland CR, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101:1244–1258. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Weitzman ER, Zapka J, Estabrook B, Goins KV. Risk and reluctance: understanding impediments to colorectal cancer screening. Prev Med. 2001;32:502–513. doi: 10.1006/pmed.2001.0838. [DOI] [PubMed] [Google Scholar]
- 8.Gluecker TM, Johnson CD, Harmsen WS, Offord KP, Harris AM, Wilson LA, Ahlquist DA. Colorectal cancer screening with CT colonography, colonoscopy, and double-contrast barium enema examination: prospective assessment of patient perceptions and preferences. Radiology. 2003;227:378–384. doi: 10.1148/radiol.2272020293. [DOI] [PubMed] [Google Scholar]
- 9.Schroy PC, Heeren TC. Patient perceptions of stool-based DNA testing for colorectal cancer screening. Am J Prev Med. 2005;28:208–214. doi: 10.1016/j.amepre.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334:155–159. doi: 10.1056/NEJM199601183340304. [DOI] [PubMed] [Google Scholar]
- 11.Ahlquist DA, McGill DB, Fleming JL, Schwartz S, Wieand HS, Rubin J, Moertel CG. Patterns of occult bleeding in asymptomatic colorectal cancer. Cancer. 1989;63:1826–1830. doi: 10.1002/1097-0142(19900501)63:9<1826::aid-cncr2820630928>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 13.Ahlquist DA. Next-generation stool DNA testing: expanding the scope. Gastroenterology. 2009;136:2068–2073. doi: 10.1053/j.gastro.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Berger BM, Ahlquist DA. Stool DNA screening for colorectal neoplasia: biological and technical basis for high detection rates. Pathology. 2012;44:80–88. doi: 10.1097/PAT.0b013e3283502fdf. [DOI] [PubMed] [Google Scholar]
- 15.Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, Butz ML, Thibodeau SN, Rabeneck L, Paszat LF, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142:248–256; quiz e25-26. doi: 10.1053/j.gastro.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lidgard GP, Domanico MJ, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, Allawi H, Yab TC, Taylor WR, et al. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013;11:1313–1318. doi: 10.1016/j.cgh.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Exact Sciences Corporation. Multi-Target Colorectal Cancer Screening Test for the Detection of Colorectal Advanced Adenomatous Polyps and Cancer (DeeP-C). In Clinical Trials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2013. Available from: http://clinicaltrials.gov/ct2/show/NCT01397747. [Google Scholar]
- 18.Wolf RL, Basch CE, Brouse CH, Shmukler C, Shea S. Patient preferences and adherence to colorectal cancer screening in an urban population. Am J Public Health. 2006;96:809–811. doi: 10.2105/AJPH.2004.049684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health. 2007;10:415–430. doi: 10.1111/j.1524-4733.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128:192–206. doi: 10.1053/j.gastro.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Schroy PC, Lal S, Glick JT, Robinson PA, Zamor P, Heeren TC. Patient preferences for colorectal cancer screening: how does stool DNA testing fare? Am J Manag Care. 2007;13:393–400. [PubMed] [Google Scholar]
- 22.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 23.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38:536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Gorin SS. Correlates of colorectal cancer screening compliance among urban Hispanics. J Behav Med. 2005;28:125–137. doi: 10.1007/s10865-005-3662-5. [DOI] [PubMed] [Google Scholar]
- 25.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46:S10–S16. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 27.Brouse CH, Basch CE, Wolf RL, Shmukler C, Neugut AI, Shea S. Barriers to colorectal cancer screening with fecal occult blood testing in a predominantly minority urban population: a qualitative study. Am J Public Health. 2003;93:1268–1271. doi: 10.2105/ajph.93.8.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasser KE, Ayanian JZ, Fletcher RH, Good MJ. Barriers to colorectal cancer screening in community health centers: a qualitative study. BMC Fam Pract. 2008;9:15. doi: 10.1186/1471-2296-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, Snover DC, Schuman LM. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 30.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 31.Smith RA, Cokkinides V, Eyre HJ. Cancer screening in the United States, 2007: a review of current guidelines, practices, and prospects. CA Cancer J Clin. 2007;57:90–104. doi: 10.3322/canjclin.57.2.90. [DOI] [PubMed] [Google Scholar]
- 32.Heresbach D, Manfredi S, D’halluin PN, Bretagne JF, Branger B. Review in depth and meta-analysis of controlled trials on colorectal cancer screening by faecal occult blood test. Eur J Gastroenterol Hepatol. 2006;18:427–433. doi: 10.1097/00042737-200604000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Seeff LC, Nadel MR, Klabunde CN, Thompson T, Shapiro JA, Vernon SW, Coates RJ. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 34.Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, Marcus AC, Steiner JF, Ahnen DJ. Predictors of nonadherence to screening colonoscopy. J Gen Intern Med. 2005;20:989–995. doi: 10.1111/j.1525-1497.2005.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harewood GC, Wiersema MJ, Melton LJ. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97:3186–3194. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]


