Abstract
AIM: To compare prevalence rates of non-alcoholic fatty liver disease (NAFLD) between Hispanics of Mexican origin and Hispanics of Dominican and Puerto Rican origin.
METHODS: We evaluated prevalence rates of NAFLD between the two largest sub-populations of Hispanics in the United States; Hispanics of Mexican origin and Hispanics of Caribbean origin (Dominican and Puerto Rican), in the multi-ethnic study of atherosclerosis (MESA) cohort. MESA is a large, population based, multi-center cohort study comprised of 6814 healthy Caucasian, African-American, Hispanic, and Asian men and women aged 45-84. We utilized the baseline serum, anthropometric and radiographic measurements obtained between 2000 and 2002. NAFLD was measured via computed tomography scan and was defined as liver/spleen attenuation ratio < 1.
RESULTS: There were 788 Hispanic participants included in the study after exclusions. The prevalence of NAFLD was 29% (n = 225). Hispanics of Mexican origin had a significantly higher prevalence of NAFLD (33%), compared to Hispanics of Dominican origin (16%), (P < 0.01) and Hispanics of Puerto Rican origin (18%), (P < 0.01). After controlling for age, sex, BMI, waist circumference, hypertension, serum HDL, triglyceride and CRP level and insulin resistance, Hispanics of Mexican origin remained significantly more likely to have NAFLD than those of Dominican and Puerto Rican origin.
CONCLUSION: United States Hispanics of Mexican origin have a significantly higher prevalence of NAFLD when compared to United States Hispanics of Dominican or Puerto Rican origin after controlling for known risk factors. Care should be taken when performing risk assessment in Hispanic populations not to make assumptions of homogeneity.
Keywords: Non-alcoholic fatty liver disease, Prevalence, Hispanic subpopulations
Core tip: Hispanics have a significantly higher prevalence of non-alcoholic fatty liver disease (NAFLD) and evidence of more advanced disease when compared to other ethnic groups. As a consequence it has been proposed that clinicians perform biopsies on Hispanics diagnosed with NAFLD given the increased of fibrosis development. Most of the studies examining Hispanics with NAFLD evaluated those of Mexican descent. It is unknown if this increased propensity to develop NAFLD is uniform among all people classified as Hispanics or if certain subpopulations are at higher risk. This study aims to compare the prevalence rates of NAFLD between US Hispanic subgroups.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common etiology of chronic liver disease in the United States as well as worldwide[1-4]. While the true prevalence of NAFLD is unknown, it is thought that up to 30% of the adult United States population may have steatosis due to NAFLD[1,5]. NAFLD is projected to be a major cause of liver-related morbidity and mortality over the next several decades in parallel with the obesity crisis with a prevalence predicted to increase 50% by the year 2030[6]. While not all individuals with NAFLD develop liver-related complications, it is clear that up to 30% will develop non-alcoholic steatohepatitis (NASH) and be at risk for fibrosis, cirrhosis, portal hypertension and hepatocellular carcinoma[7-11].
While central obesity, insulin resistance and dyslipidemia are well-established risk factors for the development of NAFLD, not all individuals with these risk factors develop hepatic steatosis[12]. There also appears to be racial-ethnic variations in the propensity to develop NAFLD with Hispanics being overrepresented in population studies when compared to blacks and whites[13-15]. Furthermore, when compared to blacks and whites, Hispanics with NASH appear to have more advanced histology on liver biopsy[16,17]. There is evidence that the increased prevalence of NAFLD in Hispanics does not appear to be solely attributable to an increased presence of metabolic risk factors associated with NAFLD such as insulin resistance and obesity[15,18] The mechanism by which Hispanics are at a higher risk to develop NAFLD and NASH is unclear but may be attributable to increased intraperitoneal fat distribution[18]. Genetic polymorphisms related to steatosis and hepatocyte injury may also explain such variation in the prevalence of NAFLD among Hispanics[19]. The single amino acid isoleucine to mehtionine polymorphism in the Patatin-like phospholipase domain containing 3 gene (PNPLA3) has been strongly associated with hepatic steatosis across multiple racial-ethnic groups and has a higher frequency among Hispanics when compared to European and African American individuals[20,21].
What is unclear is if the propensity to develop NAFLD is uniform among all subgroups that fall under the umbrella term Hispanic. The term Hispanic is non-specific in that it encompasses a culturally and genetically diverse collection of individuals, illustrated by the poor concordance between genetic based ancestry models and self-reported ethnicity among Hispanics[22-24]. Given such genetic variability and the observed differences in the prevalence of metabolic diseases among Hispanic subgroups, such as type 2 diabetes, we hypothesize that the tendency to develop NAFLD is not uniform among all Hispanics[25]. The aim of this study is to compare prevalence rates of NAFLD between the two largest US Hispanic subgroups: Hispanics of Mexican origin and Hispanics of Caribbean origin (Dominican and Puerto Rican) in the multi-ethnic study of atherosclerosis (MESA) database.
MATERIALS AND METHODS
Study population
The MESA is a large, population based, multi-center cohort study designed to describe risk factors related to the development and progression of subclinical coronary atherosclerotic heart disease. The cohort is comprised of 6814 Caucasian, African-American, Hispanic, and Asian men and women aged 45-84 who were clinically free from cardiovascular disease at baseline and followed for a period of eight years. Study participants were recruited from six communities (Los Angeles County, CA; Manhattan, New York, NY; Baltimore, MD; Chicago, IL; St. Paul, MN and Forsyth County, NC) in the United States. Internal Review Boards at each participating centers approved the studies and each participants gave written informed consent. Participants with active cancer, cognitive impairment, or weight greater than 300 pounds (136 kg) and pregnant individuals were excluded. This study has been described in detail elsewhere[26].
In addition to the MESA exclusion criteria, we additionally excluded for the following: significant alcohol consumption (defined as > 14 drinks on average per week in men and > 7 drinks on average per week in women), a history of Hepatitis C and the absence of a visible liver and/or spleen on cardiac CT (Figure 1). This study focused on comparing Hispanics of Mexican and Caribbean Origin (Dominican and Puerto Rican). Hispanics of Cuban Origin were excluded due to the small number of individuals with NAFLD; n = 2 out of total n = 41 total participants. Hispanics that were described as of Central American origin (n = 46), South American origin (n = 66) and “other” were excluded (n = 12) in an effort to maintain homogeneity.
Figure 1.
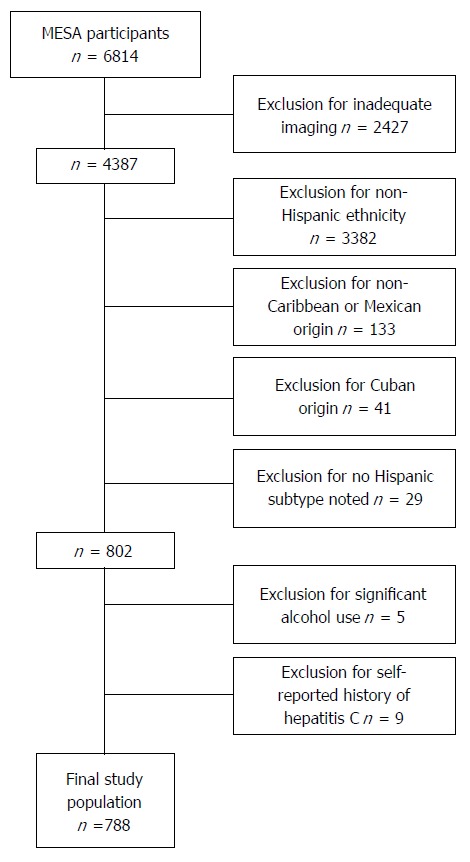
Multi-ethnic study of atherosclerosis participants: Exclusion criteria. Study exclusion criteria. Participants without adequate imaging for liver:spleen ratio calculations were excluded as were individuals with self-reported history of hepatitis C and significant alcohol consumption (> 14 drinks per week in men, > 7 drinks a week in women); Non-Hispanic and Hispanics not of Caribbean origin were excluded; Cubans, due to small number with non-alcoholic fatty liver disease (n = 2) were also excluded. MESA: Multi-ethnic study of atherosclerosis.
Clinical parameters
We utilized the baseline serum, anthropometric and radiographic measurements obtained between 2000 and 2002 of the MESA study. Information on sociodemographic factors (age, sex, and education), lifestyle factors (alcohol consumption and smoking status) and self-reported medical history (hypertension, diabetes, liver disease and cirrhosis) were collected at the baseline examination using standardized questionnaires. A central laboratory (University of Vermont, Burlington) measured levels of total and HDL cholesterol, triglycerides, plasma glucose, and high-sensitivity C-reactive protein in blood samples obtained after a 12 h fast. Waist circumference was measured horizontally at the level of the umbilicus. Hip girth was measured at the maximum circumference of the buttocks. Body mass index was calculated as weight in kilograms divided by height in meters squared. Homeostatic model assessment was used to measure insulin resistance (HOMA-IR) calculated as fasting glucose (mg/dL) × fasting insulin (μU/mL) /405[27].
NAFLD definition and radiographic method
All participants in the MESA study obtained non-contrast cardiac computed tomography (CT) scans at baseline that included areas of the upper abdomen. Attenuation coefficients in HU were measured in four areas of the liver and two areas of the spleen. Measurements for each area included the minimum, maximum and mean HU for a 2 cm round or ellipse region of interest (ROI) in the right and left lobes of the liver, as well as in the spleen. If sufficient tissue was not available for a 2 cm measure, a 1 cm measurement was obtained. If less than 1 cm of tissue was evident on the cardiac scan, the study was deemed not sufficient for measure. A final HU value for each liver was calculated by averaging the four ROI analyzed. Similarly a final HU value for the spleen was calculated by averaging the two ROI analyzed. A liver/spleen attenuation ratio (LS ratio) was then calculated comparing the final HU measurement between the liver and spleen on each CT scan. NAFLD was defined as a LS ratio of < 1. LS ratio < 1 has an area under the receiver operating curve (AUROC) of 0.991 when measuring hepatic steatosis > 30%, corresponding to moderate to severe steatosis on histology[28-31].
Statistical analysis
Results of summary outcome measures were reported as mean ± standard deviation and proportions. Differences between groups were tested using χ2 analyses for categorical data and two sample Student’s t test for continuous variables. Logistic regression was used to determine predictors of NAFLD using univariate and multivariate analysis. In our analytical models we included a core set of covariates which included age (as a continuous measure), gender and education (measured dichotomously; completed high school yes or no). We included the variables that were significant with a P value of 0.20 or smaller on univariate analysis into our multivariate model of the entire population. All analyses were performed using Stata version 11.0 (StataCorp LP, College Station, Texas).
RESULTS
Baseline characteristics of the study population
After applying our exclusion criteria there were a total of 788 participants available for analysis, baseline descriptive characteristics are reported in Table 1. The mean age (years) of participants was 61 ± 10 and 54% of participants were female. Of the final study population, 67% (n = 524) were of Mexican (MX) origin, 15 % (n = 121) of Dominican (D) origin and 18% (n = 143) of Puerto Rican (PR) origin. Diabetes was reported in 19% of the population. A total of 225 (29%) participants had radiographic NAFLD at baseline as defined by LS ratio < 1. Radiographic NAFLD was found in 34% of participants of Mexican origin (n = 179), 17% (n = 21) of Dominican origin and 17% (n = 25) of Puerto Rican origin (Figure 2).
Table 1.
Descriptive characteristics of the study populations n (%)
| Variable | Value (n = 788) |
| NAFLD | 225 (29) |
| Age (yr) | 61 ± 10.4 |
| Male gender | 361 (46) |
| Puerto Rican | 143 (18) |
| Dominican | 121 (15) |
| Mexican | 524 (67) |
| BMI (kg/m2) | 29.6 ± 5.1 |
| Waist circumference (cm) | 100.9 ± 12.9 |
| Hypertension | 280 (36) |
| C-reactive protein (mg/dL) | 4.3 ± 5.3 |
| Total cholesterol (mg/dL) | 198.5 ± 37.0 |
| Triglyceride (mg/dL) | 162.0 ± 103.0 |
| LDL (mg/dL) | 119.8 ± 33.0 |
| HDL (mg/dL) | 47.2 ± 13.0 |
| Diabetes | 148 (19) |
| HOMA-IR | 2.2 ± 2.7 |
| Metabolic syndrome | 355 (45) |
| High school completion | 416 (53) |
Data are expressed as n (%) or mean ± SD. NAFLD: Non-alcoholic fatty liver disease; LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
Figure 2.
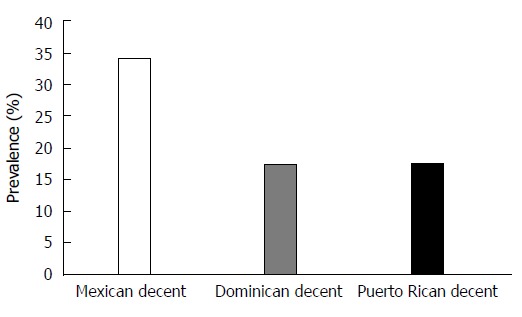
Prevalence of non-alcoholic fatty liver disease by Hispanic subgroup.
There were several differences in the descriptive baseline variables between participants of Mexican and Dominican origin. When compared to participants of Dominican origin, Hispanics of Mexican origin were older, more likely to be male, have a higher BMI and have a larger waist circumference. There were also several physiologic and metabolic differences between these two groups. When compared to participants of Dominican origin, Hispanics of Mexican origin had lover HDL levels, higher triglyceride levels and HOMA insulin resistance scores. They were more likely to have metabolic syndrome but less likely to have hypertension (Table 2).
Table 2.
Baseline demographic, anthropomorphic and physiologic characteristics of the study population: Hispanics of Mexican and Hispanics of Dominican origin n (%)
| Variable | Mexican (n = 524) | Dominican (n = 121) | P value |
| NAFLD | 179 (34) | 21 (17) | < 0.001 |
| Age (yr) | 62 ± 10.3 | 59 ± 10.7 | 0.005 |
| Male gender | 256 (49) | 45 (37) | 0.02 |
| BMI (kg/m2) | 30.1 ± 5.1 | 27.8 ± 4.6 | < 0.001 |
| Waist circumference (cm) | 102.0 ± 12.4 | 96.5 ± 12.8 | < 0.001 |
| Hypertension | 172 (33) | 54 (45) | 0.01 |
| C-reactive protein (mg/dL) | 4.5 ± 5.7 | 3.8 ± 5.6 | 0.25 |
| Total cholesterol (mg/dL) | 198.7 ± 37.8 | 197.9 ± 34.6 | 0.84 |
| Triglyceride (mg/dL) | 176.7 ± 113.9 | 124.8 ± 61.3 | < 0.001 |
| LDL (mg/dL) | 118.4 ± 32.5 | 124.4 ± 34.1 | 0.07 |
| HDL (mg/dL) | 46.1 ± 12.4 | 48.8 ± 12.7 | 0.03 |
| Diabetes | 108 (21) | 15 (12) | 0.10 |
| HOMA insulin resistance | 2.4 ± 3.2 | 1.5 ± 1.0 | 0.002 |
| Metabolic syndrome | 266 (51) | 36 (30) | < 0.001 |
| High school completion | 266 (51) | 59 (49) | 0.69 |
Data are expressed as n (%) or mean ± SD. NAFLD: Non-alcoholic fatty liver disease; LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
Participants of Puerto Rican and Mexican origin were similar with respect to descriptive baseline variables except that when compared to those of Puerto Rican origin, Hispanics of Mexican origin had a lower rate of high school completion. There were several differences with respect to physiologic and metabolic parameters. When compared to participants of Puerto Rican origin, Hispanics of Mexican origin had higher triglyceride and HOMA insulin resistance levels, lower HDL levels and higher rates of metabolic syndrome (Table 3).
Table 3.
Baseline demographic, anthropomorphic and physiologic characteristics of the study population: Hispanics of Mexican and Hispanics of Puerto Rican origin n (%)
| Variable | Mexican (n = 524) | Puerto Rican (n = 143) | P value |
| NAFLD | 179 (34) | 25 (17) | < 0.001 |
| Age (yr) | 62 ± 10.3 | 60 ± 10.5 | 0.07 |
| Male gender | 256 (49) | 60 (42) | 0.14 |
| BMI (kg/m2) | 30.1 ± 51 | 29.5 ± 5.2 | 0.29 |
| Waist circumference (cm) | 102.0 ± 12.4 | 100.3±14.0 | 0.16 |
| Hypertension | 172 (33) | 54 (38) | 0.27 |
| C-reactive protein (mg/dL) | 4.5 ± 5.7 | 4.2 ± 3.6 | 0.52 |
| Total cholesterol (mg/dL) | 198.7 ± 37.8 | 198.2 ± 36.6 | 0.89 |
| Triglyceride (mg/dL) | 176.7 ± 113.9 | 139.5 ± 73.1 | < 0.001 |
| LDL (mg/dL) | 118.4 ± 32.5 | 121.0 ± 33.7 | 0.40 |
| HDL (mg/dL) | 46.1 ± 12.4 | 49.6 ± 14.6 | 0.01 |
| Diabetes | 108 (21) | 25 (17) | 0.61 |
| HOMA | 2.4 ± 3.2 | 1.9 ± 1.5 | 0.05 |
| Metabolic syndrome | 266 (51) | 53 (37) | 0.003 |
| High school completion | 266 (51) | 91 (64) | 0.01 |
Data are expressed as n (%) or mean ± SD. NAFLD: Non-alcoholic fatty liver disease; LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
Hispanics of Dominican and Puerto Rican origin were similar with respect to baseline descriptive values except that participants of Dominican origin had lower BMI (kg/m2) and smaller waist circumference (cm) than participants of Puerto Rican origin as well as lower rates of high school completion. With respect to physiologic and metabolic parameters, participants of Dominican and participants of Puerto Rican origin were similar except that participants of Puerto Rican origin had higher HOMA insulin resistance scores (Table 4).
Table 4.
Baseline demographic, anthropomorphic and physiologic characteristics of the study population: Hispanics of Puerto Rican and Hispanics of Dominican origin n (%)
| Variable | Dominican (n = 121) | Puerto Rican (n = 143) | P value |
| NAFLD | 21 (17) | 25 (17) | 0.98 |
| Age (yr) | 59 ± 10.7 | 60 ± 10.5 | 0.35 |
| Male gender | 60 (42) | 45 (37) | 0.43 |
| BMI (kg/m2) | 27.8 ± 4.6 | 29.5 ± 5.2 | 0.006 |
| Waist circumference (cm) | 96.5 ± 12.8 | 100.3 ± 14.0 | 0.02 |
| Hypertension | 54(38) | 54 (45) | 0.25 |
| C-reactive protein (mg/dL) | 3.8 ± 5.6 | 4.2 ± 3.6 | 0.56 |
| Total cholesterol (mg/dL) | 197.9 ± 34.6 | 198.2 ± 36.5 | 0.95 |
| Triglyceride (mg/dL) | 124.8 ± 61.3 | 139.5 ± 73.1 | 0.08 |
| LDL (mg/dL) | 124.4 ± 34.1 | 121.0 ± 33.7 | 0.42 |
| HDL (mg/dL) | 48.8 ± 12.7 | 49.6±14.6 | 0.67 |
| Diabetes | 15 (12) | 25 (17) | 0.25 |
| HOMA | 1.5 ± 1.0 | 1.9 ± 1.5 | 0.02 |
| Metabolic syndrome | 36 (30) | 53 (37) | 0.21 |
| High school completion | 59 (49) | 91 (64) | 0.02 |
Data are expressed as n (%) or mean ± SD. NAFLD: Non-alcoholic fatty liver disease; LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
Hispanic subgroup as a risk factor for NAFLD
Univariate analysis: Being a Hispanic of Dominican origin was associated with a significantly lower crude odds ratio for the presence of NAFLD when compared to being a Hispanic of Mexican origin. Similarly Hispanics of Puerto Rican origin had significantly lower crude odds ratio of NAFLD prevalence when compared to Hispanics of Mexican origin (Table 5).
Table 5.
Crude and adjusted odds ratios for non-alcoholic fatty liver disease by Hispanic subgroup with Hispanics of Mexican origin as the reference population
| Hispanic subgroup | Total (n) | With NAFLD (n) |
Crude |
Adjusted |
||||
| OR | 95%CI | P value | OR | 95%CI | P value | |||
| Mexican | 524 | 179 | 1 (ref) | 1 (ref) | ||||
| Dominican | 121 | 21 | 0.40 | 0.24-0.67 | < 0.001 | 0.49 | 0.28-0.86 | 0.01 |
| Puerto Rican | 143 | 25 | 0.41 | 0.26-0.65 | < 0.001 | 0.44 | 0.29-0.86 | 0.002 |
Multivariate regression analysis was performed, controlling for age, sex, BMI, waist circumference, hypertension, level of education (completion of high school), serum HDL levels, serum triglycerides levels, serum C-reactive protein levels and homeostasis model assessment of insulin sensitivity. NAFLD: Non-alcoholic fatty liver disease.
We compared the predictors of NAFLD in individuals with NAFLD in each subgroup. There was no statistically significant difference in any predictor across groups (Table 6). When we compared the groups to each other, the only significant difference found was that Hispanics of Mexican origin with NAFLD had a significantly higher BMI than Hispanics of Dominican origin with NAFLD (P = 0.05) and Hispanics of Puerto Rican origin with NAFLD had significantly more individuals with hypertension than those of Mexican origin (P = 0.04) with NAFLD.
Table 6.
Comparison of predictors in Hispanic subgroups with non-alcoholic fatty liver disease n (%)
| Variable | Mexican (n = 179) | Dominican (n = 21) | Puerto Rican (n = 25) | P value |
| Age (yr) | 60 ± 9.9 | 62 ± 9.9 | 58 ± 10.5 | 0.43 |
| Male gender | 86 (48) | 7 (33) | 11 (44) | 0.43 |
| BMI (kg/m2) | 32.0 ± 5.4 | 29.5 ± 5.0 | 32.2 ± 5.4 | |
| Waist circumference (cm) | 105.7 ± 12.5 | 103.7 ± 14.0 | 107.0 ± 13.9 | 0.67 |
| Hypertension | 68 (38) | 11 (52) | 15 (60) | 0.07 |
| C-reactive protein (mg/dL) | 5.1 ± 5.3 | 7.1 ± 8.2 | 5.9 ± 4.2 | 0.24 |
| Total cholesterol (mg/dL) | 196.8 ± 41.4 | 190.0 ± 31.0 | 197.3 ± 31.6 | 0.74 |
| Triglyceride (mg/dL) | 202.4 ± 140.6 | 138.6 ± 54.8 | 173.5 ± 106.7 | 0.08 |
| LDL (mg/dL) | 114.1 ± 31.1 | 117.6 ± 26.4 | 120.0 ± 108.7 | 0.62 |
| HDL (mg/dL) | 43.3 ± 11.4 | 44.7 ± 9.5 | 44.4 ± 9.6 | 0.80 |
| Diabetes | 45 (25) | 3 (14) | 7 (28) | 0.80 |
| HOMA | 3.1 ± 2.1 | 2.3 ± 1.4 | 2.6 ± 1.5 | 0.14 |
| Metabolic syndrome | 117 (66) | 12 (57) | 16 (64) | 0.74 |
| High school completion | 87 (49) | 8 (38) | 16 (64) | 0.20 |
Data are expressed as n (%) or mean ± SD. NAFLD: Non-alcoholic fatty liver disease; LDL: Low-density lipoprotein; HDL: High-density lipoprotein.
Multivariate analysis: Hispanics of Dominican and Puerto Rican origin had a significantly lower risk for the prevalence of NAFLD compared to Hispanics of Mexican origin on multivariate analysis after controlling for age, sex, BMI, waist circumference, hypertension, level of education, serum HDL, triglyceride and CRP levels and HOMA. Adjusted odds ratios can be seen in Table 5.
DISCUSSION
While several studies have demonstrated a higher frequency of NAFLD among Hispanic individuals, little is known about the distribution of NAFLD among various Hispanic subgroups. Given the genetic and cultural variance among Hispanics, illustrated by poor concordance between genetic ancestry markers and self-reported ethnicity, we hypothesized that the distribution of NAFLD would not be uniform among Hispanic subgroups. Here we demonstrate a significant difference in the frequency of moderate to severe steatosis between the two largest United States subgroups of Hispanics (those of Mexican vs Caribbean origin) utilizing a large cohort database.
Hispanics of Mexican origin showed a higher prevalence of NAFLD when compared to both Hispanics of Dominican and Puerto Rican origin, while there was no significant difference in the frequency of NAFLD between Hispanics of Dominican and Puerto Rican origin. At baseline, Hispanics of Mexican origin were more similar to Hispanics of Puerto Rican origin than those of Dominican origin with respect to demographic, metabolic and anthropomorphic features. Despite these similarities, Hispanics of Mexican origin had a much higher frequency of NAFLD than Hispanics of Puerto Rican origin in our study suggesting perhaps that additional factors other than the presence of the traditional risk factors for NAFLD is related to the development of steatosis in Mexican individuals. Although Hispanics of Mexican origin were more likely to be diabetic and have metabolic syndrome than Hispanics of Dominican origin, Hispanics of Mexican origin were still more likely to have NAFLD when these traditional NAFLD risk factors were controlled for on multivariate analysis, again suggesting other factors at play perhaps genetic increasing the predisposition of Hispanics of Mexican origin to develop NAFLD compared to other Hispanic groups. PNPLA3 has been shown to account for up to 72% of the ethnic differences in the prevalence of NAFLD[32]. Further studies are necessary to see if this explains the differences in prevalence we have found among Hispanic subtypes living in the US.
Our study does have some limitations. This study is a cross-sectional analysis of a prospective cohort population and causality cannot be determined. In our study, NAFLD was defined indirectly by using radiographic methods. Although there is a strong concordance between moderate to severe steatosis (> 30%) and LS ratios, the gold standard is liver biopsy and less severe steatosis may have been present in individuals but not seen by radiographic methods. Additionally, due to the lack of histologic data, we cannot comment on the prevalence of NASH in Hispanics of Caribbean origin which has been shown to be more severe in Hispanics of Mexican origin[16,17]. Individuals with a weight greater than 300 lbs were excluded from the MESA database and we were not able to assess the prevalence rates of NAFLD in very obese individuals based on Hispanic subgroups.
As far as we know, this is the first study to compare the frequency of NAFLD between the major Hispanics subgroups living the United States. The majority of studies describing NAFLD in Hispanic populations were performed predominantly on Hispanics of Mexican origin. Given that the current American Association for the Study of Liver Disease guidelines recommend liver biopsy in individuals with NAFLD deemed to be at high risk of NASH and fibrosis, and count a Hispanic ethnic background as a risk factor, it is important to determine which specific Hispanic individuals are in fact are at high risk for NAFLD. Such risk stratification could significantly decrease the number of invasive procedures performed. Our findings help clinicians to better determine and ascribe the risk of NAFLD to the Hispanic patients they see based on region of origin. Further studies are needed to explore if there are similar differences in the prevalence of NAFLD between other Hispanics subgroups not included in this study as well as determine what genetic factors are contributing to these differences seen. For now, care should be taken in generalizing studies related to NAFLD that involves Hispanics, especially of Mexican origin, to all groups that fall under the umbrella term Hispanic.
COMMENTS
Background
Nonalcoholic fatty liver disease (NAFLD) is the most common etiology of chronic liver disease and is projected to soon be the leading cause of liver transplants. Hispanics are significantly more likely to have NAFLD when compared to other ethnic groups. Hispanics with NAFLD are also more likely to have advanced disease steatosis and fibrosis on histology compared to other ethnic groups. As a result, it is recommended that liver biopsy should be obtained in Hispanic patients found to have NAFLD according to American Association for the Study of Liver Diseases guidelines. The term “Hispanic” applies to a very diverse group of people and it is unclear if this increased risk and therefore the need for liver biopsy is uniform.
Research frontiers
NAFLD is the leading cause of chronic liver disease. A research hotspot in this field is being able to better define which individuals are at risk.
Innovations and breakthroughs
The majority of studies evaluating NAFLD in United States Hispanics are performed on Mexican Americans. This is the first study to compare Hispanic subgroups in the United States.
Applications
Currently guidelines recommend liver biopsy in those individuals deemed high risk for non-alcoholic steatohepatitis. Their findings allow for appropriate risk stratification among a culturally and genetically diverse group of people.
Terminology
According to the US census bureau The term “Hispanic” is used to describe people who classify themselves as being of “Mexican, Mexican Am., Chicano” or ”Puerto Rican” or “Cuban” - as well as those who indicate that they are “another Hispanic, Latino, or Spanish origin”. They further state that origin can be viewed as the heritage, nationality group, lineage, or country of birth of the person or the person’s ancestors before their arrival in the United States and people who identify their origin as Hispanic, Latino, or Spanish may be of any race.
Peer review
This is a cross-sectional observational study to compare prevalence rates of NAFLD between the two largest subpopulations of Hispanics. NAFLD was diagnosed by computed tomography. The results of this study show that Hispanics of Mexican origin had a significantly higher prevalence of NAFLD than Dominican or Puerto Rican origin.
Footnotes
Supported by National Heart, Lung, and Blood Institute at the National Institutes of Health grants R01 HL071739 and by contracts N01-HC-95159 through N01-HC-95165 and N01 HC 95169
P- Reviewers: Fan JG, Julie NL, Matsuzaki Y S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 3.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883–889. doi: 10.1111/j.1365-2036.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 5.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530.e1; quiz e60. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM. Nonalcoholic steatohepatitis. Semin Liver Dis. 2004;24:3–20. doi: 10.1055/s-2004-823098. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 10.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 11.White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342–1359.e2. doi: 10.1016/j.cgh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11:1–16, vii. doi: 10.1016/j.cld.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 14.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 15.Bambha K, Belt P, Abraham M, Wilson LA, Pabst M, Ferrell L, Unalp-Arida A, Bass N. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769–780. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty SR, Troy TN, Huo D, O’Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797–804. doi: 10.1016/j.jhep.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiskirchen R, Wasmuth HE. The genes that underlie fatty liver disease: the harvest has begun. Hepatology. 2009;49:692–694. doi: 10.1002/hep.22800. [DOI] [PubMed] [Google Scholar]
- 20.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagenknecht LE, Palmer ND, Bowden DW, Rotter JI, Norris JM, Ziegler J, Chen YD, Haffner S, Scherzinger A, Langefeld CD. Association of PNPLA3 with non-alcoholic fatty liver disease in a minority cohort: the Insulin Resistance Atherosclerosis Family Study. Liver Int. 2011;31:412–416. doi: 10.1111/j.1478-3231.2010.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caballero AE. Understanding the Hispanic/Latino patient. Am J Med. 2011;124:S10–S15. doi: 10.1016/j.amjmed.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Bryc K, Velez C, Karafet T, Moreno-Estrada A, Reynolds A, Auton A, Hammer M, Bustamante CD, Ostrer H. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci USA. 2010;107 Suppl 2:8954–8961. doi: 10.1073/pnas.0914618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wassel CL, Jacobs DR, Duprez DA, Bluemke DA, Sibley CT, Criqui MH, Peralta CA. Association of self-reported race/ethnicity and genetic ancestry with arterial elasticity: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Soc Hypertens. 2011;5:463–472. doi: 10.1016/j.jash.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flegal KM, Ezzati TM, Harris MI, Haynes SG, Juarez RZ, Knowler WC, Perez-Stable EJ, Stern MP. Prevalence of diabetes in Mexican Americans, Cubans, and Puerto Ricans from the Hispanic Health and Nutrition Examination Survey, 1982-1984. Diabetes Care. 1991;14:628–638. doi: 10.2337/diacare.14.7.628. [DOI] [PubMed] [Google Scholar]
- 26.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 27.Blaha MJ, DeFilippis AP, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, Szklo M, Lakoski SG, Bertoni AG, Kronmal RA, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 29.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–445. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, Ha HK, Lee MG, Hwang S, Lee SG, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Romeo S, Huang-Doran I, Baroni MG, Kotronen A. Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein. Curr Opin Lipidol. 2010;21:247–252. doi: 10.1097/mol.0b013e328338ca61. [DOI] [PubMed] [Google Scholar]


